Abstract
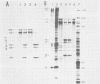
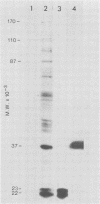
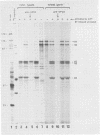
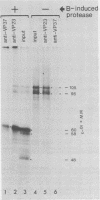
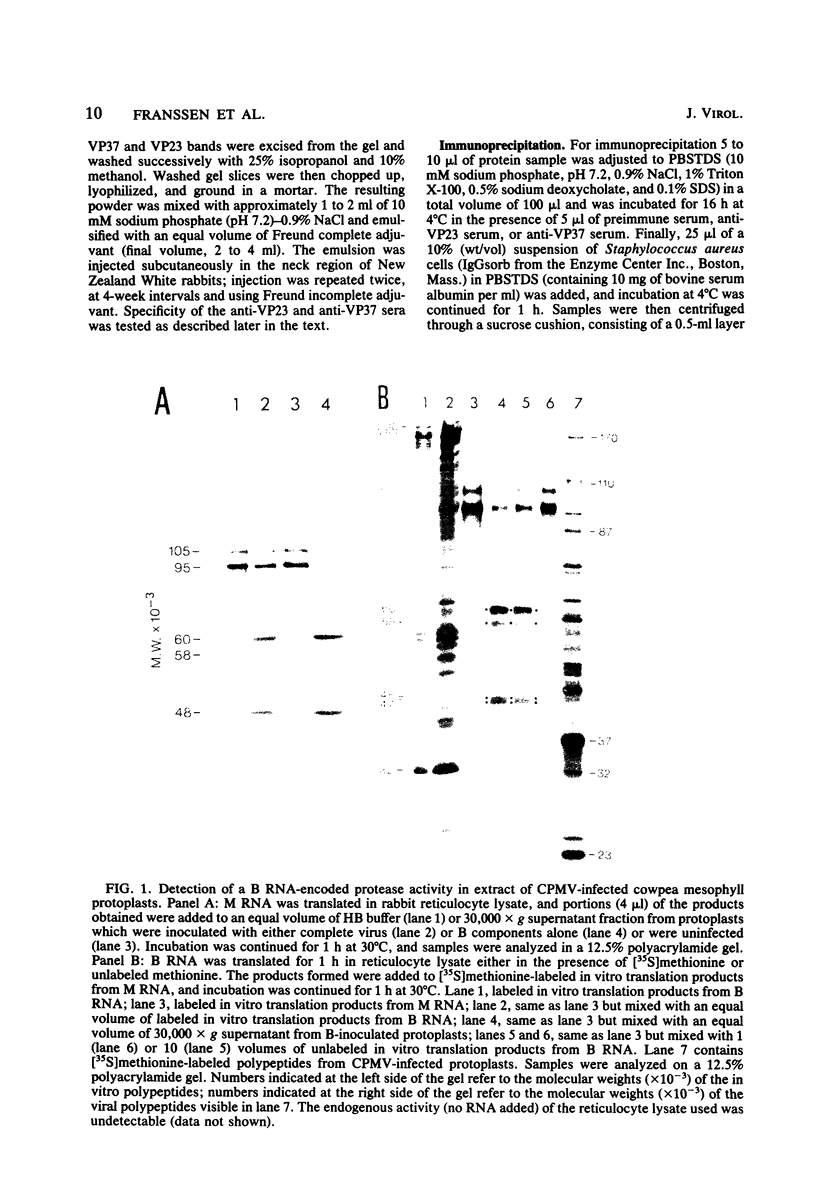
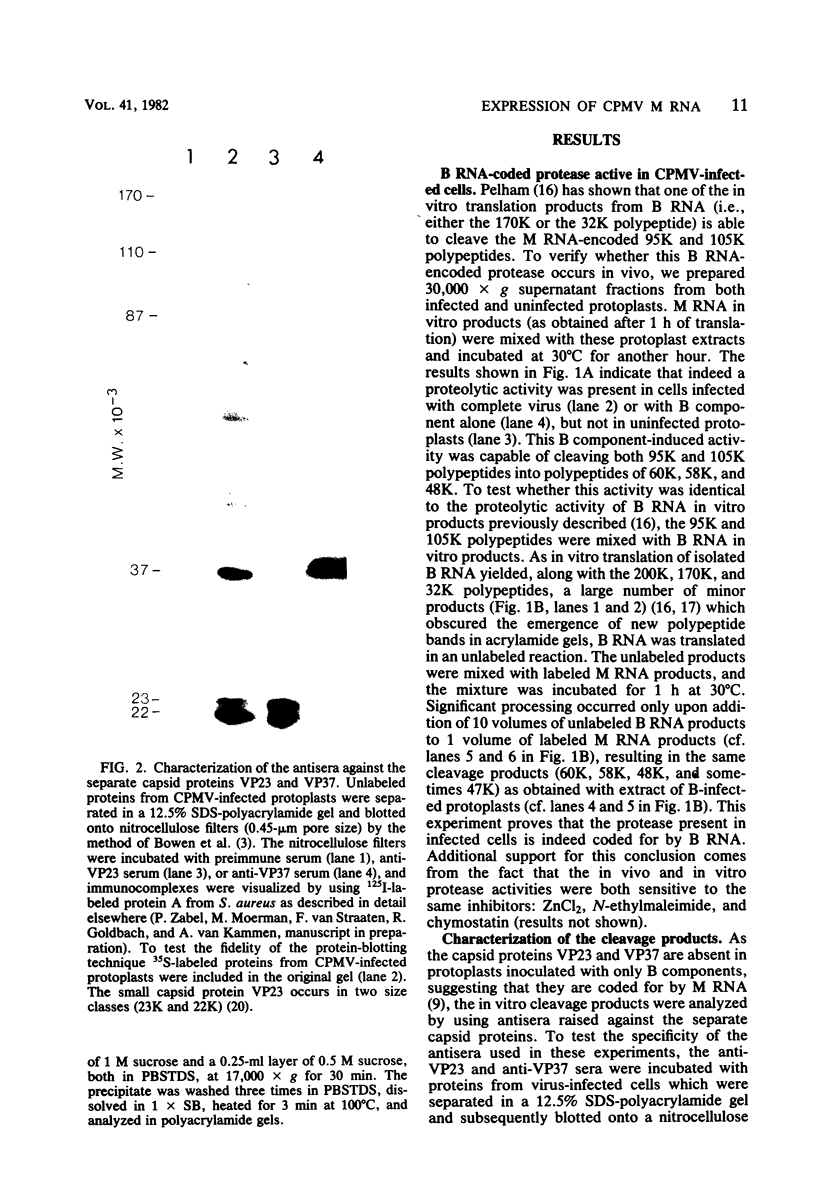
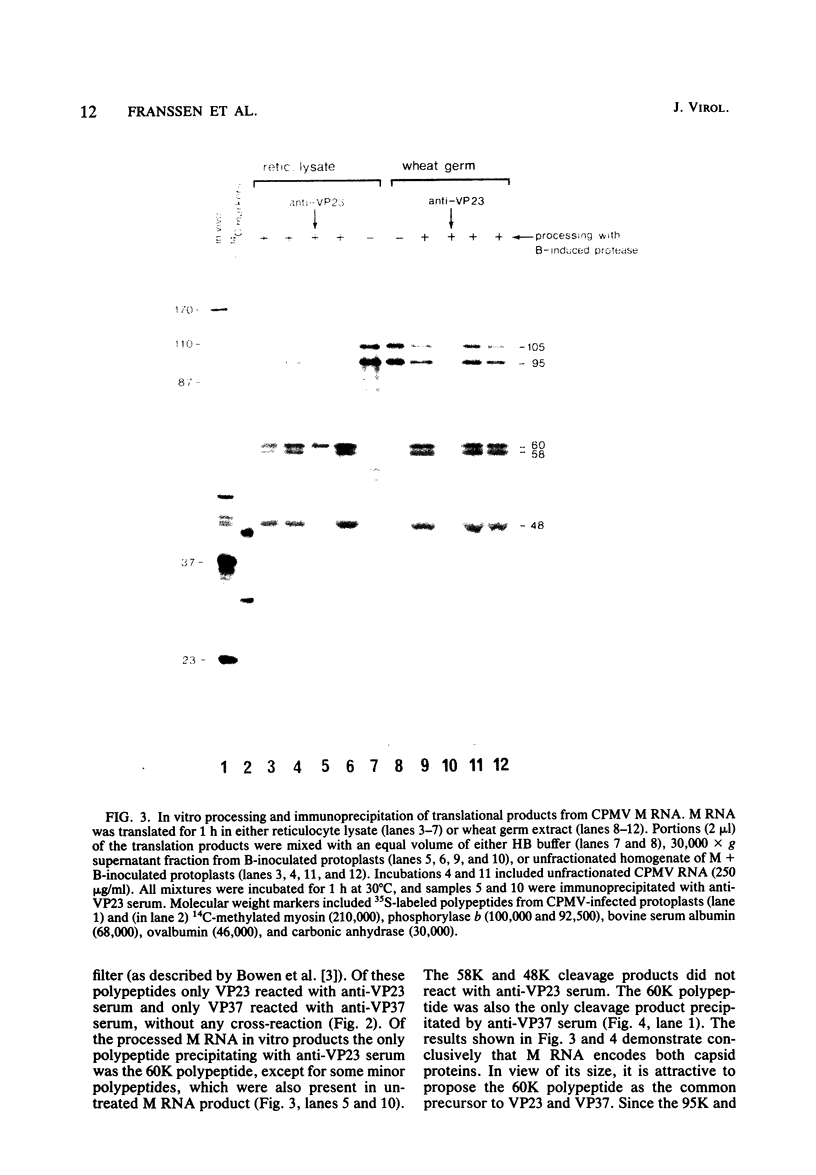
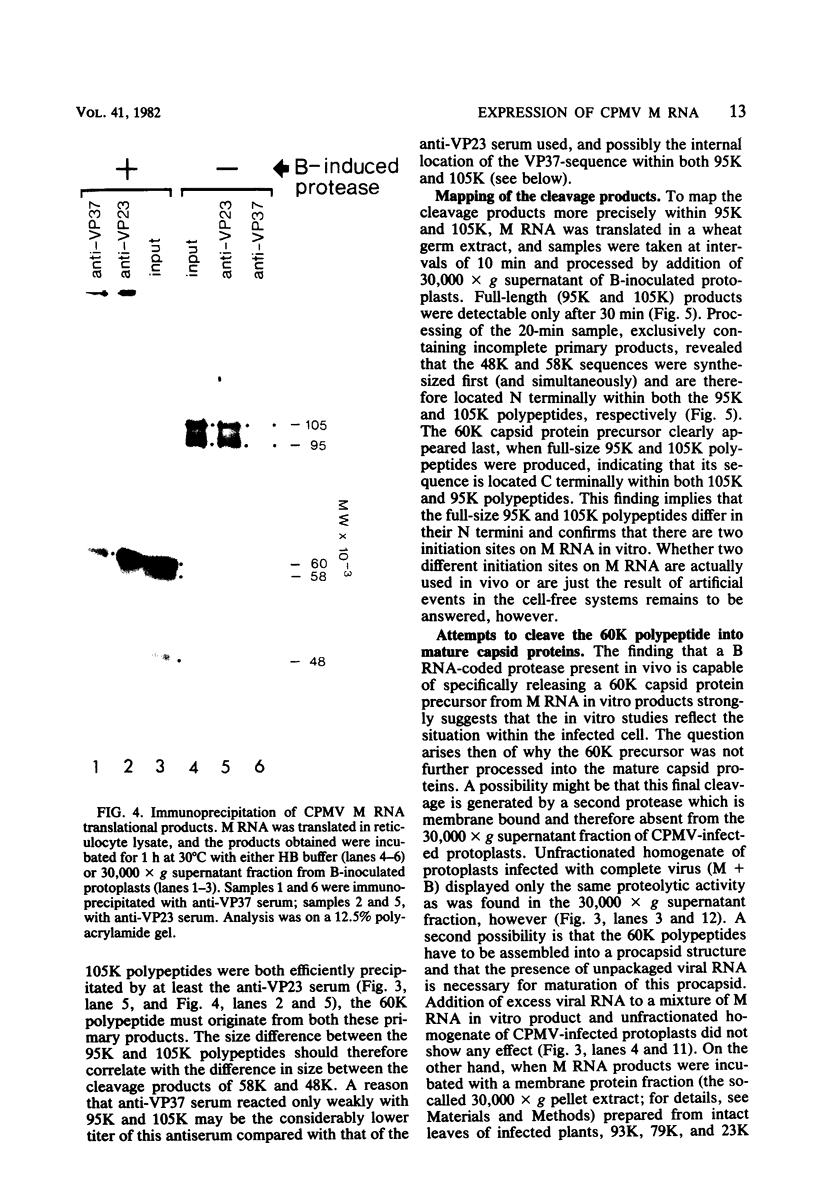
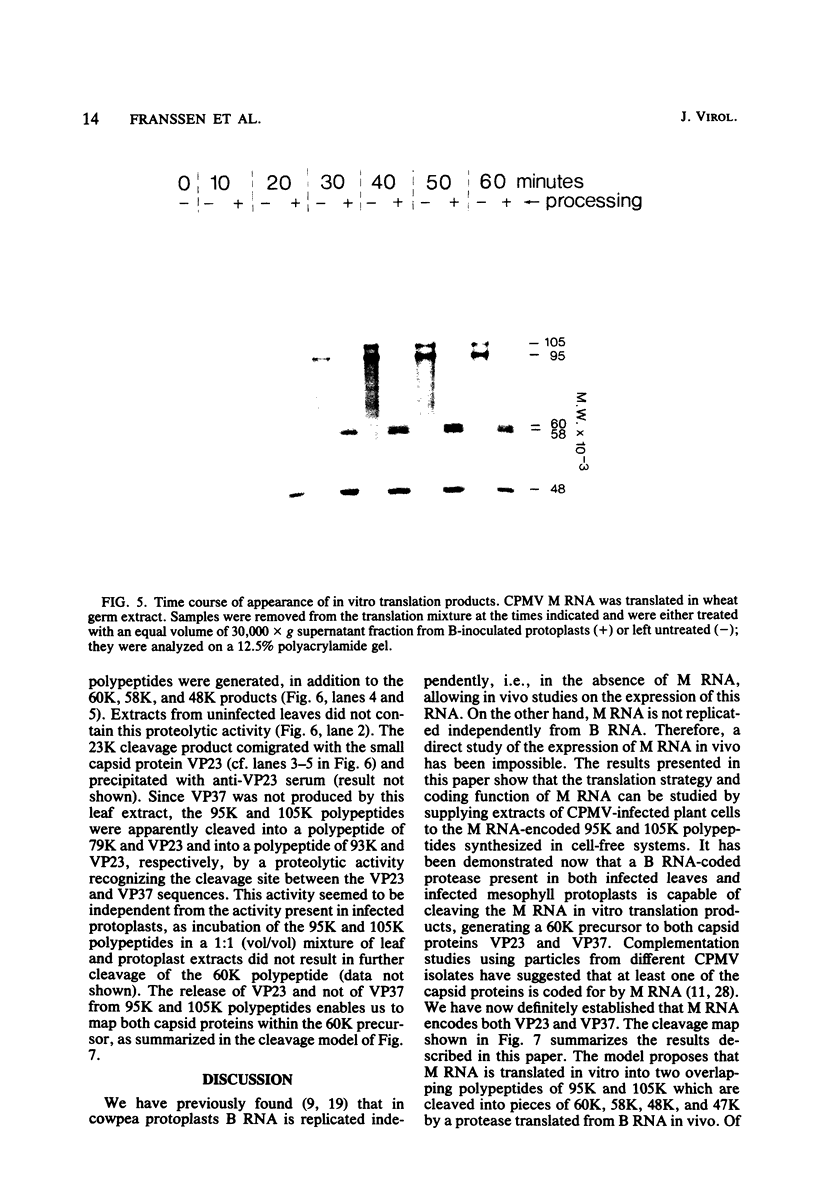
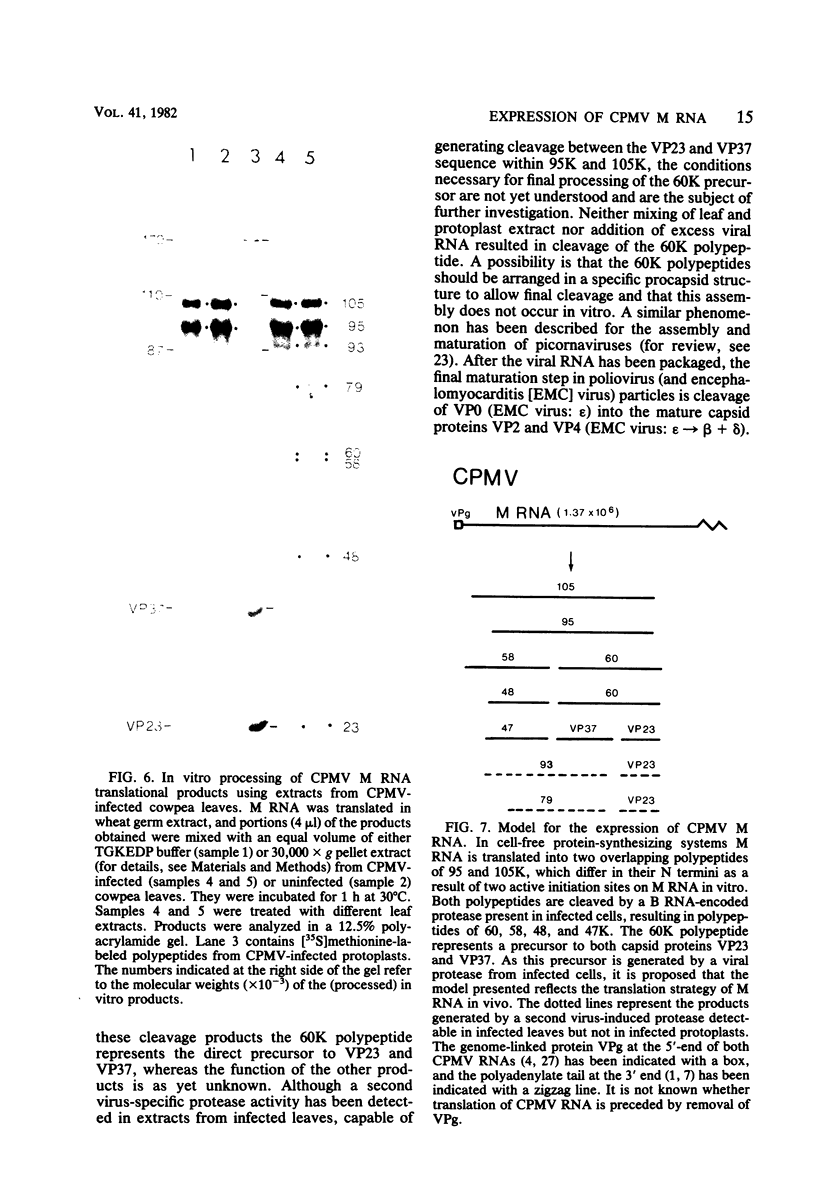
The expression of the middle-component (M) RNA of cowpea mosaic virus was studied by means of in vitro translation. In both the wheat germ extract and the rabbit reticulocyte lysate, M RNA was translated into two overlapping polypeptides of 95 and 105 kilodaltons. Incubation of these polypeptides with 30,000 × g supernatant fractions from cowpea mesophyll protoplasts inoculated with complete virus or with separate bottom (B) components alone resulted in extensive processing, yielding polypeptides of 60, 58, 48, and 47 kilodaltons. Similar proteolytic activity was found associated with the in vitro translation products from the bottom-component RNA, demonstrating that the protease present in infected cells is encoded by B RNA. Using antisera raised against the separate capsid proteins VP23 and VP37, it was shown that the 60-kilodalton cleavage product is the precursor to both capsid proteins. Cleavage of nascent 95- and 105- kilodalton polypeptides by the in vivo protease demonstrated that this capsid protein precursor is located C terminally within both polypeptides and that the synthesis of these two overlapping polypeptides is the result of two initiation sites on middle-component RNA. In addition, a second virus-induced proteolytic activity, capable of releasing VP23 from the 95- and 105-kilodalton polypeptides, was detected in leaves of infected plants, but not in infected mesophyll protoplasts. A model for the expression of the middle-component RNA is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Kaesberg P. Determination of the length distribution of poly(A) at the 3' terminus of the virion RNAs of EMC virus, poliovirus, rhinovirus, RAV-61 and CPMV and of mouse globin mRNA. Nucleic Acids Res. 1979 Nov 10;7(5):1195–1204. doi: 10.1093/nar/7.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn P. Ribonuclease inhibitor from human placenta: rapid purification and assay. J Biol Chem. 1979 Dec 25;254(24):12484–12487. [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert S. D., Bruening G., Najarian R. C. Protein bound to the genome RNAs of cowpea mosaic virus. Eur J Biochem. 1978 Dec 1;92(1):45–51. doi: 10.1111/j.1432-1033.1978.tb12721.x. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Aalbers A. M., Stuik E. J., Van Kammen A. Translation of cowpea mosaic virus RNA in a cell-free extract from wheat germ. FEBS Lett. 1977 May 15;77(2):265–269. doi: 10.1016/0014-5793(77)80248-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Verver J. W., Goldbach R. W., Van Kammen A. Efficient reverse transcription of cowpea mosaic virus RNAs. Nucleic Acids Res. 1978 Dec;5(12):4643–4661. doi: 10.1093/nar/5.12.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Schilthuis J. G., Rezelman G. Comparison of in vivo and in vitro translation of cowpea mosaic virus RNAs. Biochem Biophys Res Commun. 1981 Mar 16;99(1):89–94. doi: 10.1016/0006-291x(81)91716-2. [DOI] [PubMed] [Google Scholar]
- Gopo J. M., Frist R. H. Location of the gene specifying the smaller protein of the cowpea mosaic virus capsid. Virology. 1977 Jun 15;79(2):259–266. doi: 10.1016/0042-6822(77)90353-1. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of fragmented viral RNA in vitro: initiation at multiple sites. FEBS Lett. 1979 Apr 1;100(1):195–199. doi: 10.1016/0014-5793(79)81162-x. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Aalbers A. M., van Kammen A., Thuring R. W. Molecular weights of plant viral RNAs determined by gel electrophoresis under denaturing conditions. Virology. 1974 Aug;60(2):515–521. doi: 10.1016/0042-6822(74)90345-6. [DOI] [PubMed] [Google Scholar]
- Rezelman G., Goldbach R., Van Kammen A. Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol. 1980 Nov;36(2):366–373. doi: 10.1128/jvi.36.2.366-373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Rezelman G., van Kammen A. The inhibition of cowpea mosaic virus replication by actinomycin D. Virology. 1979 Jan 30;92(2):299–309. doi: 10.1016/0042-6822(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Scheele G., Blackburn P. Role of mammalian RNase inhibitor in cell-free protein synthesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4898–4902. doi: 10.1073/pnas.76.10.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongmeearkom P., Goodman R. M. Complementation and pseudorecombination between ribonucleic acids from two natural isolates of cowpea mosaic virus (severe subgroup). Virology. 1978 Mar;85(1):75–83. doi: 10.1016/0042-6822(78)90412-9. [DOI] [PubMed] [Google Scholar]
- Tjian R., Stinchcomb D., Losick R. Antibody directed against Bacillus subtilis rho factor purified by sodium dodecyl sulfate slab gel electrophoresis. Effect on transcription by RNA polymerase in crude extracts of vegetative and sporulating cells. J Biol Chem. 1975 Nov 25;250(22):8824–8828. [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., Van Krammen A. In vitro replication of cowpea mosaic virus RNA. II. Solubilization of membrane-bound replicase and the partial purification of the solubilized enzyme. J Virol. 1976 Mar;17(3):679–685. doi: 10.1128/jvi.17.3.679-685.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., van Kammen A. In Vitro Replication of Cowpea Mosaic Virus RNA III. Template Recognition by Cowpea Mosaic Virus RNA Replicase. J Virol. 1979 Jan;29(1):21–33. doi: 10.1128/jvi.29.1.21-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Manna M. M., Bruening G. Polyadenylate sequences in the ribonucleic acids of cowpea mosaic virus. Virology. 1973 Nov;56(1):198–206. doi: 10.1016/0042-6822(73)90299-7. [DOI] [PubMed] [Google Scholar]