Abstract
Certain cognitive functions differ in men and women, although the anatomical and functional substrates underlying these differences remain unknown. Because neocortical activity is directly related with higher brain function, numerous studies have focused on the cerebral cortex when searching for possible structural correlates of cognitive gender differences. However, there are no studies on possible gender differences at the synaptic level. In the present work we have used stereological and correlative light and electron microscopy to show that men have a significantly higher synaptic density than women in all cortical layers of the temporal neocortex. These differences may represent a microanatomical substrate contributing to the functional gender differences in brain activity.
Keywords: electron microscopy, neocortex, neuronal density, sex
It is well known that men and women display different capacities in certain cognitive functions that are unrelated to differences in the general level of intelligence. The most consistently reported differences relate to spatial and language abilities, and whereas men excel in mental rotation and spatial perception, women perform better in verbal memory tasks, in verbal fluency tasks, and in the speed of articulation (1, 2). These differences are not thought to be a only consequence of the influence of sex hormones on brain organization during development but also of genetic factors (3–5). Because higher brain functions are related to the activity of the neocortex, many studies aimed at identifying possible structural correlations for cognitive gender differences have focused on the cerebral cortex, using a variety of anatomical and brain imaging techniques. At the macroscopic level, sexual dimorphism has been reported in the cortical volume of the Wernicke and Broca areas (6), as well as in the frontal and medial paralimbic cortices (7–10), and in the thickness and density of the gray matter in the parietal lobes (for a review see ref. 10). At the microscopic level, differences have been reported in the density of neurons (11–14) and in the complexity of the dendritic arbors as well as in the density of dendritic spines in several cortical areas (15). Nevertheless, the functional significance of these differences remains unknown because no generally valid equation relates neuronal number or morphology to behavioral complexity (13, 15). For example, the intelligence of humans with brains weighing as little as half the average and with no evidence of any compensatory increase in neuron density may be normal or even above the mean.
Understanding how neuronal circuits contribute to the functional organization of the cerebral cortex requires a detailed ultrastructural analysis of neuronal connectivity. However, the difficulties encountered when attempting to apply microanatomical techniques to study the human brain explain why most studies on the structure of the neocortex have been performed at the light microscopic level. The most important problems when performing ultrastructural studies are related to the lack of suitable human brain tissue to study synaptic circuitry, for which the only source of control tissue might be autopsy material (i.e., from individuals that did not suffer brain pathologies or psychiatric illness). Unfortunately, the ultrastructural preservation of postmortem human brain tissue is usually rather poor, and it is generally unsuitable for the detailed quantitative analysis that can be performed on biopsy material. Indeed, this is one of the main reasons for the paucity of data regarding the synaptic circuitry in the normal human brain. Thus, the key question as to whether cortical synaptic circuits differ between men and women remains unsolved.
The purpose of this study was to analyze the possible gender differences in synaptic density, for which we have taken advantage of biopsy material obtained during neurosurgical treatment for epilepsy. This resected tissue represents an excellent opportunity to study the human brain at the electron microscope level, in part because the resected tissue can be immediately immersed in the fixative. Undoubtedly, this is why the quality of the immunocytochemical staining at both the light and electron microscopy levels in human biopsy material has been shown to be comparable to that obtained in experimental animals (e.g., ref. 16). Hence, using correlative light and electron microscopy coupled to stereological techniques, we show that there is significant sexual dimorphism in the density of synapses in all cortical layers of the temporal neocortex. These differences may represent a microanatomical substrate that contributes to gender functional differences in brain activity.
Results
Light Microscopy Analysis.
We analyzed the thickness and neuronal density in layers I, II, IIIA, IIIB, IV, V, and VI of 100-μm Nissl-stained sections from the tissue obtained. No significant differences were found between men and women regarding the neuronal density (Table 1 and Fig. 1A) as previously reported in the temporal neocortex (13). However, other reports have shown greater neuronal density in the posterior temporal neocortex of women when compared with men (12). The discrepancy between the study of Pakkenberg and Gundersen (13) and our present results when compared with those obtained by Witelson et al. (12) may be attributed to the cytoarchitectonic differences of the regions examined. Indeed, we examined the anterior part of the middle temporal gyrus, corresponding to area 21 of Brodmann [supporting information (SI) Fig. S1] (17), whereas they analyzed the superficial surface of the posterior part of the superior temporal gyrus, also denominated the TA1 area (18) or area 22 by Brodmann (17).
Table 1.
Number of neurons per cubic millimeter (mean ± SEM) and the percentage of synaptic junctions per layer
| Layer | Neuronal density |
Percentage of synapses |
||||
|---|---|---|---|---|---|---|
| Women |
Men |
|||||
| Women | Men | % AS | % SS | % AS | % SS | |
| I | 11,558 ± 1,200 | 11,034 ± 850 | 76 | 24 | 72 | 28 |
| II | 54,915 ± 3,200 | 49,127 ± 2,900 | 80 | 20 | 83 | 17 |
| IIIa | 18,112 ± 510 | 17,279 ± 560 | 85 | 15 | 92 | 8 |
| IIIb | 15,869 ± 430 | 15,907 ± 220 | 84 | 16 | 86 | 14 |
| IV | 49,754 ± 3,200 | 47,758 ± 970 | 86 | 14 | 89 | 11 |
| V | 26,393 ± 1,200 | 24,070 ± 830 | 88 | 12 | 92 | 8 |
| VI | 16,520 ± 430 | 16,291 ± 380 | 88 | 12 | 91 | 9 |
AS, asymmetric synapses; SS, symmetric synapses.
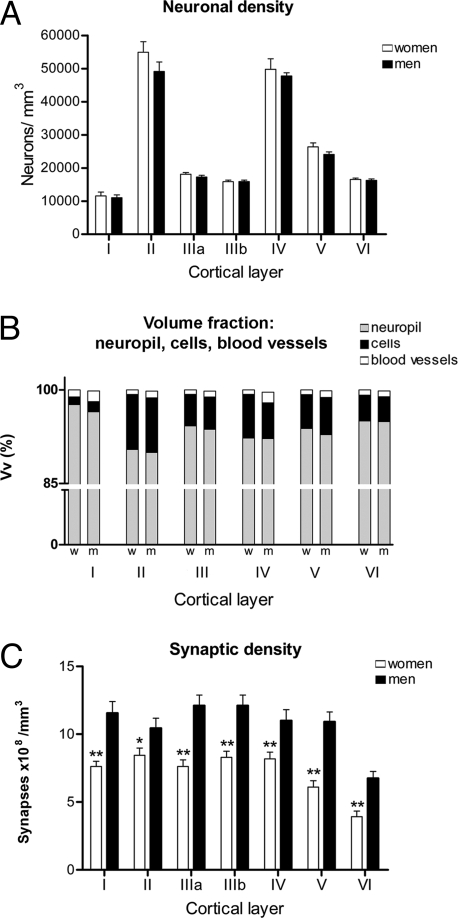
Fig. 1.
Graphs of the neuronal densities, volume fraction (Vv), synaptic densities, and synapses per neurons in men and women in each cortical layer. (A) Graph showing the neuronal densities (mean ± SEM) in each cortical layer demonstrating that there are no significant differences between men and women. (B) Comparison of the Vv between men and women, calculated for the neuropil, cell bodies (including those from glia and neurons), and blood vessels in each cortical layer. Note that the neuropil represents between 90% and 98% of the volume, for which no significant differences were found between men and women. (C) Graph showing a comparison of synaptic density (mean ± SEM) between men and women in each cortical layer. w, women; m, men. *, P < 0.05; **, P < 0.01.
Furthermore, the cell body (glia and neurons), neuropil, and blood vessel volume fractions (Vv) were also examined in each of these cortical layers in 2-μm-thick semithin sections stained with toluidine blue (Fig. S2). Again, no significant differences were found in any of the parameters examined (Fig. 1B). In summary, no cytoarchitectonic differences could be observed in the tissue obtained from men and women.
Ultrastructural Analysis.
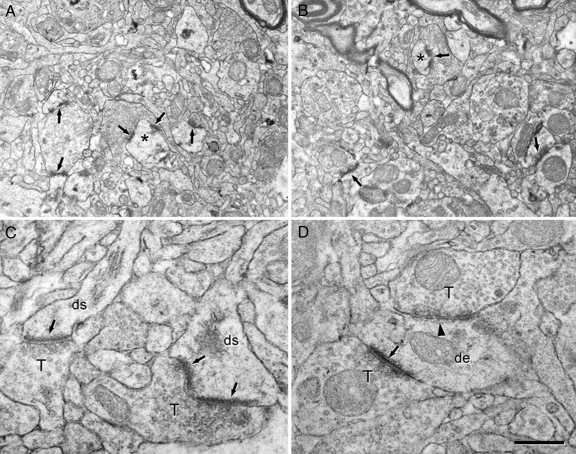
We have studied the morphology and density of synapses in each cortical layer, and the ultrastructure of the neuropil was indistinguishable in women from thatin men (Fig. 2 A and B and Fig. S3 a and b). The types of synaptic junctions were classified into three categories: asymmetric, symmetric, and uncharacterized (Tables 1 and 2). In the first two types, the synaptic cleft could be visualized and synapses were identified based on the morphology of the postsynaptic density. Thus, asymmetric synapses had a prominent postsynaptic density whereas symmetric synapses had a thin postsynaptic density (Fig. 2 C and D and Fig. S3 a and b) (19–21). In the uncharacterized synapses, the synaptic cleft could not be visualized because of the oblique plane of section. In this study, uncharacterized synapses were included in the final estimate of the total synaptic density. Furthermore, uncharacterized synapses in Fig. 1 were included as asymmetric and symmetric types according to the frequency of both types of synapses. Therefore, the proportion of each type of synapse in this work is an estimate of the real ratio (see ref. 22).
Fig. 2.
Electron micrographs to illustrate the ultrastructure of the human temporal neocortex. (A and B) Low-power electron micrographs showing the neuropil from layer IIIb of the temporal neocortex from a woman (A) and a man (B). Some synapses are indicated by arrows, and the asterisks illustrate two dendritic spines that are also shown at higher magnification in Fig. S3. (C and D) High-power electron micrographs showing the two major morphological types of synapses in the neuropil. Asymmetric synapses (arrows) had a prominent postsynaptic density, whereas symmetric synapses (arrowhead) had a thin postsynaptic density. de, dendritic shaft; ds, dendritic spines; T, axon terminals. [Scale bar (in D): 0.9 μm for A and B and 0.4 μm for C and D.]
Table 2.
Accumulated data when considering all cortical layers
| Sex | Mean cross-sectional length, μm, of: |
Mean no. (× 108/mm3) of: |
Percentage of: |
No. of neurons/mm3 |
Vv |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymmetric synapses | Symmetric synapses | All synapses | Asymmetric synapses | Symmetric synapses | All types of synapses | Asymmetric synapses | Symmetric synapses | Cell bodies (glia and neurons) | Blood vessels | Neuropil | |||
| Women | 0.30 ± 0.09 | 0.21 ± 0.10 | 0.29 ± 0.06 | 3.17 ± 2.08 | 1.06 ± 1.84 | 7.17 ± 3.29 | 86 | 14 | 27,589 ± 16,854 | 5.3 | 0.8 | 93.9 | |
| Men | 0.30 ± 0.08 | 0.20 ± 0.09 | 0.27 ± 0.05 | 4.23 ± 2.59 | 1.42 ± 2.00 | 10.61 ± 4.97 | 84 | 16 | 25,924 ± 15,110 | 5.2 | 1.2 | 93.3 | |
Data (mean ± SD) of all the synapses include asymmetric, symmetric, and uncharacterized synapses.
When the mean cross-sectional lengths of asymmetric, symmetric, and uncharacterized synapses were analyzed, no significant differences were found between men and women in any layer (Table 2).
Synapses were quantified in the neuropil (i.e., avoiding the neuronal and glial somata, blood vessels, large dendrites, and myelinated axons) (23), and we found men to have a higher synaptic density in all layers (Fig. 1C). The smallest difference in density was found in layer II, in which the synaptic density was 18% higher in men than in women (Fig. 1C), whereas the greatest difference was found in layer V, where the synaptic density in men was 52% higher than in women (678 million synapses per cubic millimeter plus). Considering all layers, men also have a significant higher average synaptic density of 12.9 × 108 per cubic millimeter, whereas in women it was 8.6 × 108 per cubic millimeter. Thus, there was a 33% difference in synaptic density between men and women.
Nevertheless, the proportion of asymmetric and symmetric synapses when considering all layers together (Tables 1 and 2) was similar in men and in women, 86% and 14% in men and 84% and 16% in women, respectively.
Discussion
The most striking finding from the present study is that despite the well known anatomical and functional interindividual variability in the brain (e.g., refs. 24 and 25), we consistently observed a lower synaptic density in women in all cortical layers of the temporal neocortex. Because we examined only relatively few cases (four women and four men), we consider that these differences must therefore be very robust in the general population.
Nevertheless, we would caution the reader that the main limitation in this kind of study is that we have virtually no data about the synaptic density in biopsy samples of the strictly normal human neocortex. Indeed, it is well known that synaptic reorganization occurs in the epileptic brain, although these changes occur in regions with neuronal loss and gliosis such as the sclerotic hippocampus (e.g., ref. 26) or the peritumoral or dysplastic cortex (e.g., refs. 27 and 28). The eight biopsies used in the present study can be considered to be close to what would be expected as normal conditions for the following reasons: first, the epileptic activity was clearly of mesial origin; second, the whole neocortex in all of these patients displayed non-spiking activity; and, third, they presented normal cytoarchitectonic and ultrastructural characteristics. In addition, although we cannot rule out that synaptic changes may also occur in the neocortex, there is no reason to believe that the differences in synaptic density observed between men and women was due to the epileptic condition because all of the subjects were epileptic. Thus, it is likely that these differences are truly due to sex differences.
Importantly, no differences in cytoarchitecture were observed. More specifically, no significant differences were found between men and women regarding the thickness of the gray matter, the volume fraction of cortical elements (neuropil, cells, and blood vessels), and neurons per volume as previously reported in the temporal neocortex (13). As a consequence, the number of synapses in each layer was greater in men than in women, and, thus, in this particular region of the neocortex the general connectivity in men appears to be more extensive than in women. Accordingly, gender appears to influence synaptic connectivity, and this phenomenon is regulated independent of other cytoarchitectonic features.
If we consider the columnar organization of the input connections, the differences in connectivity between neighboring neurons, and the combinations of the interlaminar connections of both pyramidal and nonpyramidal neurons, it is clear that neurons in different layers do not process the same information (29, 30). Furthermore, pyramidal neurons located in different layers project to different cortical and subcortical nuclei (31–33). Hence, it is likely that the differences in synaptic density between men and women observed in all cortical layers represent a microanatomical substrate for sex differences in the fine-tuning of several functions.
The larger number of synaptic connections in men does not necessarily mean that all cortical circuits in this region are more complex than in women. Rather, specific circuits may be more complex in the male brain. The temporal lobe is a complex, associative, and multiintegrative cortical region (ref. 17; for a review see ref. 34). Therefore, the functional consequences of the differences in synaptic circuitry observed here are particularly difficult, if not impossible, to correlate with specific functions related to men or women.
Interestingly, a recent study on synaptic density carried out in the monkey prefrontal cortex seems to indicate that there are no differences between males and females (35). However, many studies have shown variations between species and cortical areas in terms of density, proportion, and types of neurons, as well as in the density of synapses (e.g., ref. 22). Thus, whether these gender differences are unique to the human cerebral cortex or whether similar conditions arise in monkeys and great apes should be specifically analyzed in each species and cortical area. Finally, and in line with this consideration, we would advise the reader to exercise caution in extrapolating the present data to the whole brain. Indeed, it was reported that the anterior commissure, which connects several regions of the frontal and temporal lobes, is 12% larger in women than in men, suggesting that women would have more commissural associative connections (36). Further work will be necessary to examine whether synaptic density is similar or different in other cortical areas.
Materials and Methods
Human postoperative brain tissue was obtained from eight patients suffering pharmacoresistant mesial temporal lobe epilepsy secondary to hippocampal alterations. In each case the patient's consent was obtained in accordance with the Helsinki Declaration (37), and all protocols were approved by the ethical committee at the Hospital de la Princesa (Madrid). Tissue was obtained from four women of 26, 31, 31, and 41 years of age and from four men of 24, 27, 32, and 36 years of age, and this material has been used in previous studies (38–40). Video EEG monitoring of bilateral foramen ovale electrodes was indicative of left mesial temporal lobe epilepsy in all patients. Furthermore, during surgery the epileptogenic regions were identified through subdural recordings with a 20-electrode grid (lateral neocortex) and a four-electrode strip (uncus and parahippocampal gyrus). Intraoperative electrocorticographic recordings revealed spiking activity localized in the mesial structures, whereas the lateral neocortex of all these patients displayed normal activity. That is, no spikes, sharp waves, or slow activities were observed during intraoperative electrocorticography. All of the patients were right-handed, and they had normal IQs.
The lateral neocortex of all these patients was non-spiking, displaying normal activity in intraoperative electrocorticography, although a small portion of the anterior part of the left temporal lobe had to be removed to access the altered hippocampus. Biopsy tissue was immediately immersed in the fixation solution. Then, the lateral neocortex and mesial structures were subjected to standard neuropathological assessment. The surgical outcome of epileptic patients was evaluated after 18 months, and the patients were classified following the Engel scale as grade I (41).
The anterolateral temporal cortex tissue (T2), corresponding to area 21 of Brodmann (17) (Fig. S1), was cut into 1.5-cm-thick coronal slices and immersed in a cold solution of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 24–36 h. Coronal sections (100 μm) were cut with a Vibratome and collected in series in PB. Some sections were Nissl-stained to reveal the laminar boundaries and to carry out the histopathological assessment. All of the lateral neocortical biopsies were histologically normal.
Estimation of Neuronal Density.
Neuronal density was estimated by using optical dissectors as described by West and Gundersen (ref. 42; see also ref. 43) and with the aid of StereoInvestigator software (version 7.0; MicroBrightField). Optical dissectors were performed on every cortical layer from each case using the Nissl-stained sections adjacent to those used to count the synapses. After a starting point was randomly selected, five sections were selected at equally spaced intervals in the same cortical area. Optical dissectors were made with an oil immersion ×100 objective on a surface of 900–1,600 μm2 and with a depth of 22–25 μm, rendering a study volume of 19,800–40,000 μm3 per optical dissector. To provide the systematic area offset the movement of the stage was controlled through StereoInvestigator software (MicroBrightField). A neuron was counted only if the nucleolus was clearly identified in the height of the optical plane along the z axis.
Estimation of the Volume Fraction (Vv) of the Neuropil.
Semithin sections (2 μm) stained with toluidine blue were used to estimate the volume fraction (Vv) occupied by the neuropil, which excluded blood vessels and cell bodies (including those from neurons and glia). This was accomplished by point counting and by applying the Cavalieri principle using the integrated StereoInvestigator stereological package (see Fig. S2).
Electron Microscopy.
Sections adjacent to those used for Nissl staining were processed for electron microscopy. These sections were postfixed in 2% glutaraldehyde in PB for 1 h, treated in 1% osmium tetroxide, dehydrated, and flat-embedded in Araldite resin. Plastic-embedded sections were studied by a correlative light and electron microscopy method described in detail elsewhere (44). Briefly, sections were photographed under the light microscope and then serially cut into semithin (2-μm-thick) sections with a Reichert ultramicrotome. The semithin sections were stained with 1% toluidine blue in 1% borax, examined under the light microscope, and photographed to locate the area and layers of interest. Selected semithin sections were resectioned into serial ultrathin sections with a silver-gray interference color corresponding to a thickness of ≈60–70 nm (45). The ultrathin sections were collected on formvar-coated, single-slot grids, stained with uranyl acetate and lead citrate, and examined with a JEOL 1200 EX electron microscope. Using this correlative light and electron microscopy method, it was possible to determine the exact region of the neuropil that was analyzed by electron microscopy, and, therefore, we could accurately identify the layer analyzed. Photographs were taken randomly at ×30,000 with a digitalizing image system (Mega View III Side-mounted TEM Camera; Soft Imaging System) and by using imaging acquisition software (analiSIS 3.2; Soft Imaging System). At least 30 micrographs at a magnification of ×30,000 were obtained per layer and case (for a detailed description on the estimation of synapses see ref. 23).
The two major morphological types of cortical synapses were clearly identified in the cortical tissue analyzed, these being type I and type II according to Gray (19) or those denominated asymmetric and symmetric by Colonnier (ref. 20; for review see refs. 46 and 47). The synapses in which the synaptic cleft and associated membrane densities could not be visualized clearly (because of the oblique plane of section) were considered as uncharacterized synapses.
Synaptic density per unit area (NA) was estimated from electron microscopy samples of the neuropil from each cortical layer (for a detailed description see ref. 23). The density of synapses per unit volume of the neuropil was calculated by using the formula NV = NA/d where NA is the number of synaptic profiles per unit area and d is the average cross-sectional length of synaptic junctions. The cross-sectional length of synaptic junctions was measured by using the Image J analysis program (Scion).
Estimation of Tissue Shrinkage.
To obtain homogeneous estimates of neuronal density and synapses, tissue shrinkage was evaluated by using StereoInvestigator software to measure the cortical surface and volume in sections before and after processing for Nissl staining or electron microscopy. Initially, the surface area of the nonprocessed Vibratome sections and the thickness were measured at six random points to estimate shrinkage along the z axis (i.e., section compression). Thereafter, the sections were Nissl-stained or processed for EM, and the same measurements were taken again. As a result, the cortical tissue was estimated to have shrunken 68.7% in volume when processed for Nissl staining and 42.6% when processed for electron microscopy. These shrinkage values were taken into consideration when estimating the thickness of the cortical layers and neuronal and synaptic density, as well as for the estimation of the ratio of the number of synapses per neuron.
Statistical Analyses.
Statistical comparisons between the two groups (men and women) were performed by using the unpaired Student t test or the Mann–Whitney nonparametric U test, depending on whether the datasets fitted a normal distribution and passed the test for homogeneity of variances (48). All statistical studies were performed with the aid of the Prism statistical package (Prism 4.0; GraphPad). To assure a blind analysis of the material, all of the cases were coded, and only later were the codes broken for the statistical analyses.
Supplementary Material
Acknowledgments.
We thank Pilar Marco for her earlier contribution to this project. We also thank Dr. Rafael G. Sola (Hospital de la Princesa, Madrid) for supplying human tissue. This work was supported by the Ministry of Education and Science (Grant BFU2006-13395), Centro de Investigaciónes Biomédicas en Red sobre Enfermedades Neurodegenerativas, and the Cajal Blue Brain Project. L.A.-N. is the recipient of a postdoctoral fellowship from the European Community's 6th Framework Program for Research and Technological Development (PROMEMORIA LSHM-CT-2005-512012).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803652105/DCSupplemental.
References
- 1.Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Dev. 1985;56:1479–1498. [PubMed] [Google Scholar]
- 2.Kimura D. Sex and Cognition. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 3.Bocklandt S, Vilain E. Sex differences in brain and behavior: Hormones versus genes. Adv Genet. 2007;59:245–266. doi: 10.1016/S0065-2660(07)59009-7. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies W, Wilkinson LS. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 6.Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54:171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- 7.Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. NeuroImage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 8.Amunts K, et al. Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Amunts K, et al. Gender-specific left-right asymmetries in human visual cortex. J Neurosci. 2007;27:1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowell ER, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- 12.Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 14.Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: More neurons in males; more processes in females. J Child Neurol. 1999;14:98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs B, et al. Quantitative dendritic and spine analyses of speech cortices: A case study. Brain Lang. 1993;44:239–253. doi: 10.1006/brln.1993.1016. [DOI] [PubMed] [Google Scholar]
- 16.Del Río MR, DeFelipe J. A study of SMI 32-stained pyramidal cells, parvalbumin-immunoreactive chandelier cells, and presumptive thalamocortical axons in the human temporal neocortex. J Comp Neurol. 1994;342:389–408. doi: 10.1002/cne.903420307. [DOI] [PubMed] [Google Scholar]
- 17.Brodman K. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien Dargestellt auf Ground des Zellenbaues. Leipzig, Germany: Barth; 1909. [Google Scholar]
- 18.von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des Erwaschsenen Menschen. Berlin: Springer; 1925. [Google Scholar]
- 19.Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- 20.Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Res. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- 21.Colonnier M. In: Organization of the Cerebral Cortex. Schmitt FO, Worden FG, Adelman G, Dennis SG, editors. Cambridge, MA: MIT Press; 1981. pp. 125–152. [Google Scholar]
- 22.DeFelipe J, et al. In: Evolution of the Nervous System. Kaas JH, editor. Oxford: Academic; 2007. pp. 161–190. [Google Scholar]
- 23.DeFelipe J, Marco P, Busturia I, Merchan-Perez A. Estimation of the number of synapses in the cerebral cortex: Methodological considerations. Cereb Cortex. 1999;9:722–732. doi: 10.1093/cercor/9.7.722. [DOI] [PubMed] [Google Scholar]
- 24.Uylings HB, Rajkowska G, Sanz-Arigita E, Amunts K, Zilles K. Consequences of large interindividual variability for human brain atlases: Converging macroscopical imaging and microscopical neuroanatomy. Anat Embryol (Berlin) 2005;210:423–431. doi: 10.1007/s00429-005-0042-4. [DOI] [PubMed] [Google Scholar]
- 25.Caspers S, et al. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Houser CR. In: Jasper′s Basic Mechanism of the Epilepsies. Delgado-Escueta AV, Wilson WA, Olsen RW, Porter RJ, editors. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 743–761. [Google Scholar]
- 27.Alonso-Nanclares L, DeFelipe J. Vesicular glutamate transporter 1 immunostaining in the normal and epileptic human cerebral cortex. Neuroscience. 2005;134:59–68. doi: 10.1016/j.neuroscience.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-Nanclares L, et al. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. doi: 10.1093/brain/awh331. [DOI] [PubMed] [Google Scholar]
- 29.Rockland KS, Ichinohe N. Some thoughts on cortical minicolumns. Exp Brain Res. 2004;158:265–277. doi: 10.1007/s00221-004-2024-9. [DOI] [PubMed] [Google Scholar]
- 30.DeFelipe J. In: Neocortical Modularity and the Cell Minicolumn. Casanova MF, editor. New York: Nova Science; 2005. pp. 57–91. [Google Scholar]
- 31.Jones EG. In: Cerebral Cortex. Jones EG, Peters A, editors. New York: Plenum; 1984. pp. 1–28. [Google Scholar]
- 32.Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- 33.White EL. Cortical Circuits: Synaptic Organization of the Cerebral Cortex. Boston: Birkhäuser; 1989. [Google Scholar]
- 34.Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 35.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen LS, Gorski RA. Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol. 1991;312:97–104. doi: 10.1002/cne.903120108. [DOI] [PubMed] [Google Scholar]
- 37.World Medical Association. Declaration of Helsinki. Brit Med J. 1991;302:1194. [Google Scholar]
- 38.Marco P, et al. Inhibitory neurons in the human epileptogenic temporal neocortex. An immunocytochemical study. Brain. 1996;119:1327–1347. doi: 10.1093/brain/119.4.1327. [DOI] [PubMed] [Google Scholar]
- 39.Arellano JI, Muñoz A, Ballesteros-Yáñez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127:45–64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- 40.Sola RG, et al. Pharmacoresistant temporal-lobe epilepsy. Exploration with foramen ovale electrodes and surgical outcomes. Rev Neurol. 2005;41:4–16. [PubMed] [Google Scholar]
- 41.Engel JJ. In: Surgical Treatment of Epilepsies. Engel JJ, editor. New York: Raven; 1987. pp. 553–571. [Google Scholar]
- 42.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 43.Williams RW, Rakic P. Three-dimensional counting: An accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- 44.DeFelipe J, Fairen A. A simple and reliable method for correlative light and electron microscopic studies. J Histochem Cytochem. 1993;41:769–772. doi: 10.1177/41.5.8468459. [DOI] [PubMed] [Google Scholar]
- 45.Peachey LD. Thin sections. I. A study of section thickness and physical distortion produced during microtomy. J Biophys Biochem Cytol. 1958;4:233–242. doi: 10.1083/jcb.4.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. New York: Oxford Univ Press; 1991. [Google Scholar]
- 47.Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- 48.Motulsky H. Intuitive Biostatistics. New York: Oxford Univ Press; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.