Abstract
Recent evidence suggests that binding of agonist to its cognate receptor initiates not only classical G protein-mediated signaling, but also β-arrestin-dependent signaling. One such β-arrestin-mediated pathway uses the β1-adrenergic receptor (β1AR) to transactivate the EGFR. To determine whether β-adrenergic ligands that do not activate G protein signaling (i.e., β-blockers) can stabilize the β1AR in a signaling conformation, we screened 20 β-blockers for their ability to stimulate β-arrestin-mediated EGFR transactivation. Here we show that only alprenolol (Alp) and carvedilol (Car) induce β1AR-mediated transactivation of the EGFR and downstream ERK activation. By using mutants of the β1AR lacking G protein-coupled receptor kinase phosphorylation sites and siRNA directed against β-arrestin, we show that Alp- and Car-stimulated EGFR transactivation requires β1AR phosphorylation at consensus G protein-coupled receptor kinase sites and β-arrestin recruitment to the ligand-occupied receptor. Moreover, pharmacological inhibition of Src and EGFR blocked Alp- and Car-stimulated EGFR transactivation. Our findings demonstrate that Alp and Car are ligands that not only act as classical receptor antagonists, but can also stimulate signaling pathways in a G protein-independent, β-arrestin-dependent fashion.
Keywords: β-adrenergic receptor, G protein-coupled receptor, signaling
Beta-adrenergic receptors (βARs) are members of the seven transmembrane receptor (7TMR) family that become activated upon catecholamine binding to stimulate G protein-dependent signaling. Turning off the G protein signal by a process known as desensitization occurs through G protein-coupled receptor kinase (GRK) phosphorylation of the 7TMR, and subsequent binding of β-arrestin (1, 2). We recently showed that β-arrestin can also mediate β1AR transactivation of the EGFR, resulting in a cardioprotective effect under conditions of chronic catecholamine stimulation (3). The appreciation that chronic stimulation of βARs likely contributes to the pathogenesis of heart failure (4) has led to the use of β-adrenergic receptor antagonists (i.e., β-blockers) to competitively inhibit the harmful effects of chronic catecholamine stimulation (5). Although β-blockers have been widely used in the treatment of hypertension (6) and heart failure (7), the clinical efficacy among the different β-blockers do not seem to be equivalent (8).
Recent evidence suggests that binding of different ligands promotes distinct receptor conformations leading to specific signaling events (9–12). We hypothesized that some β-blockers may have the unique property of being able to stimulate β1AR signaling without activating G proteins, providing a possible mechanism for differences in clinical efficacy. To test this hypothesis, we assessed the ability of 20 β-blockers to induce β-arrestin-mediated EGFR transactivation using cells expressing β1ARs and EGFRs.
Results
Alp and Car Induce EGFR Internalization.
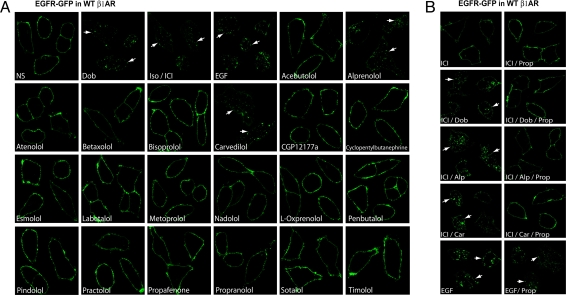
We screened β-adrenergic ligands to determine if any could lead to β1AR-mediated EGFR transactivation. Cells stably expressing WT β1ARs were transfected with GFP-tagged EGFR (EGFR-GFP) and stimulated with several agonists or 20 different β-blockers. EGFR transactivation was determined by the ability of the ligand to induce EGFR-GFP internalization as visualized by confocal microscopy. In the absence of agonist, EGFR had a uniform membrane distribution (Fig. 1A). In contrast, the βAR agonists dobutamine (Dob) and isoproterenol (Iso) resulted in internalization of EGFRs as visualized by marked redistribution into cellular aggregates, similar to that seen with EGF (Fig. 1A Upper). Among the 20 β-blockers tested, we found that only Alp and Car were able to induce EGFR internalization (Fig. 1A). Next, we examined whether Alp- or Car-induced EGFR internalization is blocked with a nonselective β-blocker. Pretreating HEK293 cells stably expressing WTβ1AR with propranolol (Prop) blocked the Alp- or Car-induced EGFR internalization (Fig. 1B).
Fig. 1.
Alp and Car induce EGFR internalization. (A) HEK293 cells stably expressing WTβ1AR are transfected with EGFR-GFP. EGFR internalization following agonists (Dob, Iso, and EGF) or β-blocker administration is visualized using confocal microscopy. In the absence of agonist, EGFR-GFP is visualized on the membrane, whereas Dob, Iso, and EGF stimulation results in the redistribution of EGFR-GFP into cellular aggregates. Cells stimulated with Iso were pretreated with the β2AR-selective antagonist ICI-118551. Among 20 β-blockers, only Alp and Car induce redistribution of EGFR into cellular aggregates. (B) HEK293 cells stably expressing WTβ1AR with transient transfection of EGFR-GFP are pretreated with Prop and stimulated with Dob, Alp, or Car. Dob-, Alp-, or Car-induced EGFR internalization is blocked by Prop. Each confocal image is a composite of representative images.
Alp and Car Stimulate β1AR-Mediated EGFR Phosphorylation and ERK1/2 Activation.
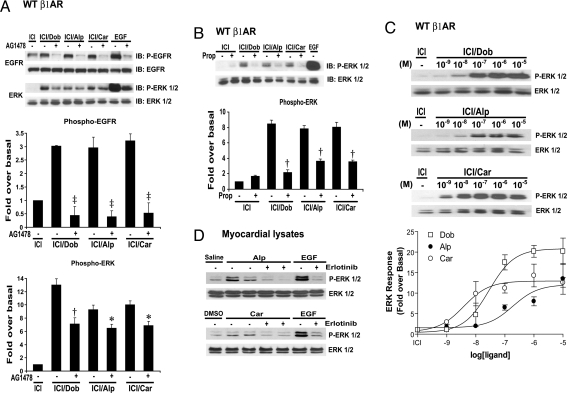
We next tested whether Alp and Car could mediate EGFR phosphorylation and induce downstream signaling. Cells stably expressing WT β1ARs were transfected with FLAG-EGFR and stimulated with Dob, Alp, Car, or EGF. Cells were pretreated with either the β2AR-specific antagonist ICI-118551 (ICI) to block endogenous β2ARs (13) or the specific EGFR inhibitor AG1478. Stimulation with Dob, Alp, or Car resulted in a significant increase in EGFR phosphorylation, along with a modest activation of phosphorylated ERK (pERK; Fig. 2A) and pAkt [supporting information (SI) Fig. S1A], suggesting that Alp and Car can activate survival signaling pathways. EGFR phosphorylation upon treatment with Dob, Alp, or Car was completely blocked by pretreatment with AG1478 (Fig. 2A). In contrast, ERK activation was partially blocked by AG1478 (Fig. 2A), consistent with the multiple known pathways leading to downstream ERK activation (14). Additionally, HEK293 cells lacking endogenous β1AR expression (Fig. S1B Top) did not elicit phosphorylation of ERK1/2, whereas those transfected with WT β1AR (Fig. S1B Bottom) elicited a Dob-, Alp-, or Car-stimulated ERK1/2 response that was sensitive to EGFR inhibition. Last, pretreating HEK293 cells stably expressing WTβ1AR with Prop blocked the Alp- or Car-induced ERK activation (Fig. 2B), again showing that the action of Alp and Car on EGFR transactivation requires ligand occupancy of the β1AR.
Fig. 2.
Alp- or Car-β1AR-stimulated EGFR transactivation and ERK1/2 activation are sensitive to EGFR inhibition. (A) HEK293 cells stably expressing WTβ1ARs with transient transfection of FLAG-EGFR are treated with Dob, Alp, Car, or EGF with or without AG1478. Both Alp and Car induced EGFR transactivation and ERK activation, which are sensitive to EGFR inhibition. (B) HEK293 cells stably expressing WTβ1AR with transient transfection of FLAG-EGFRs are pretreated with Prop and stimulated with Dob, Alp, or Car. Dob-, Alp-, or Car-induced ERK activation is blocked by Prop. (C) Dose-dependent ERK responses by Alp or Car. HEK293 cells stably expressing WTβ1ARs with transient transfection of FLAG-EGFR are treated with the indicated concentrations of Dob, Alp, or Car for 5 min. (D) WT mice are pretreated with erlotinib or DMSO followed by infusion with ligands. Myocardial lysates are immunoblotted with anti-phospho-ERK1/2 and anti-total ERK1/2 antibodies. Data represent mean ± SE of at least four independent experiments. *, P < 0.05 vs. without AG1478; †, P < 0.01 vs. without AG1478 or Prop; ‡, P < 0.001 vs. without AG1478.
Dose-Dependent ERK Activation by Alp or Car.
We next evaluated the dose-response characteristics for Alp and Car in stimulating cellular ERK1/2. We treated HEK293 cells stably expressing WT β1AR with Dob, Alp, or Car in the presence or absence of AG1478 over a range of concentrations for 5 min and analyzed whole-cell lysates for pERK and total ERK1/2 content (Fig. 2C and Fig. S2A). In the absence of AG1478, similar EC50 values were found for Dob and Alp: 39 nM and 59 nM, respectively. In contrast, Car showed a higher potency with activation of ERK. The EC50 of Car was 2 nM. Pretreatment with AG1478 decreased the Alp- or Car-stimulated ERK activation by ≈50%, suggesting that part of this β-arrestin-mediated ERK signal is independent of EGFR transactivation.
Alp or Car Induces Erlotinib-Sensitive ERK Activation in the Heart in Vivo.
We next examined whether Alp and Car can stimulate β-arrestin-mediated EGFR transactivation in vivo. Alp or Car was administered by i.v. infusion to WT mice in the presence or absence of the EGFR inhibitor erlotinib. Erlotinib was administered i.p. 1 h before βAR ligands. Hearts were removed after 5 min, and myocardial lysates were immunoblotted for pERK. Consistent with our cell culture experiments, Alp or Car significantly increased ERK activation in the heart, which was blocked by erlotinib pretreatment (Fig. 2D).
Alp and Car Display very Weak Partial Agonism for Gs-Dependent Adenylyl Cyclase Activation Above 1 μM.
We tested the efficacy profiles of four selected compounds on the adenylyl cyclase signaling pathway in HEK293 cells expressing β1ARs and the cAMP biosensor ICUE2 compared with the cAMP biosensor ICUE2 alone (Fig. S2 B and C). Whereas Iso activated β1AR G protein-dependent signaling, Alp and Car displayed relatively weak potency with activation of β1AR G protein-dependent signaling given their known binding affinities for β1AR (15–17). These data suggest a weak effect of Alp and Car on the cAMP biosensor that may be mediated through receptor activation with very low potency.
Phosphorylation of GRK Sites on the β1AR Is Necessary for Alp- or Car-Induced EGFR Transactivation.
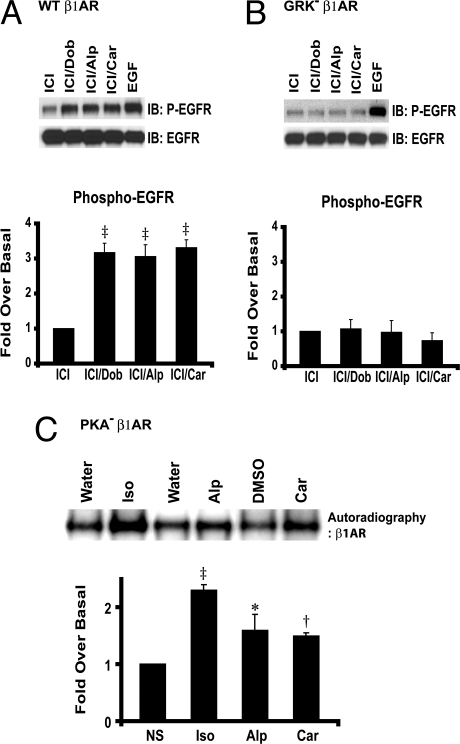
We next tested whether phosphorylation of the β1AR on GRK sites induced by the β-blockers Alp and Car is required for EGFR transactivation. Here, we used cells stably expressing GRK− β1ARs that have the 14 putative GRK phosphorylation sites within the C-terminal tail mutated to alanine (18). In HEK293 cells stably expressing WT β1ARs, Alp and Car stimulation resulted in robust phosphorylation of EGFR (Fig. 3A). In contrast, Dob, Alp, and Car were not able to stimulate EGFR transactivation in GRK− β1AR-expressing cells (Fig. 3B). WTβ1AR and GRK− β1ARs have a similar receptor expression as described in Materials and Methods.
Fig. 3.
Alp- or Car-induced β1AR-mediated transactivation of EGFR requires GRK phosphorylation. HEK293 cells stably expressing WTβ1AR (A), or GRK− β1AR (B) are transfected with FLAG-EGFR and then treated with Dob, Alp, or Car. As indicated, WTβ1AR induces an increase in phospho-EGFR in response to treatment with Dob, Alp, and Car, whereas GRK− β1AR lacks this effect. (C) Metabolically labeled HEK293 cells stably expressing PKA− β1AR are stimulated with various ligands. Alp and Car stimulate a significant increase in β1AR phosphorylation at the GRK sites. Data represent mean ± SE of at least four independent experiments. *, P < 0.05 vs. NS; †, P < 0.01 vs. NS; ‡, P < 0.001 vs. ICI or NS.
To determine whether the β-blockers Alp and Car could induce GRK-mediated phosphorylation of the β1AR, we performed metabolic labeling experiments in HEK293 cells stably expressing a mutant β1AR that is deficient in the four putative PKA phosphorylation sites (PKA−β1AR) (18). In response to either Alp or Car, we show a significant 1.5-fold increase in β1AR phosphorylation (Fig. 3C). These combined data demonstrate that the EGFR is transactivated in response to Alp and Car stimulation, and that the mechanism for transactivation requires phosphorylation of the β1AR on consensus GRK phosphorylation sites, a process known to promote β-arrestin recruitment and activation (3, 19).
Both β-Arrestin 1 and β-Arrestin 2 Are Required for Alp- or Car-Induced ERK Activation.
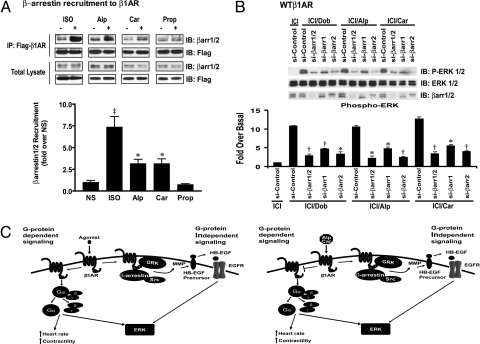
To determine if Alp or Car stimulation of the β1AR can trigger β-arrestin translocation, we performed immunoprecipitation experiments in HEK293 cells stably expressing β1ARs in the presence of chemical cross-linkers. Upon stimulation, both Alp and Car stimulated significant β-arrestin recruitment to the β1AR, whereas Prop administration had no effect (Fig. 4A). Previous studies have shown that agonist-stimulated recruitment of β-arrestin to the receptor can promote ERK activation (3, 20). To further show the importance for β-arrestins in Alp- or Car-induced ERK activation, we used siRNA to specifically knock down expression of β-arrestin 1 and β-arrestin 2 (13). Stimulation with Dob, Alp, or Car resulted in significant ERK activation in scrambled siRNA-transfected cells. In contrast, ERK activation was significantly blocked in the presence of siRNA targeting β-arrestin 1, β-arrestin 2, or both using HEK293 cells stably expressing β1ARs (Fig. 4B). Consistent with previous studies, immunoblotting for β-arrestin showed effective depletion of both β-arrestins in the presence of their silencing siRNAs (Fig. 4B) (13).
Fig. 4.
β-Arrestin is recruited to β1AR by Alp or Car and is required for Alp- or Car-induced ERK activation. (A) HEK293 cells stably expressing FLAG-WTβ1AR are stimulated with Iso, Alp, Car, or Prop. Alp or Car induces β-arrestin recruitment to the β1AR. (B) HEK293 cells stably expressing WTβ1AR are transfected with either FLAG-EGFR and scrambled siRNA (si-Con); or FLAG-EGFR and siRNAs targeting β-arrestin 1 (si-βarr1), β-arrestin 2 (si-βarr2), or β-arrestin1/2 (si-βarr1/2), respectively. Alp- or Car-induced phospho-ERK1/2 is diminished in cells transfected with siRNA targeting the β-arrestins. (C) β1AR agonists enhance cardiac contractility by G protein-dependent signaling, and they also induce β-arrestin-dependent signaling (Left). However, Alp and Car selectively induce β-arrestin-mediated β1AR transactivation of EGFR (Right). Alp and Car induce GRK-mediated phosphorylation of β1AR, and recruitment of β-arrestin and Src. This leads to MMP activation to promote HB-EGF shedding, and subsequent activation of EGFR and its downstream signaling such as ERK. Data represent mean ± SE of at least five independent experiments. *, P < 0.05 vs. NS and Prop or si-Con; †, P < 0.01 vs. si-Con; ‡, P < 0.001 vs. NS and Prop.
Alp- or Car-Induced β1AR-Mediated EGFR Transactivation Requires Src, MMP Activity, and Heparin-Binding EGF (HB-EGF) Shedding.
In our experiments, we show that both Alp and Car use β-arrestin to induce β1AR-mediated EGFR transactivation. As β-arrestin is known to interact with the cytosolic tyrosine kinase Src (20), we tested whether pharmacological inhibition of Src would block this process. HEK293 cells stably expressing β1ARs were transfected with EGFR-FLAG and pretreated with or without the Src inhibitor PP2 (4-amino-5-[4-chlorophenyl]-7-[t-butyl]pyrazolo[3,4-d]pyrimidine) before ligand stimulation. PP2 markedly blocked EGFR phosphorylation and significantly inhibited ERK activation following Dob, Alp, or Car stimulation, consistent with the concept that Src is involved in β1AR-mediated EGFR transactivation (Fig. S3 A, D, and E).
As Src has been shown to be upstream of MMP-mediated shedding of HB-EGF (21, 22), we further tested whether MMP and HB-EGF inhibition would abrogate Alp- or Car-induced β1AR-mediated transactivation. β1AR- and EGFR-FLAG-expressing HEK293 cells were pretreated with either the MMP inhibitor ilomastat (GM6001) or the HB-EGF neutralizing antibody, followed by Dob, Alp, or Car stimulation. Similar to PP2 inhibition, pretreatment with ilomastat or an HB-EGF neutralizing antibody significantly attenuated β1AR-stimulated EGFR transactivation and subsequent ERK activation (Fig. S3 B–E). We next examined whether MMP is involved in Alp- or Car-induced ERK activation in vivo. Alp or Car was administered by i.v. infusion to WT mice in the presence or absence of the MMP inhibitor ilomastat. Ilomastat was administered i.p. 1 h before βAR ligands. Consistent with our cell culture experiments, Alp or Car induces ilomastat-sensitive ERK activation in the heart (Fig. S3F).
Taken together, these data demonstrate that Src, MMP activation, and HB-EGF are required for Alp- or Car-induced β1AR-mediated EGFR transactivation, in agreement with the previously described pathway for EGFR transactivation when stimulated by a βAR agonist (3).
Discussion
In this study, we tested whether classical antagonists of the β1AR could function as agonists for β-arrestin-mediated signaling. We screened 20 β-blockers to determine whether any could activate a recently described β-arrestin-dependent signaling pathway EGFR transactivation. Indeed, we show that, among all β-blockers screened, only Alp and Car can stimulate transactivation of the EGFR. Moreover, these ligands induce phosphorylation of the receptor, recruitment of β-arrestin to the ligand occupied receptor and subsequent MMP activation to promote HB-EGF shedding and EGFR activation.
Recent data indicate that agonist-stimulated 7TMRs use G protein-dependent and G protein-independent mechanisms to activate ERK signaling (Fig. 4C) (3, 20, 23–25). In addition to agonists, it has recently been demonstrated for the β2AR that carvedilol can stimulate β-arrestin-mediated activation of ERK (26). However, it was not known whether this would occur for the β1AR and whether β-arrestin-mediated ERK activation depends on activation of the EGFR. In our study, we demonstrate that Car stimulation of the β1AR uses β-arrestin to transactivate the EGFR and induce ERK signaling (Fig. 4C). Interestingly, Car has been shown to inhibit ERK activation in monocytes (27), suggesting that Car may activate different signaling pathways depending on the cell type. Alp-induced ERK activation also uses a β-arrestin-dependent mechanism in cells expressing β1ARs, which is not the case for cells expressing β2ARs (26). Therefore, activation of β-arrestin-dependent pathways by βAR antagonists appears to be receptor subtype-specific.
In the framework of an allosteric model, unliganded G protein-coupled receptors exist in at least two states, an inactive conformation and an active conformation. Agonists are believed to stabilize the active conformation, whereas neutral antagonists compete for binding of agonists to the receptor (28, 29). Recent evidence suggests that different ligands stabilize different active conformations, resulting in distinct signaling properties (9–12). For the human β1AR, it has been suggested that two agonist activation sites exist: the classical catecholamine binding site and an allosteric site defined by binding of the ligand CGP-12177 (30–32). It has been shown that, for some β-blockers such as Alp and Car, they bind with high affinity to the catecholamine site, but also with lower affinity to a secondary site to stimulate G protein activation (30–32). Although the precise characteristics of this putative secondary site are not well characterized, it could represent an independent site within the β1AR monomer, a ligand-specific conformation (33), or a conformation that is dependent on the extent of receptor phosphorylation, dimerization (34), or association with particular scaffold proteins (e.g., AKAP79/150) (35). Nonetheless, our data in this study do not support a secondary site for the action of Alp and Car in stimulating β-arrestin-mediated β1AR transactivation of the EGFR. This is based on the facts that CGP-12177 did not induce β-arrestin-mediated β1AR transactivation of the EGFR and that the ability of Alp or Car to induce ERK activation was blocked by the nonselective β-blocker Prop, which binds to only the high-affinity catecholamine site (31). Thus, our data suggest that the β-receptor ligands Alp and Car are unique in their ability to promote a β1AR conformation that readily induces β-arrestin signaling.
We observed that Car and Alp stimulate very weak activation of Gs-dependent adenylyl cyclase in HEK293 cells expressing mouse β1ARs. Previous studies have reported inconsistent findings regarding activation of Gs-dependent adenylyl cyclase by Car. Whereas Car acted as an agonist toward the adenylyl cyclase pathway in HEK293S cells expressing human β1AR in one study (36), another study found that Car showed marked inverse agonist effects in HEK293 cells expressing human β1AR (37) and the β2AR (26). These discrepancies are likely attributed to differences in expression levels of receptor and sensitivity of the different cAMP assay systems.
β-Blockers are a class of drugs that are used in the treatment of common cardiovascular disorders such as hypertension, coronary artery disease, and heart failure (38). They comprise various ligands with potentially different clinical efficacies; however, it remains to be established whether these agents really differ in their effectiveness, and what might be the molecular basis of such differences (39). Given that β-arrestin-mediated β1AR transactivation of EGFR may render cardioprotection, as we recently showed in an experimental model system (3), β-blockers that activate this pathway might possess superior efficacy in treating cardiovascular disorders. Among 20 β-blockers screened in our study, only Car and Alp were identified to stimulate this potentially cardioprotective pathway. Interestingly, Car is one of three agents currently approved for the treatment of heart failure (36), and may possess a survival advantage over other βAR antagonists (40). Although Alp is used in the treatment of hypertension, angina, and arrhythmia (41–43), its clinical efficacy in treating heart failure remains to be tested.
In conclusion, we demonstrate that Alp and Car stimulate β-arrestin-mediated β1AR-EGFR transactivation, a pathway we recently have shown to be cardioprotective. Our finding that Alp and Car stabilize a receptor conformation to allow binding and activation of β-arrestin has important therapeutic implications. We postulate that biased ligands of this type may represent a new generation of therapeutic agents that will block the deleterious effects of β1AR overstimulation while simultaneously being able to provide cardioprotection via β-arrestin-mediated β1AR-EGFR transactivation.
Materials and Methods
siRNA Experiments Targeting β-Arrestins.
siRNA targeting β-arrestins were generated and the sequence of the 21-nt siRNAs has been previously described (13, 44). Thirty to 40% confluent HEK293 cells stably expressing WT β1AR in six-well dishes were transfected with 0.2 μg of FLAG-EGFR and 3.5 μg of siRNA using the GeneSilencer Transfection reagent (Gene Therapy Systems) as previously described (45). All assays were performed 60 to 72 h after siRNA transfection. Cells were serum-starved for 12 h before stimulation as described previously (3, 45).
Immunoprecipitation with Dithiobis(succinimidyl) Propionate Cross-Linking.
HEK293 cells stably expressing FLAG-β1AR were used for these experiments as previously described (25). Cells on 100-mm dishes were incubated in Dulbecco PBS solution plus 10 mM Hepes for 1 h at 37°C and subsequently stimulated with 10 μM ligands for 5 min. A membrane-permeable, hydrolyzable covalent cross-linker, dithiobis(succinimidyl) propionate (0.2 mg/ml from Pierce) was added to the dishes with slow and constant agitation. After 30 min incubation at room temperature, the dithiobis(succinimidyl) propionate reaction was quenched by adding Tris-HCl, pH 7.5. Cells were lysed, and receptors were immunoprecipitated with FLAG beads (Sigma). Co-immunoprecipitated β-arrestins were detected by immunoblotting.
Metabolic Labeling Assay.
Metabolic labeling assay was accomplished according to previously described protocols (18, 25). Assays were performed at 37°C in phosphate-free DMEM. Labeling was conducted for 1 h in medium containing 100 μCi of 32Pi/ml (inorganic phosphate, Pi). After stimulation with 10 μM ligands for 30 min, equivalent amounts of β1AR were immunoprecipitated from each sample and were resolved by SDS/PAGE in 10% gels. Dried gels were subjected to autoradiography and analyzed quantitatively with an Amersham Biosciences PhosphorImager.
Treatment Protocol for Mice.
C57BL/6 mice were pretreated for 1 h with erlotinib (20 mg/kg), ilomastat (25 mg/kg), or 10% DMSO i.p., followed by infusion of saline solution, 10% DMSO, Alp (20 mg/kg), Car (16 mg/kg), or EGF (100 μg/kg). Five minutes after drug administration, hearts were excised and flash-frozen in liquid N2 for biochemical assays as described previously (3).
For more information see SI Text.
Supplementary Material
Acknowledgments.
We thank Weili Zou for excellent technical assistance. This work was supported by National Institutes of Health Grant HL56687 and HL-75443 (to H.A.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804745105/DCSupplemental.
References
- 1.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 2.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 3.Noma T, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 5.Fowler MB, Bristow MR. Rationale for beta-adrenergic blocking drugs in cardiomyopathy. Am J Cardiol. 1985;55:120D–124D. doi: 10.1016/0002-9149(85)91066-5. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. Br Med J. 2004;328:634–640. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 8.Cruickshank JM. Are we misunderstanding beta-blockers? Int J Cardiol. 2007;120:10–27. doi: 10.1016/j.ijcard.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 9.Kenakin T. Agonist-receptor efficacy. I: mechanisms of efficacy and receptor promiscuity. Trends Pharmacol Sci. 1995;16:188–192. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- 10.Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc Natl Acad Sci USA. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaminath G, et al. Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 12.Granier S, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 13.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes-characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- 16.Ponicke K, Groner F, Heinroth-Hoffmann I, Brodde OE. Agonist-specific activation of the beta2-adrenoceptor/Gs-protein and beta2-adrenoceptor/Gi-protein pathway in adult rat ventricular cardiomyocytes. Br J Pharmacol. 2006;147:714–719. doi: 10.1038/sj.bjp.0706674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauwels PJ, Gommeren W, Van Lommen G, Janssen PA, Leysen JE. The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers. Mol Pharmacol. 1988;34:843–851. [PubMed] [Google Scholar]
- 18.Rapacciuolo A, et al. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 19.Barnes WG, et al. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 20.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter G. EGF receptor transactivation mediated by the proteolytic production of EGF-like agonists. Sci STKE. 2000;2000 doi: 10.1126/stke.2000.15.pe1. [DOI] [PubMed] [Google Scholar]
- 22.Prenzel N, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 23.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFea KA, et al. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenoy SK, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 26.Wisler JW, et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuochi Y, et al. Carvedilol, a nonselective beta-blocker, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. Transl Res. 2007;149:223–230. doi: 10.1016/j.trsl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Bond RA. Do recent operational studies indicate that a single state model is no longer applicable to G protein-coupled receptors? Ann N Y Acad Sci. 1997;812:92–97. doi: 10.1111/j.1749-6632.1997.tb48149.x. [DOI] [PubMed] [Google Scholar]
- 29.Strange PG. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol Sci. 2002;23:89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- 30.Baker JG. Site of action of beta-ligands at the human beta1-adrenoceptor. J Pharmacol Exp Ther. 2005;313:1163–1171. doi: 10.1124/jpet.104.082875. [DOI] [PubMed] [Google Scholar]
- 31.Baker JG, Hall IP, Hill SJ. Agonist actions of “beta-blockers” provide evidence for two agonist activation sites or conformations of the human beta1-adrenoceptor. Mol Pharmacol. 2003;63:1312–1321. doi: 10.1124/mol.63.6.1312. [DOI] [PubMed] [Google Scholar]
- 32.Joseph SS, Lynham JA, Colledge WH, Kaumann AJ. Binding of (-)-[3H]-CGP12177 at two sites in recombinant human beta 1-adrenoceptors and interaction with beta-blockers. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:525–532. doi: 10.1007/s00210-004-0884-y. [DOI] [PubMed] [Google Scholar]
- 33.Seifert R, Wenzel-Seifert K, Gether U, Kobilka BK. Functional differences between full and partial agonists: evidence for ligand-specific receptor conformations. J Pharmacol Exp Ther. 2001;297:1218–1226. [PubMed] [Google Scholar]
- 34.Salahpour A, Angers S, Bouvier M. Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol Metab. 2000;11:163–168. doi: 10.1016/s1043-2760(00)00260-5. [DOI] [PubMed] [Google Scholar]
- 35.Fraser ID, et al. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 36.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 37.Rochais F, et al. Real-time optical recording of beta1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975;37:1022–1036. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bristow M. Antiadrenergic therapy of chronic heart failure: surprises and new opportunities. Circulation. 2003;107:1100–1102. doi: 10.1161/01.cir.0000054530.87613.36. [DOI] [PubMed] [Google Scholar]
- 40.Poole-Wilson PA, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 41.Adolfsson L, Areskog NH, Furberg C, Johnsson G. Effects of single doses of alprenolol and two cardioselective beta-blockers (H 87–07 and H 93–26) on exercise-induced angina pectoris. Eur J Clin Pharmacol. 1974;7:111–118. doi: 10.1007/BF00561324. [DOI] [PubMed] [Google Scholar]
- 42.Jones A, LoBrutto R, Kazakevich Y. Effect of the counter-anion type and concentration on the liquid chromatography retention of beta-blockers. J Chromatogr A. 2002;964:179–187. doi: 10.1016/s0021-9673(02)00448-x. [DOI] [PubMed] [Google Scholar]
- 43.Ranade VV, Somberg JC. Chiral cardiovascular drugs: an overview. Am J Ther. 2005;12:439–459. doi: 10.1097/01.mjt.0000167429.37357.0c. [DOI] [PubMed] [Google Scholar]
- 44.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 45.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.