Abstract
Monkeypox virus (MPV) is a virulent human pathogen that has gained increased attention because of its potential use as a bioterrorism agent and inadvertent introduction into North America in 2003. The US outbreak also provided an important opportunity to study MPV-specific T cell immunity. Although MPV-specific CD4+ and CD8+ T cells could recognize vaccinia virus (VV)-infected monocytes and produce inflammatory cytokines such as IFNγ and TNFα, they were largely incapable of responding to autologous MPV-infected cells. Further analysis revealed that, unlike cowpox virus (CPV), MPV did not interfere with MHC expression or intracellular transport of MHC molecules. Instead, MPV-infected cells were capable of preventing T cell receptor (TcR)-mediated T cell activation in trans. The ability to trigger a state of nonresponsiveness represents a unique MHC-independent mechanism for blocking antiviral T cell activation and inflammatory cytokine production and is likely an important attribute involved with viral dissemination in the infected host.
Keywords: immune evasion, orthopoxvirus, T cell immunity
Smallpox [Variola (VAR)] was eradicated from nature in 1977, but the threat of deliberate or accidental release of VAR or other virulent orthopoxviruses (OPV) has raised concern in recent years (1–4). Monkeypox virus (MPV) is second only to VAR in terms of OPV virulence—with mortality rates of ≈10% (5–8). Inadvertent importation of this virus into the US in 2003 raised MPV awareness and demonstrated first-hand how global travel can quickly lead to unexpected outbreaks of zoonotic diseases (9–11). Although MPV does not spread efficiently by human-to-human contact (12–14), it serves as an important model for smallpox (15–18) and shares several key features of pathogenesis. For instance, unlike vaccinia (VV) (19), both VAR and MPV disseminate through their infected hosts mainly by a cell-associated viremia (15, 20–23). Moreover, evasion of host immune responses is well documented; VAR infection of previously vaccinated humans and MPV infection of non-human primates can result in infectious virus persisting for prolonged periods of time as an asymptomatic infection in apparently healthy individuals (24–32).
The mechanisms underlying these forms of immune evasion are not well understood. Many viruses employ a battery of immune evasion strategies (33–38) and poxviruses in particular are equipped to evade antiviral cytokines, chemokines, and/or antigen presentation (35, 39). We have shown that cowpox virus (CPV) interferes with intracellular transport of MHC class I, a process that correlated with evasion of antiviral CD8+ T cell responses by CPV (40). It was recently demonstrated that CPV open reading frame 203 retains MHC class I in the ER (41), and, because MPV encodes a close homologue of CPV203, we expected to find a similar mechanism of immune evasion by MPV. In contrast, we observed that MPV did not down-regulate MHC class I, but instead used a mechanism of evasion that inhibited CD4+ and CD8+ T cell activation after cognate interactions with MPV-infected cells. This mechanism of abrogating local T cell responses may avoid systemic immune suppression, while at the same time protecting the viral reservoir from immune surveillance. Identification of the factor or factors involved with MPV-induced T cell inhibition could prove useful for developing new biologics aimed at preventing or alleviating T cell-mediated diseases (42, 43).
Results
Antiviral CD4+ and CD8+ T Cells Recognize VV-Infected Monocytes but Not MPV-Infected Monocytes.
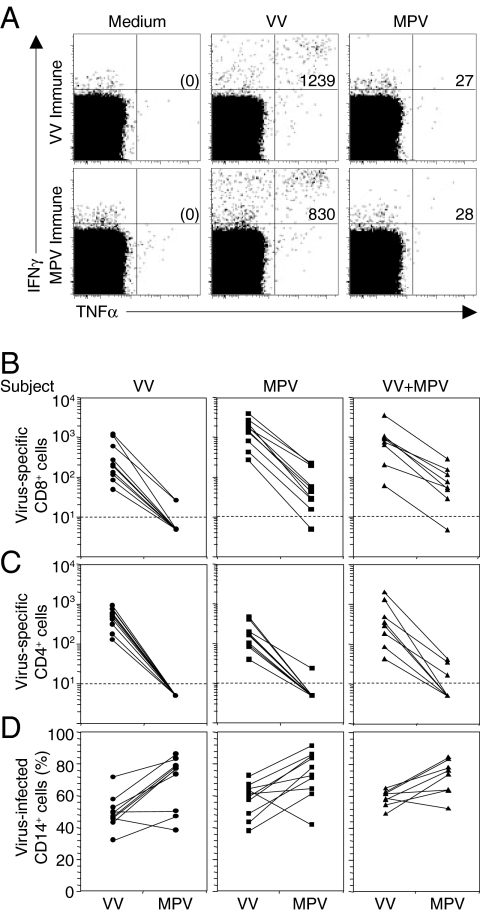
Several human HLA-binding peptide epitopes are conserved between OPV such as vaccinia virus (VV) and VAR (44), which led us to test the cross-reactive activity of VV-specific T cells from recently vaccinated donors exposed to MPV-infected cells (Fig. 1). After infecting PBMCs with an optimized concentration of VV or MPV, we measured virus-specific T cell responses by intracellular cytokine staining analysis (ICCS) (10, 40, 45). CD14+ monocytes represent the main cell type infected by these viruses (40) (data not shown), and they have the capacity to present peptides to both virus-specific CD4+ and CD8+ T cells. In Fig. 1A, we measured the frequency of IFNγ+TNFα+ CD8+ T cells after culture in medium alone (negative control used for background subtraction) or after stimulation with VV or MPV. Although VV-specific T cells were clearly able to recognize and respond to VV-infected cells (1239 IFNγ+TNFα+ T cells per million CD8+ T cells), they were largely incapable of responding to MPV-infected cells (27 IFNγ+TNFα+ T cells per million CD8+ T cells). This result indicated that either the immunodominant peptides of MPV differed from VV or that MPV was evading virus-specific T cells. To distinguish between these possibilities, we examined the T cell responses of MPV-immune individuals as well. Similarly to the results observed with the VV-specific T cells, MPV-specific T cells could recognize VV-infected cells but were unable to respond to MPV-infected cells (830 vs. 28 IFNγ+TNFα+ T cells per million CD8+ T cells).
Fig. 1.
MPV immune evasion from orthopoxvirus-specific T cells. (A) Antiviral CD8+ T cell responses from a VV-immune (6 months post-VV infection) or a MPV-immune (4 months post-MPV infection) subject were measured by ICCS after 18 h of stimulation with VV or MPV (MOI of 0.3) with Brefeldin A added for the last 6 h of stimulation. PBMCs were gated on CD8β+CD4− T cells. The numbers in the upper right quadrants depict the frequency of virus-specific IFNγ+TNFα+ T cells per million CD8+ T cells identified after background subtraction from control wells containing Medium alone (shown in parenthesis). (B) Virus-specific CD8+ T cell responses against VV or MPV were determined as in A, using PBMCs from VV-immune subjects (VV; 4–6 months postinfection, n = 10), MPV-immune subjects (MPV; 3–31 months post-MPV infection, n = 10) or VV-immune subjects who contracted MPV infection (VV+MPV; 3–13 months post-MPV infection, n = 8). (C) Virus-specific CD4+CD8β− T cell responses against VV or MPV were determined as in A. (D) Monocytes were identified based on forward and side scatter characteristics and CD14 surface expression. The percentage of CD14+ monocytes infected with VV or MPV was determined after 18 h of infection, using a polyclonal anti-orthopoxvirus antibody (40).
To determine whether the poor recognition of MPV-infected cells represented a common finding among genetically diverse individuals, we measured OPV-specific T cell responses against VV and MPV, using PBMCs from a total of 28 subjects who were VV-immune, MPV-immune, or VV+MPV-immune (i.e., VV-immune subjects who contracted MPV) (Fig. 1 B and C). Similar to Fig. 1A, all subjects demonstrated VV-stimulated CD8+ T cell responses that were reduced by >90% if stimulated with MPV instead of VV (Fig. 1B). Not only were virus-specific CD8+ T cell responses lower after exposure to MPV, but virus-specific CD4+ T cell responses were also reduced by >90% in comparison with VV (Fig. 1C). The reduced antiviral T cell response elicited after exposure to MPV was not due to decreased infection rates because both viruses readily infected CD14+ cells (Fig. 1D). These observations indicate that MPV is efficient at infecting primary human monocytes but does not trigger inflammatory cytokine production (IFNγ or TNFα) by virus-specific T cells.
MPV Does Not Down-Regulate MHC Class I or MHC Class II on Human Monocytes.
In previous studies, we discovered that cowpox virus (CPV) evaded antiviral T cell responses by down-regulating MHC class I (40). To determine whether MPV used a similar evasion strategy, we examined surface expression levels of MHC class I (HLA-A, -B, and -C) and MHC class II (HLA-DR) on primary human monocytes that were infected with VV, CPV, or MPV (Fig. 2A). CPV infection resulted in substantial down-regulation of MHC class I surface expression, whereas VV infection had no effect. Although MPV infection resulted in minor MHC class I down-regulation in certain cell lines (data not shown), MPV infection did not down-regulate MHC class I expression on primary human monocytes despite highly effective evasion from CD8+ T cell recognition (Fig. 1). In contrast to MHC class I, MHC class II expression was not altered by VV, CPV, or MPV infection (Fig. 2A).
Fig. 2.
Inhibition of antiviral T cell responses by MPV is not mediated by MHC down-regulation. (A) After 16 h of infection, virus-infected CD14+ cells were identified by intracellular staining for OPV antigens as described in ref. 40. Surface expression of MHC class I (HLA-A, -B, and -C) on virus-infected primary human monocytes was down-regulated by CPV, but not by VV or MPV. Surface expression of MHC class II (HLA-DR) was unaltered by infection with CPV, VV, or MPV. (B) MHC class I assembly and transport was measured in OPV-infected cells. HeLa cells were uninfected (uninfected* indicates a lighter scanned image of the same experiment) or infected with VV, MPV, or CPV for 5 h (starved for the last 1 h) and pulse-labeled for 20 min, and the labels were chased for 0, 30, or 60 min as indicated. More than 90% of HeLa cells were infected (data not shown), and cell lysates were immunoprecipitated with anti-MHC class I antibody, W6/32. The precipitated material was treated with EndoH and separated by SDS/PAGE. HC, heavy chain; ER, EndoH-resistant band; ES, EndoH-sensitive band. These data show one of four independent experiments, each with comparable results. (C) Stability of MHC class I heavy chain was determined in OPV-infected cells. HeLa cells were infected with VV or MPV for 5 h; starved for the last 1 h in the presence or absence of proteasomal inhibitor, MG132; and pulse-labeled for 20 min, and the labels were chased for 0, 30, or 60 min in the continued presence or absence of MG132. The lysates were then immunoprecipitated with polyclonal anti-MHC class I antibody, K455, followed by EndoH treatment and SDS/PAGE. The open arrowheads indicate the presence of nonspecific low molecular weight proteins that were immunoprecipitated with either anti-MHC class I or anti-CD44 antibodies (see Fig. S1).
Although we observed little or no reduction of MHC class I surface expression on MPV-infected monocytes, it was still possible that MPV interfered with intracellular transport of newly synthesized MHC class I. To test this possibility, we examined the fate of newly synthesized MHC class I molecules by pulse–chase labeling and immunoprecipitation. Uninfected HeLa cells, or cells infected for 5 h with VV, MPV, or CPV, were metabolically labeled for 20 min followed by a 0-, 30-, or 60-min chase period (Fig. 2B). MHC class I was immunoprecipitated with the conformation-specific antibody, W6/32, which recognizes assembled MHC class I. The precipitated material was treated with Endoglycosidase H (EndoH) to monitor the maturation and addition of glycan residues to MHC class I upon passage through the Golgi network. MHC class I from uninfected cells rapidly exited the ER as reflected by the acquisition of EndoH resistance (ER). In VV-infected cells, MHC class I molecules exited the ER with similar kinetics as in uninfected cells. In contrast, MHC class I remained EndoH sensitive (ES) in CPV-infected cells, consistent with our previous studies (40) and the observation of decreased surface MHC class I expression (Fig. 2A). MHC class I molecules in MPV-infected cells, however, did not show signs of retention; instead they matured similarly to uninfected and VV-infected cells. These data indicate that the exit of MHC class I molecules from the ER is normal in MPV-infected cells. Low molecular weight proteins (arrowheads) coprecipitated with MHC class I in MPV-infected cells, but the same bands were also observed with control immunoprecipitations of an unrelated protein, CD44, indicating that this is not an MHC class I-specific interaction [supporting information (SI) Fig. S1]. In those experiments, we found that CD44 exit from the Golgi was unaffected by VV, CPV, or MPV, consistent with the conclusion that infection with these viruses does not result in a general interference with glycoprotein trafficking. In all virus-infected cells we observed a reduction in overall levels of immunoprecipitated MHC class I compared with control, which is likely due to OPV shutoff of host cell transcription (Fig. 2B). Because MPV-infected cells showed low MHC class I recovery by immunoprecipitation, we also explored the possibility that MPV preferentially degrades newly synthesized MHC class I molecules. Therefore, we performed pulse–chase experiments in the presence of a proteasomal inhibitor, MG132 (46), and immunoprecipitated virus-infected cell lysates with conformation-independent polyclonal antiserum K455 (47) to recover both folded and unfolded MHC class I heavy chains. Although we observed a stabilizing effect on MHC class I in the presence of proteasome inhibitor in control experiments with cells transduced with recombinant adenovirus expressing HCMV-US2 (48) (data not shown), we recovered similar amounts of MHC class I in the presence or absence of MG132 from both VV and MPV-infected cells (Fig. 2C). Taken together, these data suggest that expression and maturation of MHC class I is not substantially different between MPV-infected and VV-infected cells and that interference with antigen presentation is unlikely to account for the dramatic difference in T cell stimulation by these two viruses.
MPV Inhibits T Cell Responses Against Other Viruses.
To determine whether MPV was able to prevent T cell stimulation by VV in a dominant fashion, we performed coinfection experiments with MPV and VV (Fig. 3). An optimized dose of VV (MOI of 0.3) or MPV (MOI of 0.3) was added to individual wells containing PBMCs from a VV-immune subject. Alternatively, VV was mixed with MPV at a ratio of 10:1, 1:1, or 1:10 (MPV MOI of 0.03, 0.3, and 3.0, respectively), and antiviral T cell responses were measured by calculating the number of virus-specific IFNγ+TNFα+ T cells (Fig. 3A). Analysis of antiviral T cell responses by four more VV-immune subjects confirmed that T cell activation was highest after stimulation with VV alone (Fig. 3B). In contrast, T cell-mediated cytokine responses were decreased by nearly 80% when a low dose of MPV was added (VV:MPV ratio of 10:1), and they dropped by ≈95% when cells were coinfected with a 1:1 mixture of VV and MPV. Similar results were observed with PBMCs from a MPV-immune subject (data not shown). The observation that MPV inhibits T cell activation by VV suggested that MPV encodes an immunomodulatory protein that is absent from VV. Moreover, because this inhibition occurred even at low ratios of MPV/VV, it suggested that this mechanism operates in trans, i.e., that T cell stimulation by VV-infected cells was inhibited by MPV-infected bystander cells.
Fig. 3.
MPV inhibits T cell responses to VV and Epstein–Barr virus (EBV) in trans. (A) PBMCs from a representative VV-immune subject (16 months after infection) were infected with VV (MOI of 0.3), MPV (MOI of 0.3), or a mixture of VV and MPV at the indicated ratios with VV maintained at an MOI of 0.3 in each case. OPV-specific CD4+ T cell responses (Upper) and CD8+ T cell responses (Lower) were determined by ICCS after 18 h of stimulation with Brefeldin A added for the last 6 h of stimulation. The numbers in the upper right quadrants represent the frequency of virus-specific IFNγ+TNFα+ T cells per million T cells identified after background subtraction from control wells containing Medium alone. (B) PBMCs from 4 VV-immune subjects (1 month postinfection) were stimulated with VV and/or MPV as in A. The antiviral T cell response was determined by ICCS and normalized to 100% based on the response to VV alone. The data depict the average ± SD. Statistical significance was determined using a two-tailed paired Student's t test. (C) To determine whether the inhibitory effect of MPV occurred in cis or in trans, EBV-transformed LCLs (EBV) from 3 EBV-seropositive VV-immune subjects were infected for 15 h with VV (VV+EBV) or MPV (MPV+EBV), washed extensively to remove secreted proteins, and then added separately to autologous PBMCs or mixed at a 1:1 ratio (VV+MPV+EBV) before mixing with autologous PBMCs for 6 h in the presence of Brefeldin A to stimulate EBV-specific and OPV-specific T cell responses.
To test this hypothesis, we infected Epstein–Barr virus (EBV)-transformed lymphocytic cell lines (LCLs) separately with either MPV or VV, washed the cells extensively, and then mixed them together immediately before culturing them with autologous PBMCs in the presence of Brefeldin A for 6 h. By infecting the target cells separately, we could determine whether the inhibitory properties of MPV were associated only with MPV-infected cells or whether they could inhibit T cell responses to neighboring cells that were infected with VV and EBV but not directly infected with MPV (Fig. 3C). The LCLs and PBMCs used in these experiments were obtained from EBV-seropositive subjects (data not shown). For this reason, a readily detectable IFNγ+TNFα+ CD4+ or CD8+ T cell response was observed after incubation with autologous EBV-transformed LCLs alone and this represented the EBV-specific T cell response. Mixing EBV+ LCLs at a 1:1 ratio with VV-infected EBV+ LCLs allowed measurements of the combined EBV-specific and VV-specific T cell responses. Mixing EBV+ or EBV+VV+ LCLs at a 1:1 ratio with MPV+ LCLs resulted in a marked decrease in virus-specific T cell activation. The immunosuppressive factor (or factors) produced by MPV does not appear to be a secreted protein because extensive down-regulation of autologous T cell responses occurred rapidly and in the presence of Brefeldin A, which blocks secretion of most proteins that traffic through the Golgi. These studies demonstrate that MPV both blocks VV-specific T cell responses and sharply reduces antiviral T cell responses to a common γ-herpesvirus (EBV). Moreover, this indicates that MPV-infected cells can inhibit T cell responses in trans, thereby blocking T cell activation against other virus-infected cells that are not directly infected with MPV.
MPV Inhibits T Cell Responses to Anti-CD3 Stimulation.
The ability of MPV to block T cell activation in trans suggested that this virus is both immune-evasive and immunosuppressive. To verify this suppressive effect by an independent approach, PBMCs from OPV-naïve subjects were infected with VV or MPV or were cultured without added virus for 12 h; then the T cells were activated by anti-CD3 stimulation for 6 h (Fig. 4). Plate-bound anti-CD3 stimulation (Fig. 4A) triggers polyclonal activation of memory T cells directly through the T cell receptor (TcR) in the absence of costimulation and without requiring endogenous processing/presentation of peptide antigens. There were only marginal changes in CD4+ and CD8+ T cell responses if PBMCs were infected with VV before anti-CD3 stimulation (P = 0.02 and P = 0.09, respectively). MPV infection, however, was suppressive, resulting in nearly 80% reduction in CD4+ T cell responses and ≈60% reduction in CD8+ T cell responses (P = 0.001 and P = 0.002, respectively) (Fig. 4B). As another approach to determine whether the immunosuppressive factor produced by MPV was a cell-associated protein or a secreted protein, PBMCs were stimulated with anti-CD3 in the presence of supernatants from VV-infected or MPV-infected autologous LCLs (Fig. 4C). In contrast to the results obtained in the presence of MPV-infected cells (Figs. 3 and 4 A and B), supernatants from virus-infected LCLs did not elicit a significant (P ≥ 0.2) immunosuppressive effect on T cells stimulated with anti-CD3. This indicates that MPV produces a cell-associated factor (or factors) that can inhibit T cell activation independently of MHC class I or class II processing/presentation.
Fig. 4.
MPV-induced immune suppression of T cells is not MHC-dependent. To determine whether T cell inhibition by MPV occurred independently from MHC class I or class II processing/presentation, T cells were stimulated directly through the TcR with anti-CD3 antibody. (A) The percentage of IFNγ+TNFα+ T cells from the PBMCs of one representative OPV-naïve subject was determined after 12 h incubation in medium or infection with VV or MPV (MOI of 0.3) before transfer to new wells and stimulation for an additional 6 h with plate-bound anti-CD3 in the presence of Brefeldin A. (B) PBMCs from five OPV-naïve subjects were cultured as in A. The bar graphs depict the average (± SD) response normalized to a 100% maximum based on the number of IFNγ+TNFα+ T cells observed after anti-CD3 stimulation in the absence of viral infection. (C) To determine whether MPV secreted a soluble factor that could inhibit host T cell responses, LCLs from three subjects were cultured in medium (uninfected) or infected with VV or MPV (MOI of 0.3) for 15 h before harvesting the supernatant. Autologous PBMCs were resuspended in the described LCL supernatants and incubated for 25 min before addition of anti-CD3 and Brefeldin A for 6 h. The bar graphs depict the average response (±SD) normalized to a 100% maximum based on the number of IFNγ+TNFα+ T cells observed after anti-CD3 stimulation in supernatants from uninfected autologous LCLs. Statistical significance in B and C was determined using a two-tailed paired Student's t test.
Inactivation of MPV Allows Antigen Presentation to Virus-Specific T Cells.
The impressive ability of MPV to evade virus-specific T cell responses (Fig. 1) led us to suspect that an early gene product may be involved. To investigate this possibility, PBMCs were uninfected or infected with VV or MPV in the presence or absence of cytosine arabinoside (AraC) to block late gene expression (Fig. 5 A and B). In comparison with medium alone, AraC partially inhibited T cell responses to anti-CD3 stimulation. Similar effects were seen with VV-infected PBMCs, whereas PBMCs infected with MPV again showed the greatest inhibition of anti-CD3 responses even in the presence of AraC. This indicates that an early gene product is responsible for MPV-induced immunosuppression of CD4+ and CD8+ T cell responses because inactivation of late gene expression had no measurable effect on the suppressive mechanism invoked by MPV. This result also renders it unlikely that proteins released because of cytopathic effects were responsible for T cell suppression.
Fig. 5.
MPV immune evasion requires active viral replication. (A and B) To determine whether early gene expression was required for MPV immune suppression of TcR-mediated cytokine responses, PBMCs were cultured in medium or infected with VV or MPV for 12 h in the presence or absence of Arabinoside C (Ara-C) to prevent late gene expression. Anti-CD3 and Brefeldin A were added for an additional 6 h and INγ+TNFα+ responses in CD4+ T cells (A) or CD8+ T cells (B) were determined by ICCS and normalized to the values obtained after anti-CD3 stimulation of uninfected cultures. (C) To determine whether nonreplicating MPV was capable of suppressing T cell responses, PBMCs from a representative MPV-immune subject (4 months postinfection) were cultured in Medium or with VV, MPV, UV-inactivated VV (UV-VV), or UV-inactivated MPV (UV-MPV) at a MOI of 0.3. After 12 h stimulation followed by an additional 6 h incubation in the presence of Brefeldin A, CD4+ and CD8+ T cell responses were determined by ICCS. Dotplots were pregated on CD4+ or CD8+ T cells and the numbers in the upper right quadrants depict the frequency of virus-specific IFNγ+TNFα+ T cells per million T cells after background subtraction. Statistical significance in A and B was determined using a two-tailed paired Student's t test.
MPV-immune individuals mount OPV-specific CD4+ and CD8+ T cell responses that are equal or higher than that observed after VV infection (Fig. 1 B and C, and data not shown). Thus, despite our in vitro data showing efficient T cell evasion in monocytes, MPV induces a strong T cell response in vivo. One possibility is that antiviral T cells are elicited to peptide antigens presented through nonclassical antigen presentation or “cross-presentation.” To determine whether alternative antigen presentation could overcome MPV immune evasion, PBMCs from an MPV-immune individual (representative of 12 OPV-immune subjects; data not shown) were stimulated with live VV, live MPV, UV-inactivated VV or UV-inactivated MPV (Fig. 5C). Similar to the results shown in Fig. 1, live infection with MPV resulted in nearly complete immune evasion in comparison with VV. In sharp contrast, UV-inactivation of MPV resulted in efficient priming of MPV-specific CD4+ T cell responses that were similar to that observed after live VV or UV-inactivated VV stimulation. This may be expected because MHC class II presentation typically involves the exogenous pathway of antigen presentation. MHC class I presentation to CD8+ T cells, however, is typically most efficient after endogenous processing and presentation. Live MPV infection allows synthesis of early gene product(s) that precluded CD8+ T cell activation (Fig. 5B), but nonclassical antigen presentation of UV-inactivated MPV induced readily detectable CD8+ T cell responses (Fig. 5C). These results indicate that alternative antigen presentation of viral proteins is one mechanism in which MPV evasion may be overcome in vitro and possibly in vivo.
Discussion
In this study, we demonstrate a unique immune evasion mechanism that is used by MPV to simultaneously evade antiviral CD4+ and CD8+ T cell responses. This stealth tactic is MHC-independent and relies on MPV-infected cells to trigger a state of unresponsiveness in T cells. This appears to require direct cell-to-cell contact because supernatants from MPV-infected cells were not inhibitory and T cell inhibition was observed in the presence of brefeldin A, a potent (albeit not universal) inhibitor of the secretory pathway. This immunosuppressive effect required transcription of an early virus gene product and could be overcome by UV-inactivating the virus before incubation with PBMCs. This indicates that MPV encodes a new type of immune modulator that directly or indirectly (through the potential induction of an uncharacterized cellular factor) suppresses antiviral T cell responses elicited by the host. This suppression is likely to play an important role in viral pathogenesis and systemic spread of cell-associated virus.
The evasion tactics used by MPV to counter immune surveillance by virus-specific T cells may explain why this virus is able to spread efficiently as a cell-associated viremia (15, 23). Moreover, because neutralizing antibody plays a major role in protection against virulent OPV infections (18, 49, 50), systemic spread within circulating monocytes may also protect the virus from the effects of virus-specific humoral immunity. The T cell suppressive effect described here may explain why vaccinated monkeys are not protected from lethal MPV challenge by OPV-specific memory T cells in the absence of neutralizing antibodies (18). VAR also spreads systemically as a cell-associated viremia (20, 21) and may use a similar evasion mechanism to overcome cellular immune responses of the host. Elucidation of the gene or genes in MPV that elicit this effect is an area of active investigation and will be the first step in determining whether a related homolog exists in the VAR genome.
Individuals who recover from MPV infection are able to mount antiviral T cell responses that appear similar or higher in overall magnitude to that elicited by VV infection (Fig. 1), despite the existence of an immune evasion mechanism that severely blocks T cell recognition of MPV-infected monocytes or LCLs (Figs. 1, 3, and 5). One explanation for this is that induction of the T cell response in vivo may occur indirectly through alternative antigen presentation (Fig. 5) and/or cross-priming. Alternatively, it is possible that certain cell types may be able to overcome the immunoevasive effects of MPV infection and directly present peptide antigens to T cells (I. Messaoudi and S. Wong, personal communication). Future experiments will elucidate which cell types are susceptible to this form of viral manipulation. Moreover, identifying the factor (or factors) involved with this process could lead to improved treatments for virulent OPV infections and the development of potential new biologics that could be used to abrogate destructive T cell responses in certain disease settings (42, 43).
Methods
Subjects.
MPV-immune adults contracted MPV during the 2003 outbreak in Wisconsin (10). VV-immune adults from Oregon were vaccinated with DryVax at 1–16 months before sample collection, and VV-naïve subjects were recruited from Wisconsin. Each subject provided informed written consent before participation and the Institutional Review Board of Oregon Health and Science University approved all clinical studies.
Intracellular Cytokine Staining (ICCS).
In vitro stimulation conditions are detailed in SI Text. ICCS was performed as described in refs. 10 and 45.
Pulse–chase.
Pulse–chase experiments were performed as described in ref. 40. Please see SI Text for details.
Statistics.
Statistical significance was tested in Microsoft Excel using a two-tailed Paired Student's t test. P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank the subjects for the generous gift of their time and participation. This work was supported in part by National Institutes of Health Contract HHSN266200400080C/N01 AI40080 (to C.P. and P.N.), National Institutes of Health Grants AI064843 (to K.F.) and AI063675 (to M.K.S.), and Oregon National Primate Research Center Grant RR00163 (to M.K.S. and K.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800589105/DCSupplemental.
References
- 1.Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 2.O'Toole T, Mair M, Inglesby TV. Shining light on “Dark Winter.”. Clin Infect Dis. 2002;34:972–983. doi: 10.1086/339909. [DOI] [PubMed] [Google Scholar]
- 3.Smith GL, McFadden G. Smallpox: Anything to declare? Nat Rev Immunol. 2002;2:521–527. doi: 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK. The future of smallpox vaccination: Is MVA the key? Med Immunol. 2005;4:2. doi: 10.1186/1476-9433-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: Clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 6.Jezek Z, Grab B, Paluku KM, Szczeniowski MV. Human monkeypox: Disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop Geogr Med. 1988;40:73–83. [PubMed] [Google Scholar]
- 7.Hutin YJ, et al. Outbreak of human monkeypox. Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer H, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the democratic republic of congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed KD, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 10.Hammarlund E, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 11.Rimoin AW, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jezek Z, et al. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 13.Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 14.Fleischauer AT, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 15.Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jezek Z, Fenner F. In: Monographs in Virology. Melnick JL, editor. Vol 17. Basel: Karger; 1988. p. 140. [Google Scholar]
- 17.Earl PL, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 18.Edghill-Smith Y, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JF, et al. Lack of vaccinia viremia after smallpox vaccination. Clin Infect Dis. 2004;38:456–458. doi: 10.1086/381101. [DOI] [PubMed] [Google Scholar]
- 20.Downie AW, McCarthy K, MacDonald A, MacCallum FO, Macrae AD. Virus and virus antigen in the blood of smallpox patients. Their significance in early diagnosis and prognosis. Lancet. 1953;265:164–166. doi: 10.1016/s0140-6736(53)90107-x. [DOI] [PubMed] [Google Scholar]
- 21.Jahrling PB, et al. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc Natl Acad Sci USA. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenner HA, Cho CT, Bolano C, Kamitsuka PS. Monkey pox IV modification of disease pattern by antilymphocytic sera. J Infect Dis. 1969;120:318–331. doi: 10.1093/infdis/120.3.318. [DOI] [PubMed] [Google Scholar]
- 23.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verlinde JD, Van Tongeren HA. Isolation of smallpox virus from the nasopharynx of patients with variola sine eruptione. Antonie Van Leeuwenhoek. 1952;18:109–112. doi: 10.1007/BF02538595. [DOI] [PubMed] [Google Scholar]
- 25.Bras G. The morbid anatomy of smallpox. Doc Med Geogr Trop. 1952;4:303–351. [PubMed] [Google Scholar]
- 26.Kempe CH, Dekking F, Saint Vincent L, Rao AR, Downie AW. Conjunctivitis and subclinical infection in smallpox. J Hyg (London) 1969;67:631–636. doi: 10.1017/s002217240004208x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiner GG, et al. A study of inapparent infection in smallpox. Am J Epidemiol. 1971;94:252–268. doi: 10.1093/oxfordjournals.aje.a121319. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar JK, Mitra AC, Mukherjee MK, De SK. Variola virus in the throat of healthy contacts of small-pox cases. Bull Calcutta Sch Trop Med. 1972;20:61–62. [PubMed] [Google Scholar]
- 29.Sarkar JK, Mitra AC, Mukherjee MK. Serial isolation of variola virus in the throat of household contacts of smallpox cases. Bull Calcutta Sch Trop Med. 1973;21:21–22. [PubMed] [Google Scholar]
- 30.Sarkar JK, Mitra AC, Mukherjee MK. Duration of virus excretion in the throat of asymptomatic household contacts of smallpox patients. Indian J Med Res. 1974;62:1800–1803. [PubMed] [Google Scholar]
- 31.Arita I, Henderson DA. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39:277–283. [PMC free article] [PubMed] [Google Scholar]
- 32.Gispen R, Brand-Saathof B. “White” poxvirus strains from monkeys. Bull World Health Organ. 1972;46:585–592. [PMC free article] [PubMed] [Google Scholar]
- 33.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 34.Cornell CT, Kiosses WB, Harkins S, Whitton JL. Coxsackievirus B3 proteins directionally complement each other to down-regulate surface major histocompatibility complex class I. J Virol. 2007;81:6785–6797. doi: 10.1128/JVI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fruh K, Bartee E, Gouveia K, Mansouri M. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 2002;88:55–69. doi: 10.1016/s0168-1702(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 36.Hill A, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 37.Fruh K, et al. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 38.Pande NT, Powers C, Ahn K, Fruh K. Rhesus cytomegalovirus contains functional homologues of US2, US3, US6, and US11. J Virol. 2005;79:5786–5798. doi: 10.1128/JVI.79.9.5786-5798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seet BT, et al. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 40.Dasgupta A, Hammarlund E, Slifka MK, Fruh K. Cowpox virus evades CTL recognition and inhibits the intracellular transport of MHC class I molecules. J Immunol. 2007;178:1654–1661. doi: 10.4049/jimmunol.178.3.1654. [DOI] [PubMed] [Google Scholar]
- 41.Byun M, Wang X, Pak M, Hansen TH, Yokoyama WM. Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface. Cell Host Microbe. 2007;2:306–315. doi: 10.1016/j.chom.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Johnston JB, Rahman MM, McFadden G. Strategies that modulate inflammasomes: Insights from host-pathogen interactions. Semin Immunopathol. 2007;29:261–274. doi: 10.1007/s00281-007-0080-5. [DOI] [PubMed] [Google Scholar]
- 43.Amati L, et al. Taking advantage of viral immune evasion: Virus-derived proteins represent novel biopharmaceuticals. Curr Med Chem. 2006;13:325–333. doi: 10.2174/092986706775476106. [DOI] [PubMed] [Google Scholar]
- 44.Oseroff C, et al. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc Natl Acad Sci USA. 2005;102:13980–13985. doi: 10.1073/pnas.0506768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 46.Lee DH, Goldberg AL. Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 47.Andersson M, Paabo S, Nilsson T, Peterson PA. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985;43:215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 48.Tomazin R, et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 49.Slifka MK. Immunological memory to viral infection. Curr Opin Immunol. 2004;16:443–450. doi: 10.1016/j.coi.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Couzi G, Kircher JP. Immunotherapie de la Variole. Bulletin de l'Institut d'hygiene du Maroc. 1941;1:59–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.