Abstract
Although CD8+ T cells do not contribute to protection against the blood stage of Plasmodium infection, there is mounting evidence that they are principal mediators of murine experimental cerebral malaria (ECM). At present, there is no direct evidence that the CD8+ T cells mediating ECM are parasite-specific or, for that matter, whether parasite-specific CD8+ T cells are generated in response to blood-stage infection. To resolve this and to define the cellular requirements for such priming, we generated transgenic P. berghei parasites expressing model T cell epitopes. This approach was necessary as MHC class I-restricted antigens to blood-stage infection have not been defined. Here, we show that blood-stage infection leads to parasite-specific CD8+ and CD4+ T cell responses. Furthermore, we show that P. berghei-expressed antigens are cross-presented by the CD8α+ subset of dendritic cells (DC), and that this induces pathogen-specific cytotoxic T lymphocytes (CTL) capable of lysing cells presenting antigens expressed by blood-stage parasites. Finally, using three different experimental approaches, we provide evidence that CTL specific for parasite-expressed antigens contribute to ECM.
Keywords: dendritic cells, malaria, antigen presentation, cytotoxic T lymphocyte, cerebral malaria
It is well established that immune protection against the sporozoite and liver stages of Plasmodium infection depends on CD8+ T cell responses (1). In contrast, immunity to blood stages is largely humoral, although CD4+ T cells alone can be protective (1). Though CD8+ T cells do not protect against blood-stage infection, there is mounting evidence in murine models that they contribute to the pathology of experimental cerebral malaria (ECM) (2). Mice depleted of CD8+ T cells (3–5), or deficient in CD8 (3) or β2-microglobulin (5), are protected from ECM, although the precise mechanisms of CD8+ T cell-mediated pathology remain unclear. CD8+ T cells might contribute via perforin-dependent destruction of cerebral microvascular endothelial cells (6–8), or potentially through localized production of proinflammatory cytokines such as TNF-α, IFN-γ, IL-2, or LT-α, implicated in the pathogenesis of ECM (5, 9–12).
There is limited understanding of CD8+ T cell responses to blood-stage malaria infection and, in particular, whether CD8+ T cell with specificity for Plasmodium (versus nonspecific) are responsible for ECM (2). In fact, some studies implicating CD8+ T cells in ECM may require reinterpretation in light of recent evidence demonstrating that dendritic cells (DC) expressing CD8α mediate priming of T cell responses to pathogens such as viruses (13, 14) and bacteria (14). For example, earlier studies using depletion with anti-CD8α antibody to implicate CD8+ T cells in pathology might be reinterpreted as implicating CD8α DC in CD4+ T cell priming. Similarly, studies implicating CD8+ T cells in ECM that used perforin-deficient mice might reflect a role for NK cells rather than CD8+ T cells in disease. Together, these deficiencies in our understanding of CD8+ T cell responses to Plasmodium infection call for definitive evidence that a specific effector CD8+ T cell response is induced to blood-stage infection.
To examine Plasmodium-induced CD8+ T cell responses to blood-stage malaria infection in the absence of known MHC class I (MHC I)-restricted epitopes, we generated transgenic P. berghei parasites expressing a variety of model T cell epitopes for which T cell receptor (TCR) transgenic mice are available. Using these parasites, we demonstrated that antigens expressed by blood-stage P. berghei parasites are captured and cross-presented by CD8α DC to stimulate naive CD8+ T cell proliferation and lytic function.
Results
Transgenic Parasites Express Model T Cell Epitopes.
We generated a P. berghei transgenic parasite expressing model T and B cell epitopes fused to GFP under the control of the P. berghei elongation factor (EF)-1α promoter [Fig. 1A and supporting information (SI) Fig. S1], which is active throughout the life cycle (15). The T cell epitopes chosen were MHC I- and MHC II-restricted epitopes presented in C57BL/6 (B6) and BALB/c mice, which are differentially sensitive to P. berghei-mediated ECM (9). For B6 mice, which are susceptible to ECM, we included MHC I- and II-restricted epitopes from chicken ovalbumin (OVA) and an MHC I-restricted epitope from glycoprotein B (gB) of herpes simplex virus-1 (Fig. 1A and Fig. S1). For ECM-resistant BALB/c mice, MHC I- and II-restricted epitopes from hemagglutinin (HA) of the influenza virus PR8 were included, whereas the MHC II-restricted OVA epitope can also be presented on I-Ad of BALB/c mice (Fig. 1A and Fig. S1). Corresponding TCR transgenic mice specific for each epitope were available.
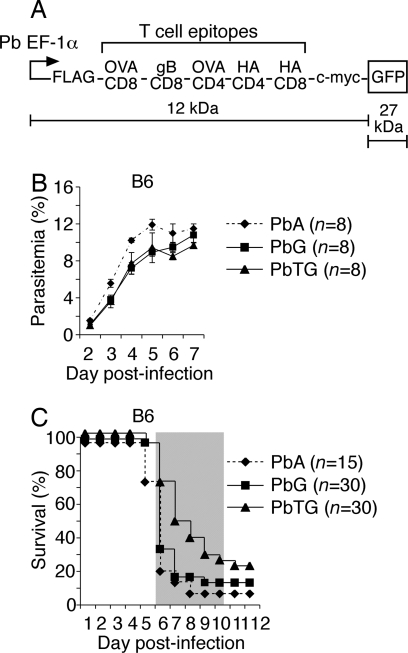
Fig. 1.
Transgenic P. berghei express model T and B cell epitopes and GFP. (A) Schematic of the polytope encoding model T and B cell epitopes fused to GFP. The fusion protein was placed under the P. berghei EF-1α promoter. (B) Parasitemia curves for P. berghei ANKA (PbA), PbG, and PbTG in B6 mice. Data pooled from two experiments; n represents total number of mice per group. Error bars represent SEM. (C) Survival curves for PbA, PbG, and PbTG in B6 mice. Data pooled from three to five experiments; n represents total number of mice per group. The shaded area indicates the time when mice displayed ECM symptoms. Mice dying after this time were killed on ethical grounds due to anemia associated with hyperparasitemia.
Transgenic P. berghei parasites were termed PbTG, and control parasites expressing only GFP were termed PbG. Transgenes were maintained as episomal plasmids under pyrimethamine selection in vivo, where growth rates of transfectants in B6 and BALB/c mice were only marginally slower than the parental P. berghei ANKA (PbA) (Fig. 1B and Fig. S2A), the slight reduction most likely a result of uneven segregation of episomally replicating plasmids during mitosis (16). Like PbA, transfectants caused lethal ECM in B6 mice as measured by host survival between days 6 and 10 post-infection (Fig. 1C). Fluorescence microscopy showed that GFP was expressed throughout the blood-stage in PbTG (Fig. S2B) and PbG (data not shown), whereas expression of the 39-kDa polytope-GFP fusion protein by PbTG was confirmed by Western blot analysis using antibodies to GFP, FLAG, or c-myc (Fig. S2C). As expected, only GFP (27 kDa) was detected for PbG (Fig. S2C).
To address whether blood-stage infection causes specific stimulation of CD8+ T cells in vivo, B6 and BALB/c mice were injected intravenously with CFSE-labeled transgenic T cells specific for each MHC I-restricted epitope within the fusion protein and, 1 day later, infected with PbTG or PbG. T cell proliferation was analyzed 3–4 days later in the spleen. This showed efficient proliferation of OT-I and gBT-I CD8+ T cells in B6 mice and CL4 CD8+ T cells in BALB/c mice (Fig. 2A). Responses were antigen specific, as T cell proliferation was not detected in mice infected with PbG (Fig. 2A). These findings provide direct evidence that antigens expressed during the blood-stage can be presented on MHC I to induce parasite-specific CD8+ T cell proliferation.
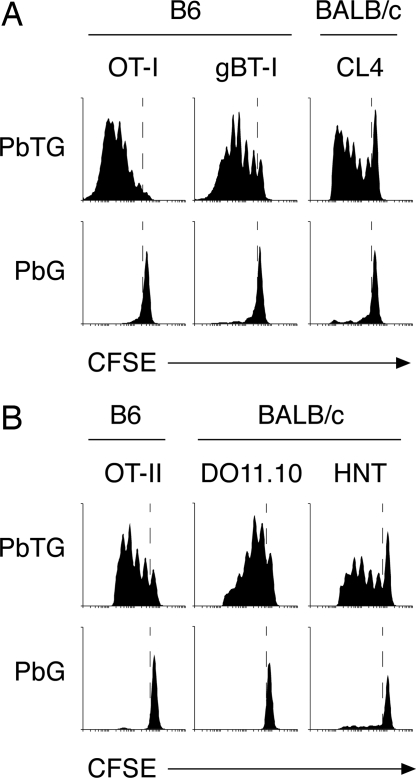
Fig. 2.
Transgenic P. berghei antigens are functionally expressed and presented to pathogen-specific T cells during blood-stage infection. Proliferation of CD8+ transgenic T cells (A) and CD4+ transgenic T cells (B) in B6 or BALB/c mice after infection with PbTG or PbG.
To test whether the CD4+ T cell epitopes were also presented in vivo, we used the same approach with MHC II-restricted transgenic T cells. This showed that the MHC II-restricted OVA epitope was presented in both B6 and BALB/c mice, stimulating proliferation of OT-II and DO11.10 CD4+ T cells, respectively, as was the HA epitope to HNT CD4+ T cells in BALB/c mice (Fig. 2B). Again, responses were specific, as proliferation was not detected in mice infected with PbG (Fig. 2B). Overall, these experiments demonstrated that the polytope was functionally expressed, correctly processed, and presented by the host immune system for specific stimulation of CD4+ and CD8+ T cells during blood-stage infection.
CD8+ Cytotoxic T Cell Responses Are Generated to Antigens Expressed During Blood-Stage Infection.
To test whether responding endogenous CD8+ T cells developed into bona fide effectors, an 18-h in vivo cytotoxicity assay to measure OVA-specific killing was performed in B6 mice infected with PbTG for 3–6 days (Fig. 3A). This involved measuring the selective lysis of adoptively transferred CFSE-labeled OVA257–264 peptide-pulsed splenocytes. Individual mice showed lytic activity in the spleen as early as day 3 after infection, and this increased somewhat over the course of infection (Fig. 3A). As B6 mice succumb to ECM after day 6, later time points were not examined.
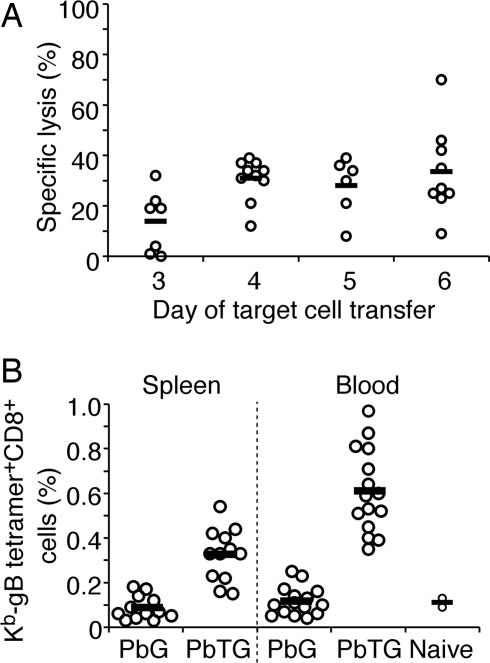
Fig. 3.
Transgenic P. berghei blood-stage infection induces functional CTL. (A) Percentage specific lysis of OVA257–264 peptide-pulsed target cells by endogenous CTL generated in the spleens of B6 mice infected with PbTG for 3–6 days. All values are relative to naive mice, which were designated as 0% lysis. (B) Expansion of endogenous gB-tetramer-specific CD8+ T cells in the spleen and blood of B6 mice infected with PbTG or PbG for 7 days. Open circles represent values for individual mice; horizontal bars represent values of the mean.
To extend these findings, we measured expansion of gB-specific CD8+ T cells in the spleens and peripheral blood of B6 mice infected with PbTG or PbG using Kb-gB-specific tetramer staining. In these experiments, mice were cured of infection by treatment with chloroquine on days 4–6 to allow examination of a relatively late time point when tetramer-positive cells would be clearly detectable. By day 7 post-infection, 0.3% of the CD8+ T cell repertoire in the spleen and 0.6% in the peripheral blood were specific for the MHC I-restricted gB epitope expressed by PbTG (Fig. 3B).
Combined, the in vivo proliferation data (Fig. 2A), the parasite-specific lytic activity (Fig. 3A), and the tetramer-positive cells (Fig. 3B) indicated that parasite-specific CD8+ T cell effector responses were generated to blood-stage infection.
To decipher whether resistance of BALB/c mice to ECM might relate to the inability to generate CTL effectors to parasite-expressed antigens, we examined lytic activity to the HA epitope expressed by PbTG (Fig. S3A). Though control BALB/c mice infected with influenza virus PR8 induced lytic activity to this epitope, no responses were detected to PbTG. This supports the view that differences in ECM resistance may relate to the capacity to generate lytic effectors. However, it is possible that responses by endogenous CD8+ T cells to authentic parasite antigens might compete with HA-specific responses to the transgenic antigen. In support of the capacity of BALB strain mice to generate CTL to PbTG, we were able to detect OVA-specific lytic activity in BALB.H-2b mice (Fig. S3B).
DC Prime T Cell Responses to Antigens Expressed During Blood-Stage Infection.
Though it has been shown that DC initiate protective immunity to sporozoite challenge (17) and are involved in the pathogenesis of ECM (18), it was important to demonstrate that DC were the major antigen-presenting cells during blood-stage P. berghei infection. To achieve this, B6 mice were lethally irradiated and reconstituted with bone marrow from CD11c-DTR transgenic mice, which express the primate diphtheria toxin receptor (DTR) and GFP under the control of the CD11c promoter (expressed predominantly by DC). After 8 weeks, chimeric mice were left untreated or were treated with diphtheria toxin (DT) every 2 days to deplete DC. One day after the first treatment, mice were infected with PbTG. The following day, mice received CFSE-labeled OT-I (CD8+) or OT-II (CD4+) T cells; 60–72 h later, T cell proliferation was measured in the spleen. Consistent with previous reports (17), systemic administration of DT depleted virtually all CD11c+GFP+ DC from the spleen (Fig. S4A). In the absence of CD11c+ DC, OT-I and OT-II T cell proliferation in vivo was greatly reduced (Fig. S4B), indicating that bone marrow-derived DC were critical for the induction of T cell responses during blood-stage infection.
Presentation of Transgenic P. berghei Antigens to CD8+ T Cells Occurs Primarily via CD8α DC.
Given that DC were required for priming antigen-specific T cell responses during blood-stage infection, it was important to determine which specific DC subtypes presented P. berghei-expressed antigens. At least four distinct populations of DC have been identified in the murine spleen (19), broadly classified into the plasmacytoid DC (pDC) and three subtypes of conventional DC—the latter distinguished by their expression of CD4 and CD8α surface markers (19). Initial studies excluded antigen presentation by pDC (data not shown). To determine whether the remaining DC subtypes cross-presented MHC I-restricted Plasmodium antigens during blood-stage infection, these conventional DC were purified from the spleens of B6 or BALB/c mice 3 days after infection with PbTG and then stained with antibodies against CD11c, CD8α, and CD4 surface markers. Live cells were gated on the CD11c+ population, sorted by flow cytometry into CD4 DC (CD4+CD8α−), CD8α DC (CD4−CD8α+), and double-negative (DN) DC (CD4−CD8α−) populations, and then cocultured with CFSE-labeled OT-I or CL4 CD8+ T cells in vitro to detect T cell proliferation as a measure of antigen presentation. As shown (Fig. 4 A and B), CD8α DC efficiently presented transgenic P. berghei antigens to both sets of CD8+ T cells. CD4 DC derived from B6 mice were only moderately stimulatory for OT-I cells (at very high numbers; 50–100 × 103 DC/well) (Fig. 4A) and failed to stimulate CL4 cells (Fig. 4B). When cytokine profiles were examined for OT-I T cells stimulated by either DC type (Fig. S5), a similar hierarchy of TNFα > IL-2 > IFNγ was observed for both DC subsets, with all cells making IFNγ contained within those able to make IL-2, and all cells able to make IL-2 contained within those able to make TNFα.
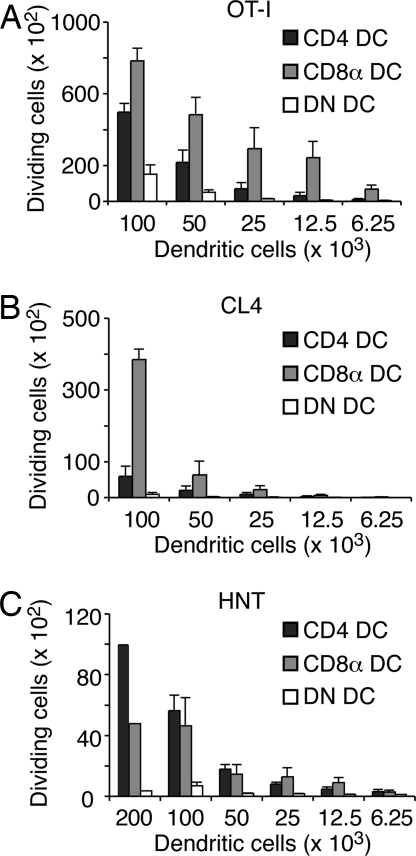
Fig. 4.
CD8α DC cross-present P. berghei-expressed transgenic antigens to stimulate CD8+ T cells. Proliferation of OT-I (A), CL4 (B), and HNT (C) transgenic T cells in the presence of conventional DC subtypes isolated from the spleens of B6 or BALB/c mice on day 3 after infection with PbTG. Data pooled from two to four experiments. Error bars represent SEM.
To examine whether CD8α DC also played a role in MHC II-restricted antigen presentation, we performed a similar assay using CFSE-labeled OT-II or HNT CD4+ T cells as responders. Though OT-II responses were unable to be measured ex vivo, presumably due to the limited sensitivity of this system (data not shown), we were able to detect presentation to HNT cells by both CD4 DC and CD8α DC subsets (Fig. 4C). Combined, these findings suggest that CD8α DC are the major antigen-presenting cells for CD8+ T cells during blood-stage infection, whereas CD4 DC play a more extensive role in CD4+ T cell stimulation.
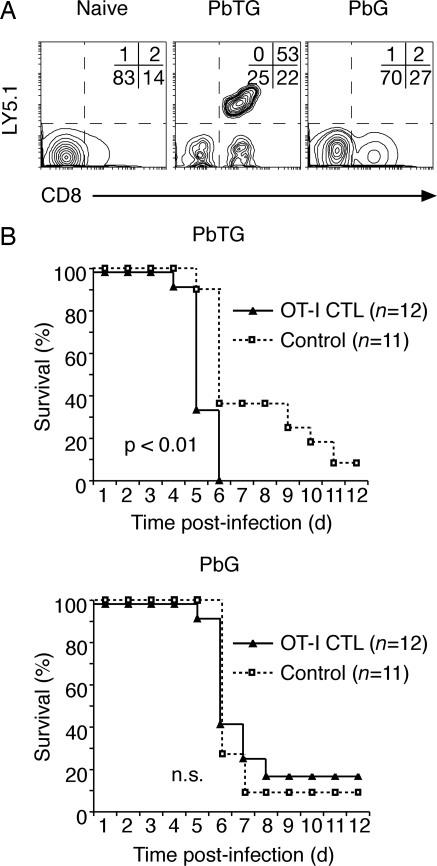
Parasite-Specific CD8+ T Cells Infiltrate the Brain and Cause Lethal Disease.
To directly examine the role of parasite-specific CD8+ T cells in cerebral malaria, B6 mice were infected with PbTG or PbG, and naïve OT-I and OT-II T cells were injected the following day. On day 5 post-infection, brains were harvested, and sequestered leukocyte populations analyzed by flow cytometry. Though substantial numbers of CD8+ T cells infiltrated the brain on day 5, OT-I T cells were only detected in the brains of mice infected with PbTG (Fig. 5A). OT-II T cells were not detected (data not shown), although this corresponded to a lower infiltration rate by CD4+ T cells in general, as reported previously (3, 6, 20).
Fig. 5.
CTL specific for parasite-expressed antigens are capable of infiltrating the brain and causing lethal disease. (A) Representative flow cytometry profiles of brain-infiltrating lymphocytes isolated from B6 mice left uninfected (naive) or infected with PbTG or PbG for 5 days. Mice were adoptively transferred with Ly5.1+ OT-I and OT-II T cells on day 1 post-infection. The percentage of cells in each quadrant is indicated. (B) Survival curves for B6 mice after adoptive transfer of OT-I CTL on day 2 post-infection with PbTG or PbG. Control mice did not receive OT-I CTL. Data pooled from three experiments; n represents total number of mice per group. The p values calculated using the Wilcoxon rank-sum test are indicated.
To further address the issue of specificity during the effector phase, we bypassed requirements for specificity in the priming phase by transferring in vitro-activated OT-I T cells into B6 mice infected with PbTG or PbG (Fig. 5B). This led to marginal though significant acceleration of ECM in mice infected with PbTG, suggesting a requirement for antigen specificity at the effector phase. To provide further evidence, RAG-1-deficient mice were used as hosts because they lack an endogenous T cell repertoire and are resistant to ECM. Strikingly, lethal disease was induced in a proportion of RAG-1-deficient mice infected with PbTG that also received OT-I CTL (Fig. S6A). RAG-1-deficient mice infected with PbG did not develop lethal disease, despite the addition of CTL (Fig. S6A). Though the sensitivity of this experimental approach was somewhat variable (Fig. S6B), in three of six independent experiments performed, lethal disease was induced in 100% of mice given parasite-specific CTL (n = 15), indicating that CD8+ T cells specific for parasite-expressed antigens contribute to lethal disease.
Discussion
The generation of transgenic P. berghei parasites expressing well defined T cell antigens provides a powerful approach to study the activation, fate, and function of T cell responses generated during blood-stage infection. In this report, we have shown that transgenic antigens expressed by blood-stage parasites are captured and presented by DC to stimulate naive CD4+ and CD8+ T cell responses. Though both CD4 DC and CD8α DC had the capacity to present MHC II-restricted antigens to CD4+ T cells, CD8α DC were most efficient at cross-presenting MHC I-restricted antigens to CD8+ T cells. This not only led to CD8+ T cell proliferation but also to the generation of CTL effectors capable of mediating ECM.
Though CD8+ T cells have been implicated in ECM, clear evidence that these cells are cytotoxic effectors and that they have specificity to P. berghei is lacking. Our study provides direct evidence that effector CTL with specificity for parasite-expressed antigens can be generated during blood-stage infection. Although MHC I-restricted epitopes for antigens expressed during Plasmodium blood-stages are yet to be defined (2, 20), the existence of authentic CTL epitopes is supported by studies reporting selective expansion of CD8+ T cells bearing the Vβ8.1,2 TCR in the brain and peripheral blood during ECM (21). In this case, however, specificity may be attributed to superantigen-like structures on the parasites and is therefore not necessarily a reflection of peptide specificity. Our study clearly shows that CTL specific for proteins expressed during the blood-stage of infection can be processed and presented in a manner capable of inducing effector CTL, at least for transgene-expressed antigens.
The generation of CD11c-DTR mice provided evidence that DC were important in immunity to sporozoite challenge (17) but did not address the role of these cells in immunity to blood-stage infection. Here, we have used CD11c-DTR bone marrow chimeric mice, in conjunction with transgenic parasites and antigen-specific T cells, to demonstrate that DC are central to antigen presentation during blood-stage infection. Our study supports a recent report that CD11c+ DC are required for the induction of ECM associated with P. berghei infection (18).
Examination of antigen presentation by the four DC populations in the spleen revealed that two subtypes had the capacity to present P. berghei antigens to naive T cells ex vivo: (1) the CD8α DC subset, which have been reported to present many other forms of antigens, including viral, bacterial, and even cell-associated antigens, and (2) the CD4 DC subset, which have yet to be assigned an antigen-presenting role during infection. Consistent with these findings, a recent report examining antigen presentation by conventional DC subsets during P. chabaudi infection in mice demonstrated that CD8α− DC (which include CD4 DC) were able to present an MHC II-restricted epitope from merozoite surface protein (MSP)-1 to CD4+ transgenic T cells on day 7 post-infection (22). These authors also demonstrated antigen presentation by CD8α DC to MSP-1-specific CD4+ T cell hybridomas, but this subset failed to stimulate proliferation of naive transgenic T cells at the time point examined (22). The failure of CD8α DC to stimulate P. chabaudi-specific CD4+ T proliferation may be a species- or antigen-specific phenomenon or relate to the later time point examined in this infection—a time when B6 mice would have succumbed to ECM in P. berghei infection. In our studies, though CD4 DC were equivalent to CD8α DC in their presentation of antigens to CD4+ T cells, CD8α DC were superior in presentation to CD8+ T cells. This latter difference probably reflects the dominant capacity of CD8α DC to cross-present exogenous antigens in the MHC I pathway (23, 24), supporting the view that CD4 DC are poorly endowed with cross-presenting capacity.
Although various reports have implicated CD8+ T cells in ECM, there has been no direct evidence that this is mediated in an antigen-specific fashion (2). Here, we have clearly demonstrated that CD8+ T cells specific for parasite-expressed antigens are detectable in the brains of infected mice bearing the appropriate antigens, suggesting that specificity is required for infiltration of damaged tissues. The precise requirement for antigen-specific CTL in ECM was, however, best illustrated by our ability to accelerate disease onset in B6 mice or to induce lethal disease in RAG-1-deficient mice, simply by transferring antigen-specific CTL. These studies now provide an avenue to dissect the target tissue and effector requirements for CTL-dependent ECM.
Better knowledge of how CTL responses are induced to blood-stage infection may also provide beneficial approaches to the generation of liver-stage vaccines, as liver cells containing merozoites (at the end of the liver-stage life cycle) may be susceptible to destruction by CTL specific for blood-stage antigens. The protective capacity of such an approach would, however, need to be balanced by consideration for the potential ability of such CTL to increase cerebral pathology. Our transgenic parasite model should help to elucidate this balance.
Materials and Methods
Generation of T and B Cell Epitopes Linked to GFP.
Sequences of overlapping oligonucleotides designed for PCR amplification of the selected CD4+ and CD8+ T cell epitopes (OVA257–264 [SIINFEKL], H-2Kb restricted; OVA323–339 [KISQAVHAAHAEINEAG], I-Ab and IAd restricted; gB498–505 [SSIEFARL], H-2Kb restricted; HA518–526 [IYSTVASSL], H-2Kd restricted; and HA126–138 [HNTNGVTAACSHE], I-Ad restricted), B cell epitopes FLAG (DYKDDDK) and c-myc (EQKLISEEDL), and restriction sites to facilitate cloning are illustrated in Fig. S1. To construct the polytope, oligonucleotides (F1–F6 and R1–R6) were denatured at 94°C for 2 min and then annealed at 37°C for 10 min before the addition of Klenow enzyme for 30 min at 30°C. After a 10-min incubation at 75°C, a PCR was performed on the annealed oligonucleotides using PLATINUM TaqDNA polymerase High Fidelity Enzyme and oligonucleotides F1 (5′-AGGATCCATGGATTACAAGGATGACGAACGATAAGTTAG-3′) and R1 (5′-TGGATCCTCAAGATCTTCTAGACAGATCCTCTTCAGAGATTAG-3′). PCR conditions were 94°C denaturation, 50°C annealing, and 68°C for nucleotide extension, incubating for 30 sec at each step for a total of 30 cycles. BamHI restriction sites (bold) were introduced in F1 and R1 to facilitate cloning into the expression vector PbGFPCON (15). Unique BglII (italicized) and XbaI (italicized in bold) sites were introduced in R1 to enable fusion to GFP and orientation screening, respectively.
P. berghei Expression Plasmids.
Plasmid PbGFPCON (15) contained an expression cassette regulated by the P. berghei elongation factor-1α (Pb EF-1α) promoter and a selection cassette encoding a mutated form of the dihydrofolate reductase synthase (DHFR-TS) gene of Toxoplasma gondii that confers resistance to pyrimethamine. To fuse the polytope to GFP, the polytope PCR product was first subcloned into the multicloning site of vector pGEM-Teasy (Promega). Digestion of this vector with BglII then created a compatible restriction site for the in-frame fusion of GFP (Fig. S1), released as a BamHI fragment from vector PbGFPCON. The final polytope-GFP fusion product was introduced as a BamHI fragment into the PbGFPCON expression cassette, to create vector PbTGFPCON.
P. berghei Transfection.
Transfection of P. berghei ANKA was performed essentially as described in ref. 25. Transformed parasites were immediately injected i.v. into BALB/c mice. Mice were treated with pyrimethamine (10 mg/kg body weight) i.p. for four consecutive days to select for drug-resistant transfectants.
Western Blotting.
Mature parasite lysates were prepared as described in ref. 26. Proteins were separated under nonreducing conditions on 12% polyacrylamide gels. Primary antibodies used were rabbit anti-GFP (1:1,000), rat anti-FLAG (1:1,000; clone 9HI) (27), and mouse anti-c-myc (1:1,000; clone 9E10) (28). Horseradish peroxidase-coupled secondary antibodies were used for detection with SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce).
Fluorescence Microscopy.
GFP fluorescence in wet mounts of P. berghei transfectants was visualised using a Carl Zeiss Axioskop microscope with EGFP filter settings at 1,000× magnification. Pictures were recorded using a PCO SensiCam, and images were produced using Adobe Photoshop software.
Mice.
The following mice were used between 6 and 12 wks of age: BALB/c (H-2d), B6 (H-2b), BALB.H-2b, B6.Ly5.1 (H-2b), RAG-1-deficient (H-2b), and the transgenic strains OT-I (29), OT-II (30), gBT-I (31), DO11.10 (32), CL4 (33), HNT (34), and CD11c-DTR (17). Mice were bred and maintained in specific pathogen-free conditions at the Walter and Eliza Hall Institute Animal Facility. All procedures were approved by the Melbourne Health Research Animal Ethics Committee.
Generation of Bone Marrow Chimeras and DC Depletion.
B6 mice were irradiated with two doses of 550 cGy 3 h apart and reconstituted with 3–5 × 106 T cell-depleted donor bone marrow cells from CD11c-DTR transgenic mice. Donor bone marrow cells were depleted of T cells using antibodies against CD4 (RL172), CD8 (30168), and Thy1 (J1j). The antibody-coated cells were removed by incubation with rabbit complement for 30 min at 37°C. The following day, residual radioresistant T cells were depleted with 100 μl of i.p. Thy1 (T24/31.7) ascites. Chimeric mice were rested for 8–10 weeks before use. For systemic DC depletion, chimeras were treated i.p. with diphtheria toxin (DT) (CSL; 4 ng/g body weight) every 48 h.
P. berghei Infection.
Mice were infected i.v. with 106 parasitized RBCs. Mice infected with transgenic P. berghei were treated with pyrimethamine in the drinking water as described in ref. 25. Parasitemia was determined from Giemsa stained tail blood smears and expressed as the percentage of parasitized RBCs. Mice were monitored daily from day 4 post-infection for neurological signs of ECM, including convulsions, ataxia, and paralysis.
CFSE-Labeled Transgenic T Cells.
CFSE-labeled transgenic T cells were prepared as described for CD8+ T cell preparations (13, 14). This was modified for CD4+ T cells by replacing GK1.5 (CD4+ cells) with 53–6.7 (CD8+ cells) before magnetic bead depletion. Mice were injected i.v. with 2 × 106 purified cells.
Flow Cytometry.
Flow cytometry was performed using a FACSCalibur, LSR, or LSR II (BD Biosciences) instrument and analyzed using Weasel (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) software. Transgenic T cells were stained with combinations of antibodies specific for CD4 or CD8α and the TCR Vα2 (OT-I, OT-II, and gBT-I), DO11.10 TCR (DO11.10), Vβ8.2 (CL4), or Vβ8.3 (HNT).
In Vivo CTL Assay.
Suspensions of lymph node cells and splenocytes from B6.Ly5.1 or BALB/c mice were depleted of RBCs and divided into two equal portions. One was pulsed with 0.1 μg/ml synthetic OVA257–264 or HA518–526 (Auspep) for 1 h at 37°C and then labeled with a high concentration (5 μM) of CFSE (CFSEhi population). The other was incubated for 1 h at 37°C without peptide and labeled with a low concentration (0.5 μM) of CFSE (CFSElo). Equal numbers of cells from each population were combined and 2 × 107 cells in total were injected i.v. into mice. Spleen cells were analyzed by flow cytometry 18 h later, and the percentage OVA- or HA-specific lysis was determined by loss of the peptide-pulsed CFSEhi population compared with the control CFSElo population. Note that in some experiments, BALB/c mice were infected with 103.9 PFU of the A/PR8/34 (PR8) influenza A virus via subcutaneous footpad infection, and anti-HA CTL responses were analyzed in the draining popliteal lymph node.
Analysis of CD8+ T cell Expansion.
B6 mice infected with PbG or PbTG were treated on days 4–6 post-infection i.p. with chloroquine (25 mg/kg body weight). On day 7, spleen cells were stained with antibodies specific for CD8α, Thy1.2, and H-2Kb-gB498–505 tetramer, and analyzed by flow cytometry.
DC Isolation and ex Vivo Antigen Presentation Assays.
DC purification from the spleen, flow cytometry, and culture of DC with CFSE-labeled T cells in vitro were performed as described (13, 14). After 60 h in culture, cytokine production by OT-I T cells was measured essentially as described in ref. 35. Briefly, OT-I cells were restimulated for 5 h with 1 μM synthetic OVA257–264 in the presence of 5 μg/ml brefeldin A. Cells were washed and stained with antibodies specific for CD8α and Ly5.1 for 30 min at 4°C. After further washing, cells were fixed, permeabilized, and stained with anti-IFN-γ, anti-TNF-α, and anti-IL-2 using a BD Cytofix/Cytoperm kit (BD Biosciences) according to manufacturer's instructions.
Purification of Brain-Sequestered Lymphocytes.
B6 mice were killed on day 5 post-infection and perfused intracardially with PBS to remove circulating leukocytes from the brain. Brains were then harvested, and sequestered lymphocytes were purified as described in ref. 36. Adoptively transferred OT-I and OT-II T cells were detected by flow cytometry using antibodies specific for CD8α or CD4 and Ly5.1.
In Vitro Activation of OT-I CTL.
To activate OT-I T cells in vitro, 2 × 107 splenocytes from B6 mice were γ-irradiated at 1,500 cGy and incubated with 1 μg/ml synthetic OVA257–264 for 1 h at 37°C. Cells were washed and cultured with 2 × 107 splenocytes from OT-I mice in complete RPMI 1640 media supplemented with 5 μg LPS and 10 U/ml IL-2. After culture for 4 days at 37°C in 5% CO2, OT-I CTL were routinely 90%–98% pure by flow cytometry. B6 mice received 2 × 106 OT-I CTL; RAG-1-deficient mice received 5 × 106 OT-I CTL i.v.
Supplementary Material
Acknowledgments.
We thank Lynn Buckingham, Chrystal Smith, Mary Camilleri, Fiona Kupresanin, Jiang-Li Tan, and Jane Langley for assistance, and Drs. Chris Janse and Andy Waters (Leiden University Medical Centre, The Netherlands) for the P. berghei plasmid pGFPCON. The National Health and Medical Research Council of Australia supported this work. R.J.L. was supported by an Australian Postgraduate Award and the Cooperative Research Centre for Vaccine Technology. L.S., G.T.B., B.S.C., and W.R.H. are International Research Scholars of the Howard Hughes Medical Institute (Chevy Chase, MD).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806727105/DCSupplemental.
References
- 1.Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999;11:412–419. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 2.Renia L, et al. Pathogenic T cells in cerebral malaria. Int J Parasitol. 2006;36:547–554. doi: 10.1016/j.ijpara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Belnoue E, et al. On the pathogenic role of brain-sequestered αβ CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–6375. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 4.Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology. 1997;114:7–12. doi: 10.1017/s0031182096008293. [DOI] [PubMed] [Google Scholar]
- 5.Yanez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol. 1996;157:1620–1624. [PubMed] [Google Scholar]
- 6.Nitcheu J, et al. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol. 2003;170:2221–2228. doi: 10.4049/jimmunol.170.4.2221. [DOI] [PubMed] [Google Scholar]
- 7.Potter S, et al. Perforin mediated apoptosis of cerebral microvascular endothelial cells during experimental cerebral malaria. Int J Parasitol. 2006;36:485–496. doi: 10.1016/j.ijpara.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Potter S, Chaudhri G, Hansen A, Hunt NH. Fas and perforin contribute to the pathogenesis of murine cerebral malaria. Redox Rep. 1999;4:333–335. doi: 10.1179/135100099101535070. [DOI] [PubMed] [Google Scholar]
- 9.de Kossodo S, Grau GE. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 10.Engwerda CR, et al. Locally up-regulated lymphotoxin-α, not systemic tumor necrosis factor-α, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–1377. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grau GE, et al. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 12.Grau GE, et al. Monoclonal antibody against interferon-γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CM, et al. Cutting edge: Conventional CD8α+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- 14.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke-Fayard B, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.van Dijk MR, et al. Replication, expression and segregation of plasmid-borne DNA in genetically transformed malaria parasites. Mol Biochem Parasitol. 1997;86:155–162. doi: 10.1016/s0166-6851(97)02843-0. [DOI] [PubMed] [Google Scholar]
- 17.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deWalick S, et al. Cutting edge: Conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J Immunol. 2007;178:6033–6037. doi: 10.4049/jimmunol.178.10.6033. [DOI] [PubMed] [Google Scholar]
- 19.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 20.Hafalla JC, Cockburn IA, Zavala F. Protective and pathogenic roles of CD8+ T cells during malaria infection. Parasite Immunol. 2006;28:15–24. doi: 10.1111/j.1365-3024.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 21.Boubou MI, et al. T cell response in malaria pathogenesis: Selective increase in T cells carrying the TCR Vβ8 during experimental cerebral malaria. Int Immunol. 1999;11:1553–1562. doi: 10.1093/intimm/11.9.1553. [DOI] [PubMed] [Google Scholar]
- 22.Sponaas AM, et al. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J Exp Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnorrer P, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 26.Waters AP, Thomas AW, van Dijk MR, Janse CJ. Transfection of malaria parasites. Methods. 1997;13:134–147. doi: 10.1006/meth.1997.0506. [DOI] [PubMed] [Google Scholar]
- 27.Wilson-Annan J, et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 30.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 31.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 32.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DJ, et al. CD8+ T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 34.Scott B, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 35.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: Correlation of cytokine profile and TCR avidity. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 36.Hansen DS, Bernard NJ, Nie CQ, Schofield L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol. 2007;178:5779–5788. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.