Abstract
There is currently no known genetic disease linked to prolactin (Prl) or its receptor (PrlR) in humans. Given the essential role of this hormonal system in breast physiology, we reasoned that genetic anomalies of Prl/PrlR genes may be related to the occurrence of breast diseases with high proliferative potential. Multiple fibroadenomas (MFA) are benign breast tumors which appear most frequently in young women, including at puberty, when Prl has well-recognized proliferative actions on the breast. In a prospective study involving 74 MFA patients and 170 control subjects, we identified four patients harboring a heterozygous single nucleotide polymorphism in exon 6 of the PrlR gene, encoding Ile146→Leu substitution in its extracellular domain. This sole substitution was sufficient to confer constitutive activity to the receptor variant (PrlRI146L), as assessed in three reconstituted cell models (Ba/F3, HEK293 and MCF-7 cells) by Prl-independent (i) PrlR tyrosine phosphorylation, (ii) activation of signal transducer and activator of transcription 5 (STAT5) signaling, (iii) transcriptional activity toward a Prl-responsive reporter gene, and (iv) cell proliferation and protection from cell death. Constitutive activity of PrlRI146L in the breast sample from a patient was supported by increased STAT5 signaling. This is a unique description of a functional mutation of the PrlR associated with a human disease. Hallmarks of constitutive activity were all reversed by a specific PrlR antagonist, which opens potential therapeutic approaches for MFA, or any other disease that could be associated with this mutation in future.
Keywords: antagonist, breast diseases, human mutation, constitutive activity, cytokine receptor
The role of prolactin (Prl) in breast physiology has been recognized for decades. In synergy with various hormones and growth factors, Prl plays a critical role in many steps of breast development (1). Given its potent activity on breast cell proliferation and differentiation, it has been long assumed that mutations affecting the properties of Prl or of its receptor (PrlR) should have clinical impact on the breast. Although genetically modified animal models fully support this assumption (2), an unequivocal answer to this question is lacking in humans. This is due in part to the fact that the rare studies that were performed to date to identify coding mutations of the PrlR gene in breast cancer patients either failed to find any (3), or reported a single nucleotide polymorphism (SNP) that remained uncharacterized at the functional level and involved too small cohorts to achieve significance (4). To date, only association studies between noncoding variations in PrlR gene and breast cancer have been reported (5, 6). More generally, there is no known loss- or gain-of-function genetic pathology yet reported for Prl or its receptor. Besides breast cancer, several benign breast diseases (BBD) affecting the human breast remain poorly understood (7, 8). These diseases are marked by lobuloalveolar growth and/or differentiation disorders, including abnormally high proliferation of the epithelium as observed in fibroadenomas (FA) (8). Multiple FA (MFA) is defined by more than 3 FA in one breast (9) (Fig. 1), which are not histologically different from isolated FAs. Receptors for estrogen, progesterone, and various growth factors have been suggested as potential candidates involved in tumor appearance/growth (8). The PrlR is another candidate, as its expression is maintained, or even increased in various benign breast lesions (10, 11). According to the poorly understood etiology of all BBDs, current treatments are mostly empirical. One of the most currently used involves progestins, although the question of whether they are beneficial or instead deleterious for the breast is still a matter of debate (12). Some have tried antiestrogen therapy with tamoxifen in BBDs, but no evaluation of such practice has ever been reported. Concerning the use of inhibitors of Prl secretion (dopamine agonists), no long-term treatment study has been reported for patients with BBDs, especially FAs or MFAs (13). In such a context, the development of new therapeutic approaches capable of reducing the proliferation observed in benign breast tumors should be relevant.
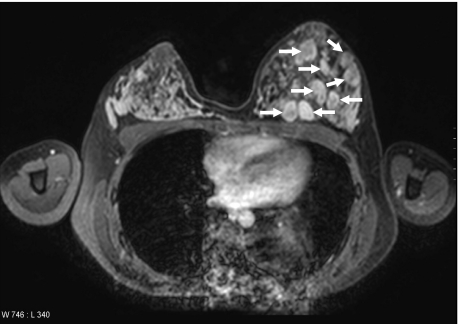
Fig. 1.
MRI of a MFA patient showing several fibroadenomas (arrows) mainly located in the left breast.
Recently, genetic predisposition has been proposed as a causal factor in benign breast disorders (7), which could apply to any regulator of breast morphogenesis, including Prl. We thus initiated a prospective clinical study aimed at identifying any coding alterations of Prl and PrlR genes in the largest MFA cohort ever reported. In four patients, we identified a heterozygous missense mutation in the PrlR gene, leading to a constitutively active receptor.
Results
Patients and Controls.
Seventy-four Caucasian women with MFA were consecutively recruited by the BBDs study group, from 9 different centers, and referred to our outpatient clinic. Inclusion was possible based on the existence of at least 3 FAs in one breast in patients receiving no treatment influencing gonadal axis for at least 1 month. All underwent basic clinical investigations. For those who underwent surgery, FA and adjacent tissue were obtained whenever possible.
A cohort of 96 control Caucasian subjects was constituted based on stringent inclusion criteria including no history of benign or malignant breast disease, no pituitary disorder, normal Prl levels. To minimize the risk of including young subjects who could later develop an MFA, we fixed the cut-off age at 35. A random population of 74 unrelated control women (no inclusion criteria) was also analyzed.
PrlR Genotype Associated with MFAs.
No missense single nucleotide polymorphism (SNP) was identified in the Prl gene of any patient. With respect to the PrlR, we found in both MFA patients and control subjects (with no difference) the sole coding SNP reported in the NCBI database (rs16871473, C/T in exon 5, encoding I76V substitution at protein level), as well as many known SNPs in intronic regions bordering exons [supporting information (SI) Text]. In four unrelated patients (5.6%), we found a coding SNP that was not reported in the NCBI database (deposit procedure in progress). It involves A-to-C substitution in exon 6 (Fig. 2A and Fig. S1), and substitutes Leu for Ile146 in the second cytokine receptor homology motif of the PrlR ligand-binding domain (Fig. 2B) (14). This SNP was found in none of 96 control women ascertained to be free of any history of Prl disorder and/or breast diseases (P = 0.034), and it was also absent in a random population of 74 women (total controls = 170).
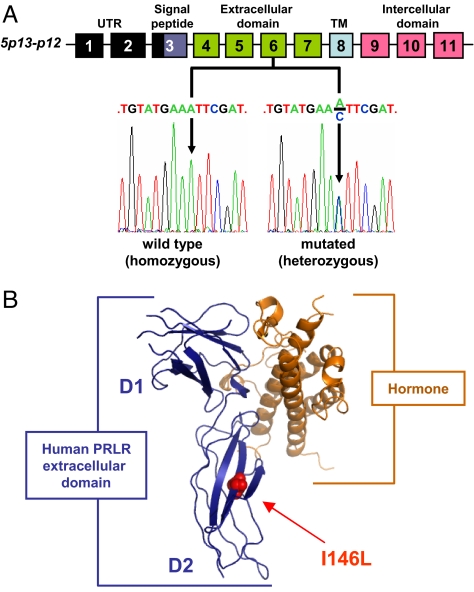
Fig. 2.
Exon 6 mutation in human PrlR gene leads to I146L missense substitution. (A) Schematic representation of the 11 exons of the human PrlR gene and the corresponding protein domains. Examples of exon 6 sense sequences obtained from one homozygous patient (2 WT alleles) and one heterozygous patient harboring both WT and A-to-C mutated alleles are shown. (B) The 3D structure of the human PrlR extracellular domain (blue) complexed to growth hormone (orange) (PDB ID code 1BP3) is used to locate the I146L substitution on the folded receptor. D1 and D2 indicate cytokine receptor homology domains 1 and 2. UTR, untranslated region; TM, transmembrane.
Establishment of Cell Models to Study I146L SNP.
The impact of I146L substitution on PrlR properties (mutant is referred to as PrlRI146L) was characterized by using transfected cell models and multiple well-established readouts for structure-function studies of the PrlR (Ba/F3 mouse lymphoid cells, HEK293 human embryonic kidney fibroblasts). To avoid any bias, stable clones (HEK) or populations (Ba/F3) to be compared were generated and selected based on similar expression levels of WT and mutated PrlRs as determined by semiquantitative PrlR immunoblotting (Fig. S2) and/or radioligand receptor assay. HEK-PrlRWT and HEK-PrlRI146L clones expressed approximately 5,000 surface receptors/cell, whereas Ba/F-PrlRWT and Ba/F-PrlRI146L populations expressed many fewer surface receptors (approximately 500 PrlR/cell as determined by ligand binding assay) which were not detectable by immunoblot. The mutated receptor exhibited unchanged affinity for human Prl compared to PrlRWT (Fig. S3 and text). To generate a model in which both WT and mutated PrlRs are co-expressed in a mammary context (as in MFAs of heterozygous patients), MCF-7 human breast cancer cells were stably transfected by using expression vector encoding PrlRI146L or PrlRWT to get comparable clones regarding the level of PrlR expression (Fig. S4). As MCF-7 express endogenous PrlRWT, stable clones were noted MCF7-PrlRWT,WT versus MCF7-PrlRWT,I146L.
Mutation I146L Encodes a Constitutively Active PrlR.
In stable HEK-PrlRWT and MCF7-PrlRWT,WT clones, Prl stimulation induced tyrosine phosphorylation of the PrlR (Fig. 3 A and B), which is known to be mediated by the receptor-associated tyrosine kinase, JAK2 (14). Otherwise, strong receptor phosphorylation was observed in non Prl-stimulated HEK-PrlRI146L cells, but not in HEK-PrlRWT cells (Fig. 3A). This was also observed in MCF7-PrlRWT,I146L (Fig. 3B), highlighting that receptor phosphorylation persisted in the heterozygous context. Signaling studies were performed in serum-free media but we failed to detect production of endogenous Prl in these cell lines (not shown), indicating that I146L mutation leads to constitutive receptor activation. Signal transducer and activator of transcription 5 (STAT5) is the main signaling target triggered by the PrlRWT (15). Accordingly, Prl induced STAT5 phosphorylation in the three cell models expressing either receptor (Fig. 3 A–C). In agreement with the constitutive activation of PrlRI146L, STAT5 was phosphorylated in the absence of Prl only in cells expressing the mutant receptor, although to different extent depending on cell lines (Fig. 3 A–C).
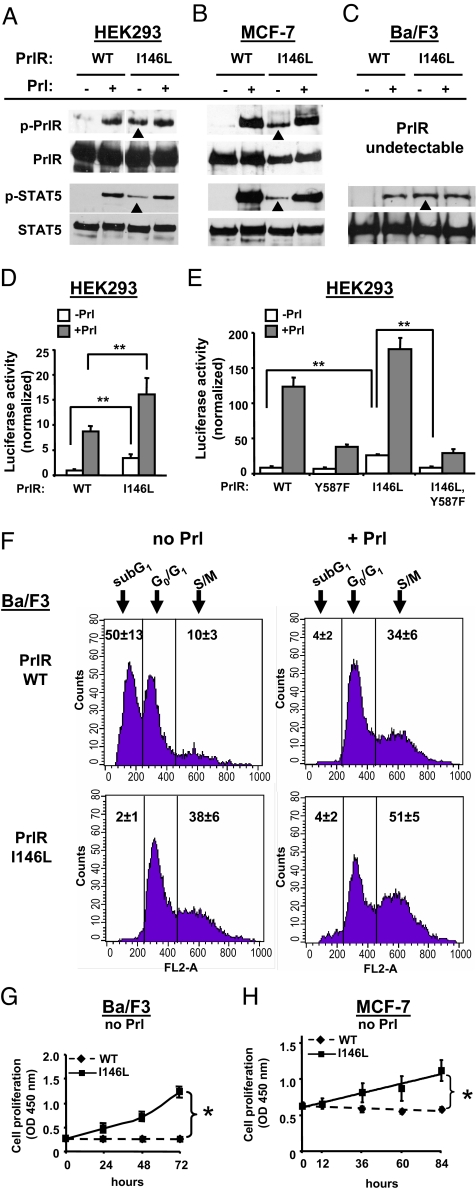
Fig. 3.
Constitutive activity of PrlRI146L in various in vitro assays. (A–C) HEK293, MCF-7, and Ba/F3 cells were stably transfected by using expression vectors encoding human PrlRWT or PrlRI146L. Each stable clone or population was serum-starved, then stimulated or not by using 40 nM human Prl (15 min) as indicated. Phosphorylated and total PrlR (Upper) and STAT5 (Lower) were analyzed by immunoblotting. Whereas tyrosine-phosphorylation of PrlR and STAT5 was observed only under Prl stimulation in cells expressing PrlRWT, constitutive phosphorylation of both proteins (arrowheads) was observed in cells expressing PrlRI146L. (D) Both HEK-PrlRWT and HEK-PrlRI146L clones also stably incorporated the Prl-responsive LHRE-luciferase gene (see SI Text). Luciferase activity was measured after 24 h treatment with (filled bars) or without (open bars) 400 nM Prl. Data normalized to basal luciferase activity of HEK-PrlRWT show significantly higher background in HEK-PrlRI146L cells (means ± SD, six independent experiments performed in triplicates). **, P < 0.01. (E) HEK293 cells were transiently cotransfected by using expression plasmids encoding (i) WT or mutated PrlR as indicated, (ii) LHRE-luciferase (firefly) reporter gene, and (iii) Renilla luciferase gene. Luciferase activities were measured after 24 h treatment with (filled bars) or without (open bars) 40 nM Prl. Data normalized to basal firefly/Renilla luciferase activities of cells expressing PrlRWT show that Y587F substitution abolishes the higher background of I146L PrlR (means ± SD, three independent experiments performed in triplicates). **, P < 0.01. (F) Cell cycle distribution of Ba/F-PrlRWT and Ba/F-PrlRI146L cells was monitored by FACS analysis. Cells were starved by Prl depletion and stimulated with 8 nM hPrl (Right) or not (Left) for 24 h. DNA content analysis of propidium iodide-stained cells is represented. Numbers indicate the average proportion of cells ± SD (seven to nine independent series) exhibiting <2n (subG1), 2n (G0/G1), and >2n (S/M). In the absence of Prl, expression of PrlRI146L protects cells from death and stimulates division (Lower Left). (G and H) Proliferation of MCF7-PrlRWT,WT and MCF7-PrlRWT,I146L cells was monitored by using WST-1 reagent. This data shows that cells expressing PrlRI146L are autonomous for growth. Error bars indicate SD (slopes differences; three independent experiments performed in triplicate). *, P < 0.05.
The relevance of Prl-independent activation of PrlRI146L/STAT5 cascade was assessed in functional assays. Despite similar levels of PrlR expression in stable clones, HEK-PrlRI146L cells exhibited significantly higher basal activity of the Prl-responsive lactogenic hormone response element (LHRE)-luciferase reporter gene compared to HEK-PrlRWT cells (Fig. 3D). The same effect was observed in other stable clones or populations (not shown), and in transient transfections (Fig. 3E). The C-terminal tyrosine of the PrlR (Tyr-587) is critical for receptor phosphorylation, activation of STAT5 and transcription of downstream target genes (16). This residue was mutated into Phe in both PrlRWT and PrlRI146L, and the cognate receptor mutants were transiently expressed in HEK cells (Fig. 3E). As expected, replacement of Tyr-587 drastically reduced the ability of Prl to activate the reporter gene via both PrlRY587F and PrlRI146L,Y587F. The Y587F mutation also reversed the higher basal activity of PrlRI146L back to the level observed for the other constructs, indicating that the constitutive activity of PrlRI146L involves phosphorylation of the C-terminal tyrosine. Besides constitutive activity, we also noticed that the activity of PrlRI146L in the presence of any concentration of Prl was systematically higher than that displayed by PrlRWT (Fig. 3D and Fig. S5).
The interleukin 3-dependence of Ba/F3 cells for proliferation/survival can be shifted to any other cytokine providing they express the cognate receptor. Accordingly, Ba/F-PrlRWT cells grew in the presence of Prl but underwent massive apoptosis in the absence thereof within 24 h (Fig. 3F). Otherwise, Ba/F-PrlRI146L survived (Fig. 3F) and even proliferated over several days (Fig. 3G) irrespective of the addition of Prl. The ability of PrlRI146L to shift cells to the S/M phase was further increased by Prl stimulation, and, as observed in the luciferase assay, it attained higher levels than in stimulated Ba/F-PrlRWT cells (Fig. 3F and Fig. S6).
MCF7-PrlRWT,WT cells were not dependent on Prl for survival but failed to proliferate in Prl/serum-deprived medium (Fig. 3H). In the same conditions, MCF7-PrlRWT,I146L cells proliferated to a submaximal level, as Prl could further enhance cell division (not shown). This demonstrates that the growth-promoting effect of PrlRI146L, as observed for receptor and STAT5 phosphorylation, occurs irrespectively of co-expression of the WT receptor, which is particularly important regarding the fact that the mutation is heterozygous in our MFA patients.
Increased Stat5 Signaling in Breast Tissue from Mutated Patients.
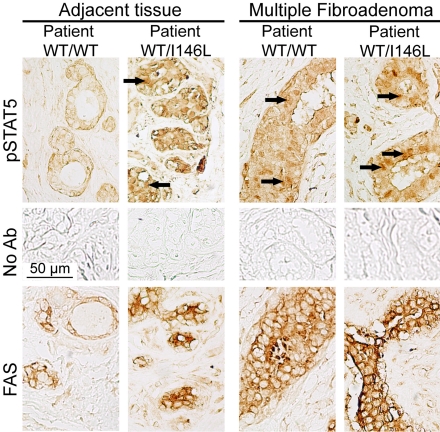
Biopsies of MFA and adjacent tissue from one of these four heterozygous patients (PrlRWT,I146L) was available for histological studies and was compared to samples from two homozygous patients (PrlRWT,WT). Activation of the PrlR-STAT5 pathway was investigated by immunohistochemical analysis of the receptor, STAT5, phospho-STAT5, and fatty-acid synthase (FAS), a downstream target gene of STAT5 in mammary cells (17). In MFA samples, irrespective of the PrlR genotype, the nuclear location of phospho-STAT5 labeling (arrows on Fig. 4) and the intense FAS labeling supported activation of that pathway in tumors. Remarkably, phospho-STAT5 and FAS labeling was also observed in adjacent tissue from the mutated patient but not in those from non mutated patients, suggesting increased activation of PrlR-triggered cascades also occurs in healthy tissue expressing the mutated receptor. PrlR and STAT5 labeling were similar in all samples analyzed (not shown), indicating that activation of STAT5 cascade was not caused by over-expression of these proteins.
Fig. 4.
Immunohistochemical analysis of breast biopsies (MFA and adjacent tissue). STAT5 phosphorylation (Top) and FAS expression (Bottom) were analyzed in MFA (Right) and adjacent tissue (Left) from one homozygous patient (PrlRWT,WT) and one heterozygous patient harboring the mutated PrlR allele (PrlRWT,I146L). (Middle) Nonspecific staining obtained without the addition of primary antibodies. Phospho-STAT5 was predominantly found in cell nuclei (arrows). Both phospho-STAT5 and FAS were up-regulated in adjacent tissue of the patient harboring PrlRI146L allele and in MFAs.
Inhibition of PrlRI146L Constitutive Activity by PrlR Signaling Inhibitors.
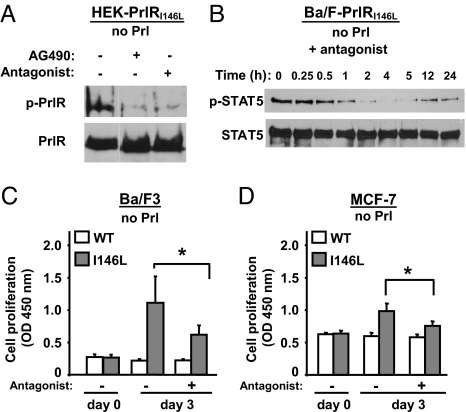
We investigated whether strategies known to inhibit Prl-induced activation of PrlRWT could down-regulate the constitutive activity of PrlRI146L. Tyrphostin B42 (AG490) is a pharmacological inhibitor of JAK2 activity and Del1–9-G129R-hPrl is a specific, competitive PrlR antagonist (18). Both inhibited constitutive phosphorylation of PrlRI146L stably expressed in HEK cells (Fig. 5A). A single treatment with the PrlR antagonist markedly reduced STAT5 activation in Ba/F-PrlRI146L cells over 24 h (Fig. 5B) as well as the number of spontaneously dividing (S/M) cells (from 41 ± 3% to 22 ± 4% in favor of G0/G1 cells; 3 independent experiments, data not shown). In agreement, three-day proliferation of Ba/F-PrlRI146L cells was also significantly reduced by a single treatment with the PrlR antagonist (Fig. 5C). Similar growth inhibition by the antagonist was observed on MCF7-PrlRWT,I146L cells (Fig. 5D).
Fig. 5.
Inhibition of PrlRI146L constitutive activity by a PrlR antagonist. (A) Constitutive phosphorylation of the mutated PrlR in HEK-PrlRI146L cells was inhibited by treating cells with the JAK2 inhibitor AG490 (50 μM, 1 h), which identifies this tyrosine kinase as involved in constitutive PrlR phosphosylation. The same effect was obtained by using the PrlR antagonist Del1–9-G129R-hPrl (0.8 μM, 1 h), which demonstrates that abolition of constitutive activation can also be achieved by this PrlR-specific inhibitor. (B) Constitutive phosphorylation of STAT5 in Ba/F-PrlRI146L cells was inhibited in a time-dependent manner by the addition of PrlR antagonist Del1–9-G129R-hPrl (0.16 μM) and remained below starting levels for at least 24 h. (C and D) Ba/F-PrlRWT and Ba/F-PrlRI146L cells (C) and MCF7-PrlRWT,WT and MCF7-PrlRWT,I146L cells (D) were treated by using the PrlR antagonist Del1–9-G129R-hPrl (0.8 μM), and cell proliferation (growth/survival ratio) was monitored after 3-day treatment using WST-1. Although the PrlR antagonist did not affect survival of cells expressing the WT PrlR (indicating absence of toxicity), it significantly reduced the growth of cells expressing the PrlR mutant (means ± SD, three independent experiments performed in triplicate). *, P < 0.05.
Discussion
Our data represent a unique functional characterization of a genetic anomaly of the PrlR gene associated with a human disease. The multiple functional assays used in this study unambiguously converge to the evidence that PrlRI146L exhibits constitutive activity, highlighting the remarkable effect of this single substitution on the biological properties of the PrlR. Importantly, these conclusions were confirmed by using mammary epithelial cells (MCF-7) co-expressing both PrlRWT (endogenous) and PrlRI146L (exogenous), which is presumably the most representative model of the situation found in the breast tissue of the heterozygous patients.
The molecular mechanism by which I146L mutation confers constitutive activity to the PrlR is currently unknown. This is not the first example of a membrane receptor on which such a conservative substitution has functional consequences (19). Based on the three-dimensional structure of the dimerized rat PrlR (20), Ile146 is located just under the surface of interaction of both receptor molecules (Fig. S7). It is reasonable to postulate that I146L mutation could force the PrlR to fold in a conformation mimicking that normally induced upon Prl binding. This is in good agreement with the ability of the PrlR antagonist to reduce significantly the constitutive activity of PrlRI146L. An engineered mutation of the topologically equivalent Ile residue in the common beta chain of the human IL-3 receptor (I374N) also led to a receptor exhibiting constitutive activity in some cell lines, suggesting that this position represents a hot spot in cytokine receptors to achieve constitutive activity (21).
Engineered PrlR variants exhibiting ligand-independent activity have also been generated by deleting half up to almost the entire extracellular domain (22, 23). The cellular effects induced by these artificial variants were very similar to those reported in this study for the natural PrlRI146L mutant. Mammary-specific transgenic mice expressing one of these variants were generated (24). Phenotypes included premature over-development of the gland in virgin and pregnant animals, and, after parturition, impaired terminal differentiation, functional failure, and delayed involution. Unfortunately, this study failed to reveal whether these morphological anomalies developed into mammary tumors because only young animals were used (24). We recently showed that transgenic mice over-expressing Prl in the differentiating/lactating mammary gland developed various benign lesions from the age of approximately 1 year (25). Using a permanently active promoter, others showed that autocrine Prl induced mammary carcinomas in virgin females from the age of approximately 15 months (26). Although the mechanisms leading to the development of benign versus malignant tumors are not yet fully understood, they may involve various parameters such as genetic background or the state of differentiation of the gland (25), these studies highlighted the ability of PrlR-triggered pathways to promote mammary tumorogenesis in rodents. In humans, the link between Prl levels and breast carcinogenesis has recently emerged from the Nurse Health study (27, 28). The description of a gain-of-function mutation of the PrlR associated with a BBD adds a new facet to the involvement of this hormonal system in the pathogenesis of breast tumors. Interestingly, this substitution has recently been reported as an uncharacterized SNP in three breast cancer patients (4, 5). Our findings strengthen the need to perform large epidemiological studies to determine whether PrlRI146L may also participate in breast carcinogenesis.
The mechanisms involved downstream constitutive PrlR activation in MFAs remain to be elucidated. The role of STAT5 transcription factor in breast tumorogenesis has been underlined in recent studies (29, 30). STAT5 appeared to be constitutively phosphorylated in the three cell models expressing PrlRI146L. Moreover, it was also activated in the mammary tissue of the mutated patient, and this was true in both the tumor and the adjacent tissue (Fig. 4). Active STAT5 was also observed in MFA from patients expressing only the WT receptor. More than being a marker of PrlRI146L constitutive activity, phosphorylated STAT5 is a thus good candidate contributing to the pathogenesis of MFAs. The molecular pathways that could mediate its effects may involve FAS, as the latter was proposed to stimulate survival and proliferation of breast cancer cells via complex mechanisms interfering with ER actions (31). Similar mechanisms could also occur in MFA, in which ER expression was also assessed (not shown). It is clear however that MFA pathogenesis remains a complex process presumably involving several other mechanisms yet to be identified (8).
Clinically, none of our mutated patients displayed any obvious sign of hyperprolactinemia, although full clinical phenotyping was not performed. Also, because serum samples for endocrine evaluation were only performed during the early follicular phase, potential luteal phase defects cannot be ruled out. In fact, germline activating mutations of receptors can lead to phenotypes that are different from those expected. For example, gain-of-function mutations of the LH receptor result in precocious puberty only in boys (19), whereas a physiological role of this receptor on the ovary is widely recognized. Also, the pathological consequences of FSH receptor activating mutations are only apparent during pregnancy in women with no apparent fertility or ovarian troubles (32). In our patients, the association of the PrlR mutation with a breast phenotype highlights this tissue as the main Prl target. In agreement, the increased risk of developing a breast cancer among women with high-normal versus low-normal Prl levels (27, 28) reinforces the idea that the first consequence of a slightly increased Prl stimulus is related to a breast phenotype. The higher sensitivity of the breast is likely related to the expression level of the PrlR, which is one of the highest of all Prl target organs, actually several fold more than seen in the ovary (33). Future investigations will be directed to identifying to what extent the increased signaling of PrlRI146L, demonstrated in various in vitro assays, participates in triggering quantitatively and/or qualitatively different downstream events that could be correlated to breast pathogenesis.
Because of the Prl-independent activity of PrlRI146L, no beneficial effect of dopamine agonists would be expected in these mutated patients. The field of BBDs therefore constitutes an opportunity to study the antiproliferative activity of alternative molecules. Competitive PrlR antagonists are a new class of potential drugs which act directly at the level of PrlR activation (34). Our study clearly shows that these molecules are able to exert inhibitory effect on PrlRI146L constitutive activity in vitro, including on STAT5 signaling. These findings encourage consideration in the future of adapting therapeutic management of patients harboring this mutated receptor.
Methods
Patients.
The study was approved by the local ethical committee and written informed consent was obtained from all patients and controls. DNA was extracted from whole blood cells, and the 11 exons of the PrlR gene were sequenced in both directions. Reference sequences were obtained from Ensembl (OTTHUMG00000090789) and National Center for Biotechnology Information online databases (NM_000949).
Cell Cultures and Transfections.
HEK293 and MCF-7 were cultured in DMEM and Ba/F3 in RPMI as detailed in SI Text. Cells were cotransfected (Fugene 6, Roche) by using plasmids encoding the human PrlR of interest and, for HEK cells only, the vector encoding firefly luciferase under control of STAT5 response elements (LHRE) (35). Stable clones were selected in growth medium containing 500 μg/ml active G-418 (geneticin).
Binding Assays.
Expression level and affinity parameters of receptors were determined by routine radioligand binding assay as described in SI Text.
Cell-Based Assays.
Luciferase assays (HEK cells) were performed as earlier described by using a luciferase kit (Promega) and a luminometer (Lumat LB 9501, Berthold) as detailed in SI Text. Cell proliferation/survival (Ba/F3, MCF-7) was measured over 3 days by daily measurement of tetrazolium salt conversion (WST-1 assay). Cell cycle distribution was checked by FACS analysis using propidium iodine labeling (35).
Signaling Studies.
Intracellular signaling was analyzed by routine immunoprecipitation/immunoblot procedures as detailed in SI Text. Immunoprecipitations were performed by using anti-human PrlR antibody (1A2B1, Zymed) or polyclonal anti-STAT5 (C-17, Santa Cruz Biotechnology). Immunoblotting involved either anti-phosphotyrosine antibody (4G10, Upstate), anti-phosphorylated STAT5 (AX1, Advantex BioReagents), and, after stripping, the anti-PrlR or anti-STAT5 antibodies referenced above.
Immunohistochemical Analysis.
The analyzes of breast biopsies were performed by using 3-μm sections from paraffin-embedded, formalin-fixed tissues, stained by using the antibodies described above, or anti-fatty acid synthase (H-300, Santa Cruz Biotechnology). Protocol is detailed in SI Text.
Statistical Analyses.
The frequencies of the mutation in both cohorts were compared by the two-tailed Fisher test. Results of bioassays are expressed as mean ± SD, and multiple groups were analyzed by the Kruskall-Wallis test with the Mann–Whitney U test as a post hoc comparison. Cell proliferation assay was analyzed by linear regression analysis, with comparison of slopes by ANCOVA analysis. Statistical analysis was performed by using PRISM software (GraphPad Software). P < 0.05 was regarded as statistically significant.
Supplementary Material
Acknowledgments.
We thank Profs. S. Lyonnet and F. Soubrier for their helpful and constructive discussions and advice; A. Rouxel, M.L. Tanguy, and A. Mallet for statistical analyses; and Dr. C. Coussieu, Dr. K. Laborde, J. Dulon, Y. Lerouzic, and V. Michaud for their technical assistance. This work was supported by Direction de la Recherche Clinique Paris, Programme Regional Hospitalier de Recherche Clinique Grant AOR03057 and Institut National de la Santé et de la Recherche Médicale. R.L.B. received an unrestricted grant from Pfizer Company, V.G. was awarded an Institut National de la Santé et de la Recherche Médicale–Assistance Publique–Hôpitaux de Paris Interface contract, and S.B. was awarded the 2007 Prix Société Française d'Endocrinologie–Ipsen.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Single Nucleotide Polymorphism database (dbSNP), www.ncbi.nlm.nih.gov/sites/entrez?db=snp (SNP ID no. ss102734470).
This article contains supporting information online at www.pnas.org/cgi/content/full/0800685105/DCSupplemental.
References
- 1.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- 2.Ormandy CJ, et al. Investigation of the transcriptional changes underlying functional defects in the mammary glands of prolactin receptor knockout mice. Recent Prog Horm Res. 2003;58:297–323. doi: 10.1210/rp.58.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Glasow A, et al. Mutational analysis of the PRL receptor gene in human breast tumors with differential PRL receptor protein expression. J Clin Endocrinol Metab. 2001;86:3826–3832. doi: 10.1210/jcem.86.8.7753. [DOI] [PubMed] [Google Scholar]
- 4.Canbay E, et al. Could prolactin receptor gene polymorphism play a role in pathogenesis of breast carcinoma? Curr Med Res Opin. 2004;20:533–540. doi: 10.1185/030079904125003232. [DOI] [PubMed] [Google Scholar]
- 5.Vaclavicek A, et al. Association of prolactin and its receptor gene regions with familial breast cancer. J Clin Endocrinol Metab. 2006;91:1513–1519. doi: 10.1210/jc.2005-1899. [DOI] [PubMed] [Google Scholar]
- 6.Lee SA, et al. A comprehensive analysis of common genetic variation in prolactin (PRL) and PRL receptor (PRLR) genes in relation to plasma prolactin levels and breast cancer risk: The multiethnic cohort. BMC Med Genet. 2007;8:72. doi: 10.1186/1471-2350-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353:275–285. doi: 10.1056/NEJMra035692. [DOI] [PubMed] [Google Scholar]
- 8.Courtillot C, et al. Benign breast diseases. J Mammary Gland Biol Neoplasia. 2005;10:325–335. doi: 10.1007/s10911-006-9006-4. [DOI] [PubMed] [Google Scholar]
- 9.Hughes LE, Mansel RE, Webster DJT. In: Benign Disorders and Diseases of the Breast—Concepts and Clinical Management. Hughes LE, Mansel RE, Webster DJT, editors. Philadelphia: WB Saunders Ltd; 2000. pp. 21–34. [Google Scholar]
- 10.Touraine P, et al. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- 11.Gill S, Peston D, Vonderhaar BK, Shousha S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: An immunohistological study. J Clin Pathol. 2001;54:956–960. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabre A, et al. Oral progestagens before menopause and breast cancer risk. Br J Cancer. 2007;96:841–844. doi: 10.1038/sj.bjc.6603618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parlati E, et al. Bromocriptine for treatment of benign breast disease: A double-blind clinical trial versus placebo. Acta Obstet Gynecol Scand. 1987;66:483–488. doi: 10.3109/00016348709015721. [DOI] [PubMed] [Google Scholar]
- 14.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin and its receptor: Actions, signal transduction pathways and phenotypes observed in prolactin receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 15.LeBaron MJ, Ahonen TJ, Nevalainen MT, Rui H. In vivo response-based identification of direct hormone target cell populations using high-density tissue arrays. Endocrinology. 2007;148:989–1008. doi: 10.1210/en.2006-1219. [DOI] [PubMed] [Google Scholar]
- 16.Lebrun JJ, Ali S, Goffin V, Ullrich A, Kelly PA. A single phosphotyrosine residue of the prolactin receptor is responsible for activation of gene transcription. Proc Natl Acad Sci USA. 1995;92:4031–4035. doi: 10.1073/pnas.92.9.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menendez JA, Peirce SK, Siddiqui L, Chen WY, Lupu R. Progesterone receptor isoform B exacerbates prolactin-induced up-regulation of fatty acid synthase (FAS) gene expression in breast cancer cells. AACR Meeting Abstracts. 2005:873–874. [Google Scholar]
- 18.Bernichtein S, et al. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;278:35988–35999. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- 19.Wu SM, Leschek EW, Rennert OM, Chan WY. Luteinizing hormone receptor mutations in disorders of sexual development and cancer. Front Biosci. 2000;5:D343–D352. doi: 10.2741/wu. [DOI] [PubMed] [Google Scholar]
- 20.Elkins PA, et al. Ternary complex between placental lactogen and the extracellular domain of the prolactin receptor. Nat Struct Biol. 2000;7:808–815. doi: 10.1038/79047. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins BJ, Bagley CJ, Woodcock J, Lopez AF, Gonda TJ. Interacting residues in the extracellular region of the common beta subunit of the human granulocyte-macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 receptors involved in constitutive activation. J Biol Chem. 1996;271:29707–29714. doi: 10.1074/jbc.271.47.29707. [DOI] [PubMed] [Google Scholar]
- 22.Gourdou I, et al. Development of a constitutively active mutant form of the prolactin receptor, a member of the cytokine receptor family. Mol Endocrinol. 1996;10:45–56. doi: 10.1210/mend.10.1.8838144. [DOI] [PubMed] [Google Scholar]
- 23.Lee RC, Walters JA, Reyland ME, Anderson SM. Constitutive activation of the prolactin receptor results in the induction of growth factor-independent proliferation and constitutive activation of signaling molecules. J Biol Chem. 1999;274:10024–10034. doi: 10.1074/jbc.274.15.10024. [DOI] [PubMed] [Google Scholar]
- 24.Gourdou I, et al. Expression by transgenesis of a constitutively active mutant form of the prolactin receptor induces premature abnormal development of the mouse mammary gland and lactation failure. Biol Reprod. 2004;70:718–728. doi: 10.1095/biolreprod.103.019448. [DOI] [PubMed] [Google Scholar]
- 25.Manhes C, et al. Local over-expression of prolactin in differentiating mouse mammary gland induces functional defects and benign lesions, but no carcinoma. J Endocrinol. 2006;190:271–285. doi: 10.1677/joe.1.06829. [DOI] [PubMed] [Google Scholar]
- 26.Rose-Hellekant TA, et al. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hankinson SE, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:629–634. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- 28.Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: An epidemiologic perspective. J Mammary Gland Biol Neoplasia. 2008;13:41–53. doi: 10.1007/s10911-008-9063-y. [DOI] [PubMed] [Google Scholar]
- 29.Cotarla I, et al. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- 30.Nevalainen MT, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Lupu R, Menendez JA. Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators? Endocrinology. 2006;147:4056–4066. doi: 10.1210/en.2006-0486. [DOI] [PubMed] [Google Scholar]
- 32.De Leener A, et al. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab. 2006;91:555–562. doi: 10.1210/jc.2005-1580. [DOI] [PubMed] [Google Scholar]
- 33.Peirce SK, Chen WY, Chen WY. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J Endocrinol. 2001;171:R1–R4. doi: 10.1677/joe.0.171r001. [DOI] [PubMed] [Google Scholar]
- 34.Goffin V, Touraine P, Culler MD, Kelly PA. Drug insight: Prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyper prolactinemia? Nat Clin Pract Endocrinol Metab. 2006;2:571–581. doi: 10.1038/ncpendmet0270. [DOI] [PubMed] [Google Scholar]
- 35.Bernichtein S, Jeay S, Vaudry R, Kelly PA, Goffin V. New homologous bioassays for human lactogens show that agonism or antagonism of various analogs is a function of assay sensitivity. Endocrine. 2003;20:177–190. doi: 10.1385/ENDO:20:1-2:177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.