Abstract
The acquisition of new genetic traits by horizontal gene transfer and their incorporation into preexisting regulatory networks have been essential events in the evolution of bacterial pathogens. An example of successful assimilation of virulence traits is Salmonella enterica, which acquired, at distinct evolutionary times, Salmonella pathogenicity island 1 (SPI-1), required for efficient invasion of the intestinal epithelium and intestinal disease, and SPI-2, essential for Salmonella replication and survival within macrophages and the progression of a systemic infection. A positive regulatory cascade mainly composed of HilD, HilA, and InvF, encoded in SPI-1, controls the expression of SPI-1 genes, whereas the two-component regulatory system SsrA/B, encoded in SPI-2, controls expression of SPI-2 genes. In this study, we report a previously undescribed transcriptional cross-talk between SPI-1 and SPI-2, where the SPI-1–encoded regulator HilD is essential for the activation of both the SPI-1 and SPI-2 regulons but at different times during the stationary phase of growth in Luria-Bertani medium. Our data indicate that HilD counteracts the H-NS–mediated repression exerted on the OmpR-dependent activation of the ssrAB operon by specifically interacting with its regulatory region. In contrast, HilD is not required for SPI-2 regulon expression under the in vitro growth conditions that are thought to resemble the intracellular environment. Our results suggest that two independent SPI-2 activation pathways evolved to take advantage of the SPI-2–encoded information at different niches and, in consequence, in response to different growth conditions.
Keywords: H-NS, OmpR, microbial pathogenesis, transcriptional regulation, salmonella
One of the major events in the evolution of pathogenic bacteria has been the horizontal transfer of large DNA fragments, which may encode for a wide variety of virulence factors (1, 2). Acquisition of pathogenicity islands is a dynamic process that can potentially compromise bacterial fitness if the expression of the newly acquired genes is not appropriately regulated by preexisting regulatory networks. Recent studies have shown that the histone-like protein H-NS has had an essential role in preventing the uncontrolled expression of A+T-rich genes contained within these incoming islands and in allowing the evolution of counteracting regulatory mechanisms to regulate the spatiotemporal expression of the gained genes (3–6). H-NS is a constitutive abundant protein, considered a global transcriptional regulator and a genome-structuring protein, which binds preferentially to A+T-rich sequences commonly present in horizontally transferred DNA (7, 8).
Salmonella enterica has evolved through the acquisition of several pathogenicity islands, two of which, Salmonella pathogenicity island (SPI)-1 and SPI-2, encode type three secretion systems that are essential for virulence (9). These islands are functionally distinct because SPI-1 genes are required for efficient invasion of the intestinal epithelium, leading to gastroenteritis, whereas SPI-2 genes are essential for Salmonella replication and survival within macrophages and, as a consequence, for systemic disease (9).
With respect to SPI-1 and SPI-2 gene regulation, almost all studies have been carried out in vitro using well-defined synthetic growth media. Although it is clear that these systems do not reconstitute the in vivo environment, they have provided valuable insights into the complex regulatory networks involved in virulence gene expression. The synthetic media used to study SPI-1 and SPI-2 regulation are high-osmolarity and nutrient-rich media, such as Luria-Bertani (LB) broth (10, 11), and low-phosphate and low-magnesium minimal media, such as N-minimal medium (10, 12), respectively. Each of these media is believed to give some approximation of the appropriate in vivo environment; the intestinal lumen for SPI-1 and the intracellular Salmonella-containing vacuole for SPI-2. The SPI-1 regulon is controlled by a regulatory cascade composed mainly of the SPI-1–encoded regulators HilD, HilA, and InvF (13–16). SPI-2 genes are expressed when Salmonella is inside host cells (17–19) and is under the control of the SPI-2–encoded two-component system SsrA/B (12, 20, 21) [see supporting information (SI) Fig. S1 for details].
Here, we developed an in vitro system to study the sequential activation of SPI-1 and SPI-2 regulons in Salmonella enterica serovar Typhimurium (S. typhimurium). Using this system, we were able to reveal a previously undescribed transcriptional cross-talk mechanism between SPI-1 and SPI-2, where the SPI-1–encoded regulator HilD differentially regulates, in a growth phase-dependent manner, both the SPI-1 and SPI-2 regulons during growth in LB medium. We show that HilD can bind directly to the regulatory region of the ssrAB operon and counteracts the repression exerted by H-NS on the OmpR- and growth phase-dependent expression of ssrAB. These results bring to light a new level of sophistication in the regulatory control of SPI-1 and SPI-2, which together form the cornerstone of Salmonella virulence. Additionally, the unveiling of a transcriptional link between a preexistent pathogenicity island and newly acquired genes highlights another important step in the evolution of pathogenic bacteria.
Results
Sequential Growth Phase-Dependent Expression of the SPI-1 and SPI-2 Regulons in LB.
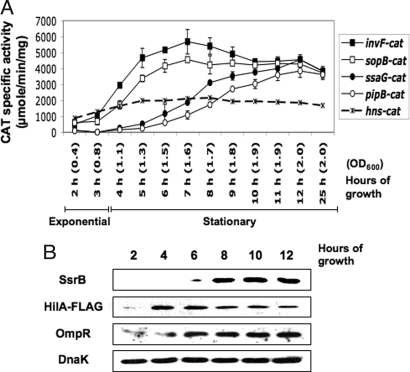
Initially, we studied the expression kinetics of cat transcriptional fusions to a number of S. typhimurium genes, including sopB and pipB, which belong to the SPI-1 and SPI-2 regulons, respectively, but are physically located within SPI-5 (22). We found that both sopB and pipB were expressed in LB, a medium commonly used to study the expression of SPI-1 genes and not known to support SPI-2 expression (10, 11), but with different expression profiles. Whereas sopB expression is activated and maximal following entry into the stationary phase, activation of pipB was observed at later time points (Fig. 1A). To test if this sequential activation was a general feature of the SPI-1 and SPI-2 regulons, we also determined the activation kinetics in LB of cat transcriptional fusions to the SPI-1–encoded invF and the SPI-2–encoded ssaG genes, which followed the same pattern observed for sopB and pipB, respectively (Fig. 1A). In N-minimal medium, as expected, growth of S. typhimurium carrying these transcriptional fusions revealed the activation of ssaG-cat and pipB-cat (SPI-2 regulon fusions) but not the SPI-1 regulon genes invF-cat and sopB-cat (data not shown).
Fig. 1.
The SPI-1 and SPI-2 regulons are expressed in LB in a growth phase-dependent manner. (A) Kinetics of expression of genes from SPI-1 (invF and sopB) and SPI-2 (ssaG and pipB) regulons as well as hns in LB cultures. Expression levels of invF-cat, sopB-cat, ssaG-cat, pipB-cat, and hns-cat transcriptional fusions were calculated by determining CAT-specific activity from samples taken hourly from LB cultures of S. typhimurium SL1344 carrying plasmid pinvF-cat1, psopB-cat1, pssaG-cat1, ppipB-cat1, or phns-cat1 (Table S1). The expression of the hns-cat fusion was analyzed as a control, which was not significantly altered along the growth curve. The data are the average of three different experiments done in duplicate. The OD600 at each time point is indicated between parentheses. Growth phases are indicated by thin bars. (B) SsrB, HilA, and OmpR production is also growth phase dependent. Immunoblots of whole-cell lysates of an S. typhimurium SL1344 strain carrying a chromosomal FLAG-tagged hilA gene grown in LB medium were performed using a polyclonal anti-SsrB or anti-OmpR antibody or a monoclonal anti-FLAG antibody. Equivalent amounts of protein were analyzed for each time point. As a loading control, DnaK was detected using a monoclonal antibody.
Because the two-component system SsrA/B is critical for the expression of the SPI-2 regulon in N-minimal medium (12, 20, 21), we next asked if it is also essential for expression of ssaG and pipB during the stationary phase in LB. Expression of the ssaG and pipB transcriptional fusions in a ssrB::kan strain was nearly abolished in either growth medium (Fig. 2 A and B). In contrast, expression of sopB in LB broth was not affected by the absence of SsrB but was drastically reduced in the absence of HilA (Fig. S2), the ToxR/OmpR-like protein that is pivotal for regulation of the SPI-1 regulon (13–15). To determine whether SsrB and HilA expression in LB was also growth phase dependent, we performed Western blot analysis of whole-cell lysates of strain JPTM7 (hilA::3×FLAG-kan) obtained from a time course of growth in LB. JPTM7 is a WT S. typhimurium SL1344 derivative expressing from its chromosomal gene location the HilA protein tagged with a C-terminal 3×FLAG epitope (HilA-FLAG), a feature that did not affect the expression of the SPI-1 or SPI-2 regulons (data not shown). SsrB was detected after 6 h of growth and was more evident toward the late stationary phase (Fig. 1B), which parallels the activation pattern of ssaG and pipB (Fig. 1A). Likewise, SsrA production was also growth phase dependent in LB (data not shown). In contrast, HilA was detected after the first 2 h at the onset of the stationary phase, reaching maximal levels between 4 and 6 h and declining during the late stationary phase (Fig. 1B), resembling the expression patterns of invF and sopB (Fig. 1A). Therefore, the growth phase-dependent expression of SPI-1 and SPI-2 regulon genes can be attributed to mechanisms regulating the expression of their respective central regulators, HilA and SsrB.
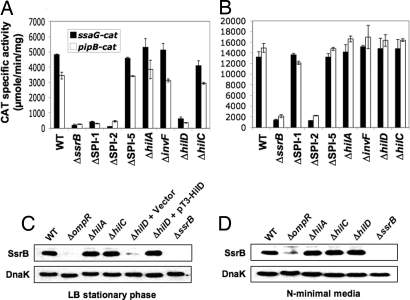
Fig. 2.
HilD is required for the stationary growth phase-dependent expression of SPI-2 regulon genes in LB but not in N-minimal medium. Expression of the ssaG-cat and pipB-cat transcriptional fusions was determined from samples taken from cultures of WT S. typhimurium and its ΔssrB, ΔSPI-1, ΔSPI-2, ΔSPI-5, ΔhilA, ΔinvF, ΔhilD, and ΔhilC derivatives carrying plasmids pssaG-cat1 or ppipB-cat1 grown for 12 h in LB medium (A) or for 16 h in N-minimal medium (B). The data are the average of three different experiments done in duplicate. SsrB in WT S. typhimurium and its ΔompR, ΔhilA, ΔhilC, ΔhilD, and ΔssrB derivatives was analyzed by Western blotting using a polyclonal anti-SsrB antibody and whole-cell lysates prepared from samples taken from LB (C) or N-minimal medium (D) cultures at the same time points as in A and B, respectively. pT3-HilD (Table S1) was used to complement the ΔhilD mutant.
These results revealed that Salmonella senses two different environmental conditions during growth in LB, which allows the sequential activation of the SPI-1 and SPI-2 regulons at different stages during the stationary phase. Thus, the stationary phase of growth in LB offers a condition where the transcriptional transition between these two regulons can be analyzed (SPI-1 turning off and SPI-2 turning on).
The SPI-1–Encoded Regulator HilD Also Regulates the SPI-2 Regulon.
To dissect the mechanism of activation of the SPI-2 regulon during the stationary phase in LB, we explored the potential role of the stationary phase σ factor RpoS as well as that of genes related to quorum sensing systems, such as luxS, which codes for the enzyme that generates an autoinducer 2-like signal, and sdiA, which codes for a LuxR-like protein (23). Mutants lacking rpoS, luxS, or sdiA showed no defect in SPI-2 gene expression (data not shown), indicating that regulatory proteins acting primarily in response to population density are not involved in this activation pathway. Furthermore, acidic pH, believed to be a major inducing signal for SPI-2 expression in minimal medium and within the Salmonella-containing vacuole (12, 19, 21, 24–26) did not induce SPI-2; indeed, acidification of the medium had an adverse effect on SPI-2 expression (data not shown). We additionally showed that the stringent signal molecule ppGpp is required for the stationary phase induction of both the SPI-1 and SPI-2 regulons but does not control the transition from SPI-1 to SPI-2 expression in LB (SI Results and Fig. S3).
We next looked at the possible involvement of elements encoded within the SPI-1, SPI-2, and SPI-5 pathogenicity islands by analyzing the expression of the ssaG-cat and pipB-cat transcriptional fusions in S. typhimurium ΔSPI-1, ΔSPI-2, and ΔSPI-5 derivatives. As expected, expression of ssaG-cat and pipB-cat fusions in the ΔSPI-2 deletion mutant was drastically reduced during the stationary phase in LB; intriguingly, it was similarly reduced in the ΔSPI-1 deletion mutant but not in the ΔSPI-5 mutant (Fig. 2A). Thus, induction of the SPI-2 regulon during the stationary phase in LB requires an SPI-1–encoded factor. To determine if this involves any of the known SPI-1–encoded regulatory proteins, we next analyzed the expression of the ssaG-cat and pipB-cat transcriptional fusions in mutants lacking HilD (ΔhilD), HilC (ΔhilC), HilA (ΔhilA), or InvF (ΔinvF). Interestingly, only the ΔhilD mutant showed reduced levels of expression of both fusions (Fig. 2A), and this was specific for these growth conditions because their expression was unaffected in the ΔhilD mutant following growth in N-minimal medium (Fig. 2B). As expected, expression of the SPI-1 regulon sopB-cat fusion was abolished in the ΔhilD, ΔhilA, and ΔinvF mutants but was not affected in the ΔhilC and ΔssrB mutants during growth in LB (Fig. S2).
To determine whether HilD regulates the SPI-2 regulon by controlling the expression of the two-component system SsrA/B, SsrA and SsrB protein levels were analyzed by immunoblotting of Salmonella lysates after growth in LB to the stationary phase. SsrB was detected directly using an anti-SsrB antibody, but an epitope tagged SsrA had to be constructed to assess SsrA expression. For this, the chromosomal ssrA gene was engineered to express a C-terminal 3×FLAG tag, generating strain JPTM9. This strain was then transferred by P22 transduction to ΔompR, ΔhilA, ΔhilC, and ΔhilD deletion backgrounds, generating strains JPTM10, JPTM11, JPTM12, and JPTM13, respectively. SsrB levels in the ΔhilA and ΔhilC strains were similar to WT, but the SsrB level was barely detected in the ΔhilD mutant unless this strain was complemented with a plasmid expressing HilD (Fig. 2C). In contrast, SsrB production was not affected in the ΔhilD mutant when grown in N-minimal medium (Fig. 2D). These results confirm the role of HilD in the regulation of the SPI-2 regulon during growth in LB. Analysis of SsrA-FLAG levels gave similar results to SsrB (Fig. S4), indicating that HilD controls both the sensor kinase and the response regulator during the stationary phase of growth in LB cultures.
Several studies have addressed the role of the response regulators OmpR and PhoP in the expression of ssrAB and SPI-2 regulon genes in Salmonella grown in low Mg2+ minimal medium or within infected epithelial or macrophage cell lines (24, 27, 28). To assess the role of these regulators in the growth phase-dependent activation of SsrA and SsrB production in LB, their levels were analyzed by immunoblotting using total cell lysates of the ΔompR and ΔphoP mutants grown in LB and in N-minimal medium. This analysis showed that OmpR but not PhoP was required for SsrA and SsrB expression under both growth conditions (Fig. 2 C and D, Fig. S4, and data not shown). Interestingly, OmpR also accumulated over time, reaching maximal levels during the late stationary phase in LB, as revealed by immunoblotting of samples from the time course of growth shown in Fig. 1B. This observation suggests that the specific HilD-dependent activation of SPI-2 expression in LB could, at least in part, be modulated by the growth phase-dependent expression of other essential regulatory proteins, such as OmpR.
HilD Counteracts the Repression Exerted by H-NS on ssrAB.
HilD has been previously shown to regulate the expression of hilA, the gene coding for the central positive regulator of the SPI-1 regulon, positively by counteracting the H-NS–mediated repression on the hilA promoter (29, 30). Furthermore, it has also been shown that expression of the ssrAB genes is thermoregulated by H-NS (31). These results are consistent with two recent reports documenting the role of H-NS in silencing the expression of genes that have been acquired by Salmonella through horizontal gene transfer, such as those belonging to the SPI-1 and SPI-2 regulons (3, 4). Based on these antecedents, we next examined whether HilD induces expression of the SPI-2 regulon by counteracting H-NS repression. Because Salmonella lacking hns shows severe growth defects and seems to be viable only after acquiring secondary mutations (4), we used an H-NS mutant that does not have DNA-binding activity but still forms heterodimers with WT monomers, and thus acts as a dominant negative mutant (32, 33). Plasmids encoding WT H-NS (pT6-HNS-WT) or the H-NS/Q92am mutant (pT6-HNS-Q92am), a C-terminal truncated H-NS derivative corresponding to the N-terminal dimerization domain (H-NSQ92am), under the control of an arabinose-inducible promoter, were transformed into strains ssrA::3xFLAG ΔompR::kan (JPTM10) and ssrA::3xFLAG ΔhilD::kan (JPTM13), which were then grown in LB in the presence or absence of 0.1% arabinose. Total lysates prepared from samples collected during the stationary phase of growth in LB were subjected to immunoblotting using anti-FLAG and anti-SsrB antibodies. As shown in Fig. 3, SsrA-FLAG and SsrB were not detected in the ΔhilD strain carrying pT6-HNS-WT with or without arabinose; however, induction of the dominant-negative H-NSQ92am from plasmid pT6-HNS-Q92am restored SsrA-FLAG and SsrB levels in the ΔhilD mutant. Interestingly, the H-NSQ92am dominant negative did not restore SsrA-FLAG or SsrB production in the ΔompR mutant (Fig. 3). These results indicated that HilD counteracts the H-NS–mediated repression on the ssrAB operon, subsequently allowing OmpR to activate transcription.
Fig. 3.
HilD positively regulates ssrA and ssrB expression by counteracting H-NS. H-NS was inactivated in S. typhimurium ssrA::3×FLAG ΔhilD::kan and ssrA::3×FLAG ΔompR::kan mutants by expressing a dominant-negative C-terminal truncated form of H-NS (see text). Plasmids pT6-HNS-WT and pT6-HNS-Q92am direct the expression of WT H-NS and H-NSQ92am, respectively, from an arabinose-inducible promoter. Strains were grown in LB medium, with or without 0.1% arabinose. SsrA-FLAG and SsrB were detected by immunoblot analysis of whole-cell lysates using a monoclonal anti-FLAG antibody or polyclonal anti-SsrB antibodies. DnaK levels were also determined as a loading control using a monoclonal antibody.
HilD and H-NS Directly Interact with the ssrAB Regulatory Region.
Consistently with the coordinated production of both SsrA and SsrB described previously, we also found that the ssrAB genes are transcribed as an operon in an HilD- and OmpR-dependent manner from the promoter located upstream of ssrA (SI Results and Fig. S5). Having shown that HilD and H-NS regulate ssrAB, we next asked whether this is mediated by direct interaction of these proteins with the regulatory region of ssrAB, analogous to HilD and H-NS regulation of hilA (29, 30, 34). Affinity-purified maltose-binding protein (MBP)-HilD and H-NS-His6 were then used to perform electrophoretic mobility shift assays (EMSAs) with fragments encompassing different regulatory regions. In agreement with other reports (30, 34, 35), MBP-HilD shifted specifically DNA fragments containing the regulatory regions of hilA or hilD at a concentration of 0.1 μM (Fig. 4A and Fig. S6A) but not those of the negative controls sopB (present in the same reaction) and sirA even at the highest concentration tested (0.4 μM) (Fig. 4 A and B and Fig. S6B), indicating that the purified MBP-HilD was functional in vitro and binds specifically. In agreement with our transcriptional fusion data showing its role as a positive regulator of the ssrAB operon, MBP-HilD also specifically shifted the ssrAB promoter fragment at concentrations ≥0.3 μM (Fig. 4B). Therefore, HilD directly interacts with the regulatory region of the ssrAB operon. Likewise, H-NS-His6 specifically bound to the fragments containing the hilA or hilD regulatory region (Fig. 4C and Fig. S6C) as well as to the ssrAB promoter fragment at concentrations ≥0.3 μM (Fig. 4D), indicating that it directly silences this promoter.
Fig. 4.
HilD and H-NS directly interact with the ssrAB regulatory region. EMSAs were performed to analyze HilD and H-NS binding to the ssrAB and hilA regulatory regions. Fragments encompassing the regulatory regions of hilA (−410 to +446), sopB (−400 to +128) and ssrAB (−300 to +478), with respect to the corresponding transcriptional start sites, were incubated with increasing concentrations of affinity-purified MBP-HilD (0, 0.05, 0.1, 0.2, 0.3, and 0.4 μM) or H-NS-His6 (0, 0.1, 0.3, 0.5, 0.6, and 0.7 μM). The DNA-protein complexes (indicated by an asterisk) were resolved in 6% polyacrylamide gels and stained with ethidium bromide. The hilA and sopB fragments were used as positive and negative controls, respectively.
Discussion
Acquisition of genomic DNA through horizontal gene transfer events could potentially impose a competitive disadvantage to the new bacterial host. Recent reports have highlighted the role that H-NS has played in bacterial evolution by silencing or downregulating, at the transcriptional level, the expression of foreign genes that have been acquired as a result of horizontal gene transfer events, thus preventing disadvantageous effects to the new bacterial host (3, 4, 6, 36). Bacterial pathogens such as Salmonella have evolved as a consequence of multiple horizontal gene transfer events at different evolutionary time points. Most of the genes acquired through these events are subjected to H-NS silencing; thus, regulatory mechanisms that alleviate this repression also evolved to induce the expression of the gained genes at particular host niches (3, 4, 6, 36).
Recent observations have suggested that the expression of SPI-1 and SPI-2 genes and their role at distinct steps during the infection process are not as independent and niche restricted as previously thought. For example, SPI-1 genes have also been shown to be important for Salmonella persistence during long-term systemic infections, phagosome maturation, and intracellular proliferation (37–39), and SPI-2 genes have been implicated in intestinal colonization, persistence during the intestinal phase, and induction of secretory and inflammatory responses (40–43). Furthermore, recent reports have suggested that expression of the SPI-1 and SPI-2 regulons is not restricted to unique and independent niches. SPI-2–encoded genes can be expressed in the intestinal lumen before penetration of the intestinal epithelium (44), whereas SPI-1 effectors can be detected in infected mice many days after infection (45) or induced intracellularly following phagocytic uptake but not following SPI-1–mediated invasion (19, 46).
Here, we describe a simple method whereby SPI-1 and SPI-2 regulons can be induced sequentially in LB medium, thus providing a unique opportunity to examine the mechanism of switching from SPI-1 to SPI-2 induction. Growth of Salmonella in LB has generally been considered to induce expression of the SPI-1 regulon and, conversely, to repress SPI-2 expression (10, 11). Although expression of SPI-2 genes has been previously observed in LB (47, 48), the mechanism involved in their activation has not been investigated. Our data show that SPI-2 genes are activated in LB in a growth phase-dependent manner once the bacteria have reached the late stationary phase and SPI-1 expression has ceased. Under these growth conditions, expression of SPI-2 genes requires the SsrA/B two-component regulatory system, whose essential role in SPI-2 regulon activation has been well documented in vitro and in vivo (12, 20, 21, 49, 50). In addition, we show that the regulatory pathway activating the SPI-2 regulon in response to stationary growth phase conditions specifically involves the SPI-1–encoded regulator HilD, which also has a hierarchical regulatory function in the activation of the SPI-1 regulon as well as a key role in vivo (51). Therefore, HilD appears to be at the center of a regulatory network that connects both SPI-1 and SPI-2 gene expression. The critical question now is how can HilD differentially activate SPI-1 and SPI-2 expression at different growth stages in the same growth medium? In theory the mechanism could depend on a number of different factors: the higher affinity that HilD shows for the hilA promoter region versus the ssrAB regulatory region; the parallel growth phase-dependent expression of other key SPI-2 regulators such as OmpR; the competitive action of a negative regulatory mechanism turning off SPI-1 expression, thus redirecting HilD toward ssrAB activation; and other possibilities that have not yet been investigated.
Analysis of different Salmonella genomes has shed light on the evolution of the Salmonella genus and suggested that SPI-2 is a more recently acquired virulence trait because it is not present in the phylogenetically older species S. bongori (52, 53). In this context, it is tempting to speculate that acquisition of SPI-2 imposed a need to coordinate its expression with preexistent virulence traits so as not to compromise bacterial fitness and efficiency to ensure the successful exploitation of different host niches. In this regard, we have shown that HilD directly binds the regulatory region of ssrAB and acts as an H-NS antagonist to allow the OmpR-dependent activation of this operon, which is consistent with its reported function in the activation of hilA (29, 30, 54). Adaptation of HilD to antagonize H-NS–dependent repression on the ssrAB operon, establishing a transcriptional cross-talk between the previously acquired SPI-1 and the incoming SPI-2, would ensure the coordinated and successive expression of both regulons under different conditions or host niches, leading to the successful evolution of Salmonella as an enteric and facultative intracellular pathogen.
Considering the pleiotropic effects of HilD on the regulation of Salmonella virulence factors, dissecting the role of HilD-mediated activation of SPI-2 regulon expression at different stages of an infection or in different hosts will require the precise definition of the ssrAB cis-acting elements in which HilD binds to exert its H-NS antagonistic function. The modification of this site at the chromosomal level to prevent HilD binding without affecting H-NS repression or ssrAB activation through HilD-independent pathways, such as those acting in N-minimal medium, will provide a strategy to generate Salmonella strains in which the HilD-mediated activation of the SPI-1 regulon remains functional. These strains could then be tested in different in vitro conditions and infection models to define the role of HilD more clearly in regulating the SPI-2 regulon.
In summary, by revealing the existence of an SPI-1 and SPI-2 transcriptional cross-talk mechanism, this work has disclosed a new level of complexity in the already intricate regulatory networks of Salmonella virulence (Fig. S1). HilD seems to coordinate the expression of both regulons in response to changing conditions that may be encountered at different stages during the progression of an infection. Once the bacterium has initiated the program that controls expression of the SPI-1 regulon in the gut lumen, the presence of HilD would help to set the activation of the SPI-2 regulon to perform, for example, its role during the enteric phase of the infection. Inside macrophages, expression of SPI-2 probably relies entirely on the PhoP/SlyA-dependent pathway, consistent with our observations that suggest these two pathways are not likely to overlap in vivo, because they are independently active under different growth conditions, and are apparently not synergistic, because each of them is active in the absence of the other. Regulation of the SPI-2 regulon is an example of the complexity of regulatory mechanisms that have evolved by integrating ancestral (e.g., H-NS, OmpR, PhoP), previously acquired (e.g., HilD), and accompanying (e.g., SsrB) regulatory elements into a complex regulatory network, which allows the spatiotemporal and coordinated expression of virulence factors during the infectious process.
Materials and Methods
Strains, Plasmids, and Primers.
Bacterial strains, plasmids, and primers used in this study are listed in Tables S1 and S2. Mutant strains were generated by the λ Red recombinase system, as reported previously (55, 56), and as briefly described in SI Materials and Methods. Construction of plasmids is also described in detail in SI Materials and Methods.
Growth Conditions.
LB medium containing 1% tryptone, 0.5% yeast extract, and 1% NaCl at pH 7.5 or N-minimal medium containing 5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 100 mM Tris-HCl (pH 7.5), 10 μM MgCl2, 0.5% glycerol, and 0.1% casamino acids was used for growth of bacterial cultures. Bacterial suspensions were prepared from overnight LB cultures that were concentrated and resuspended in fresh LB or N-minimal medium to an OD600 of 1. Then, 250-ml flasks containing 50 ml of LB or N-minimal medium were inoculated with a 50-fold dilution of the bacterial suspensions and incubated at 37°C in a shaken water bath at 200 rpm. (Gyromax 902; Amerex Instruments). Samples used to determine chloramphenicol acetyl transferase (CAT) activity or for Western blot analysis were taken hourly or at specific time points as indicated in the figure legends.
For the H-NS dominant-negative assay, duplicate LB cultures of the strains containing plasmid pMPM-T6Ω, pT6-HNS-WT, or pT6-HNS-Q92am were initiated as described previously. Two hours later, 0.1% L-(+)-arabinose (Sigma) was added to one duplicate to induce the expression of WT H-NS or the dominant-negative H-NSQ92am protein. Samples for Western blot analysis were taken 8 h after arabinose induction.
Western Blotting.
Immunoblots were performed as described in SI Materials and Methods using anti-SsrB or anti-OmpR polyclonal antibodies and anti-FLAG M2 (Sigma) or anti-DnaK (StressGen) monoclonal antibodies at 1:10,000, 1:2,000, 1:1,000, and 1:20,000 dilutions, respectively.
Expression and Purification of MBP-HilD and H-NS-His6.
MBP-HilD or H-NS-His6 fusion protein was expressed in Escherichia coli BL21/pLys containing pMAL-HilD1 or pT6-HNS-His6 and purified from a soluble extract loaded into an amylose column by eluting with column buffer containing 10 mM maltose (Bioxon) or by using a HiTrap Ni2+-chelating column, as described in SI Materials and Methods. Protein concentration was determined by the Bradford procedure.
EMSAs.
EMSAs were performed as follows. PCR fragments encompassing the regulatory regions of ssrAB, hilA, hilD, sopB, and sirA were amplified using primer pairs ssaBFBglII/ssrBRS6E, hilA1FBamHI/hilA2RHindIII, hilDFBamHI/hilDRHindIII, sigDH3R/sigDBH1F, and sirAFBamHI/sirARHindIII, respectively (Table S2), and S. typhimurium SL1344 chromosomal DNA as a template. Each PCR product (≈100 ng) was mixed with increasing concentrations of purified MBP-HilD or H-NS-His6 in a buffer containing 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1 mM DTT, 0.5 mM EDTA, 5% glycerol, and 10 μg ml−1 BSA in a final volume of 20 μl. The reactions were incubated for 20 min at room temperature and then electrophoretically separated in 6% polyacrylamide nondenaturing gels in 0.5× Tris-borate-EDTA buffer at room temperature. The DNA fragments were stained with ethidium bromide and visualized with an Alpha-Imager UV transilluminator (Alpha Innotech Corp.).
CAT Assays.
The CAT assays and protein quantification to calculate CAT-specific activities were performed as described previously (57).
Supplementary Material
Acknowledgments.
We thank A. Vázquez and M. Fernández-Mora for excellent technical assistance, L.J. Kenney for anti-SsrB and anti-OmpR antibodies, and S. Miller and F. Heffron for providing Salmonella strains. This work was supported by grants from Consejo Nacional de Ciencia y Tecnología (CONACYT) (42918Q) (to J.L.P.) and the Howard Hughes Medical Institute (75301–565101) (to J.L.P) and from Dirección General de Asuntos del Personal Académico (DGAPA) (IN227306–3) (to V.H.B). L.C.M. is supported by a predoctoral fellowship from CONACYT (No. 169380). L.A.K. and O.S.M. were supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801205105/DCSupplemental.
References
- 1.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt H, Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 2004;17:14–56. doi: 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucchini S, et al. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2006;2:e81. doi: 10.1371/journal.ppat.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarre WW, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 5.Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 2006;13:141–153. doi: 10.1093/dnares/dsl009. [DOI] [PubMed] [Google Scholar]
- 6.Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- 7.Dorman CJ. H-NS: A universal regulator for a dynamic genome. Nat Rev Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 8.Rimsky S. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr Opin Microbiol. 2004;7:109–114. doi: 10.1016/j.mib.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 10.Miao EA, Miller SI. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci USA. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundberg U, Vinatzer U, Berdnik D, von Gabain A, Baccarini M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–3437. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 13.Altier C. Genetic and environmental control of Salmonella invasion. J Microbiol. 2005;43:85–92. Spec No. [PubMed] [Google Scholar]
- 14.Jones BD. Salmonella invasion gene regulation: A story of environmental awareness. J Microbiol. 2005;43:110–117. Spec No. [PubMed] [Google Scholar]
- 15.Rhen M, Dorman CJ. Hierarchical gene regulators adapt Salmonella enterica to its host milieus. Int J Med Microbiol. 2005;294:487–502. doi: 10.1016/j.ijmm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Ellermeier JR, Slauch JM. Adaptation to the host environment: Regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol. 2007;10:24–29. doi: 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hensel M. Salmonella pathogenicity island 2. Mol Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 19.Drecktrah D, Knodler LA, Ireland R, Steele-Mortimer O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic. 2006;7:39–51. doi: 10.1111/j.1600-0854.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 21.Walthers D, et al. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol. 2007;65:477–493. doi: 10.1111/j.1365-2958.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- 22.Knodler LA, et al. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol Microbiol. 2002;43:1089–1103. doi: 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmer BM. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol Microbiol. 2004;52:933–945. doi: 10.1111/j.1365-2958.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee AK, Detweiler CS, Falkow S. OmpR regulates the two-component system SsrA-ssrB in Salmonella pathogenicity island 2. J Bacteriol. 2000;182:771–781. doi: 10.1128/jb.182.3.771-781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombes BK, Brown NF, Valdez Y, Brumell JH, Finlay BB. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J Biol Chem. 2004;279:49804–49815. doi: 10.1074/jbc.M404299200. [DOI] [PubMed] [Google Scholar]
- 26.Lober S, Jackel D, Kaiser N, Hensel M. Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int J Med Microbiol. 2006;296:435–447. doi: 10.1016/j.ijmm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Feng X, Oropeza R, Kenney LJ. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol. 2003;48:1131–1143. doi: 10.1046/j.1365-2958.2003.03502.x. [DOI] [PubMed] [Google Scholar]
- 28.Bijlsma JJ, Groisman EA. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol Microbiol. 2005;57:85–96. doi: 10.1111/j.1365-2958.2005.04668.x. [DOI] [PubMed] [Google Scholar]
- 29.Olekhnovich IN, Kadner RJ. Crucial roles of both flanking sequences in silencing of the hilA promoter in Salmonella enterica. J Mol Biol. 2006;357:373–386. doi: 10.1016/j.jmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Schechter LM, Lee CA. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol. 2001;40:1289–1299. doi: 10.1046/j.1365-2958.2001.02462.x. [DOI] [PubMed] [Google Scholar]
- 31.Duong N, et al. Thermosensing coordinates a Cis-regulatory module for transcriptional activation of the intracellular virulence system in Salmonella enterica serovar Typhimurium. J Biol Chem. 2007;282:34077–34084. doi: 10.1074/jbc.M707352200. [DOI] [PubMed] [Google Scholar]
- 32.Ueguchi C, Seto C, Suzuki T, Mizuno T. Clarification of the dimerization domain and its functional significance for the Escherichia coli nucleoid protein H-NS. J Mol Biol. 1997;274:145–151. doi: 10.1006/jmbi.1997.1381. [DOI] [PubMed] [Google Scholar]
- 33.Ueguchi C, Suzuki T, Yoshida T, Tanaka K, Mizuno T. Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H-NS. J Mol Biol. 1996;263:149–162. doi: 10.1006/jmbi.1996.0566. [DOI] [PubMed] [Google Scholar]
- 34.Olekhnovich IN, Kadner RJ. DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:4148–4160. doi: 10.1128/JB.184.15.4148-4160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olekhnovich IN, Kadner RJ. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol. 2007;189:6882–6890. doi: 10.1128/JB.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorman CJ. H-NS, the genome sentinel. Nat Rev Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- 37.Brawn LC, Hayward RD, Koronakis V. Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe. 2007;1:63–75. doi: 10.1016/j.chom.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele-Mortimer O, et al. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol. 2002;4:43–54. doi: 10.1046/j.1462-5822.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 39.Lawley TD, et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hapfelmeier S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–1685. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- 41.Coombes BK, et al. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect Immun. 2005;73:7161–7169. doi: 10.1128/IAI.73.11.7161-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coburn B, Li Y, Owen D, Vallance BA, Finlay BB. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect Immun. 2005;73:3219–3227. doi: 10.1128/IAI.73.6.3219-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bispham J, Tripathi BN, Watson PR, Wallis TS. Salmonella pathogenicity island 2 influences both systemic salmonellosis and Salmonella-induced enteritis in calves. Infect Immun. 2001;69:367–377. doi: 10.1128/IAI.69.1.367-377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown NF, et al. Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog. 2005;1:e32. doi: 10.1371/journal.ppat.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giacomodonato MN, et al. SipA, SopA, SopB, SopD and SopE2 effector proteins of Salmonella enterica serovar Typhimurium are synthesized at late stages of infection in mice. Microbiology. 2007;153:1221–1228. doi: 10.1099/mic.0.2006/002758-0. [DOI] [PubMed] [Google Scholar]
- 46.Drecktrah D, Knodler LA, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 2005;7:105–113. doi: 10.1111/j.1462-5822.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 47.Silphaduang U, Mascarenhas M, Karmali M, Coombes BK. Repression of intracellular virulence factors in Salmonella by the Hha and YdgT nucleoid-associated proteins. J Bacteriol. 2007;189:3669–3673. doi: 10.1128/JB.00002-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim S, Kim B, Choi HS, Lee Y, Ryu S. Fis is required for proper regulation of ssaG expression in Salmonella enterica serovar Typhimurium. Microb Pathog. 2006;41:33–42. doi: 10.1016/j.micpath.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Hensel M, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 50.Worley MJ, Ching KH, Heffron F. Salmonella SsrB activates a global regulon of horizontally acquired genes. Mol Microbiol. 2000;36:749–761. doi: 10.1046/j.1365-2958.2000.01902.x. [DOI] [PubMed] [Google Scholar]
- 51.Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 52.Hensel M, et al. Analysis of the boundaries of Salmonella pathogenicity island 2 and the corresponding chromosomal region of Escherichia coli K-12. J Bacteriol. 1997;179:1105–1111. doi: 10.1128/jb.179.4.1105-1111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumler AJ. The record of horizontal gene transfer in Salmonella. Trends Microbiol. 1997;5:318–322. doi: 10.1016/S0966-842X(97)01082-2. [DOI] [PubMed] [Google Scholar]
- 54.Schechter LM, Damrauer SM, Lee CA. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol. 1999;32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 55.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in. Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci USA. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Laguna Y, Calva E, Puente JL. Autoactivation and environmental regulation of bfpT expression, the gene coding for the transcriptional activator of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:153–166. doi: 10.1046/j.1365-2958.1999.01460.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.