Abstract
The bacterium Proteus mirabilis is capable of movement on solid surfaces by a type of motility called swarming. Boundaries form between swarming colonies of different P. mirabilis strains but not between colonies of a single strain. A fundamental requirement for boundary formation is the ability to discriminate between self and non-self. We have isolated mutants that form boundaries with their parent. The mutations map within a six-gene locus that we term ids for identification of self. Five of the genes in the ids locus are required for recognition of the parent strain as self. Three of the ids genes are interchangeable between strains and two encode specific molecular identifiers.
About 60 years ago, different clinical isolates of the swarming bacterial species Proteus mirabilis were shown to form visually apparent boundaries between colonies growing on agar (1). By contrast, swarms of a single strain merge with each other (2, Fig. 1A). This phenomenon is still used in diagnostic laboratories to type clinical isolates of P. mirabilis (3). Many clinical isolates of P. mirabilis secrete proteins called proticines that kill sensitive strains. An individual strain of P. mirabilis can be identified by a combination of the proticine it produces and the proticines to which it is sensitive (4, 5). Boundaries form between swarms of strains differing in proticine production and sensitivity. However some strains do not produce any proticines but still form boundaries, even with other non-proticine producing strains. Thus proticine production and sensitivity do not explain boundary formation. We sought to identify self versus non-self discrimination factors required for boundary formation by screening for and isolating mutants that recognize their parent as different from self.
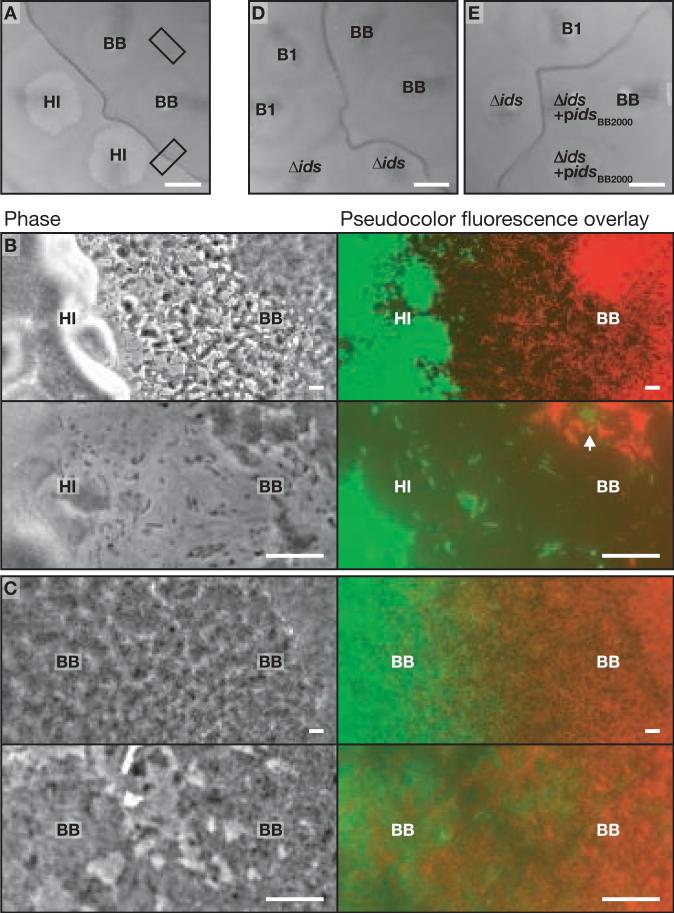
Figure 1. Images of swarm boundaries between different P. mirabilis strains.
(A) Section of an agar plate with swarms of P. mirabilis strains HI4320 (HI) and BB2000 (BB). The boxes indicate intersections visualized in panels B and C. (B) Microscopy showing (top) the boundary between a GFP-labeled HI swarm (green) and a dsRed-labeled BB swarm (red), and (bottom) a higher magnification of the boundary. The arrow indicates HI cells among BB cells in the boundary. (C) Microscopy showing (top) the merger of two BB swarms, and (bottom) a higher magnification of the merger. The left BB swarm (green) expressed GFP and the right BB swarm (red) expressed dsRed (2). (D) Section of an agar plate with swarms of BB, the Ids transposon mutant (B1) and the idsA-F deletion mutant (Δids). (E) Section of an agar plate with swarms of B1, Δids, BB, and two swarms of the idsA-F deletion mutant carrying an idsA-F expression vector (Δids+pidsBB2000). The bars are 1 cm (A, D and E) and 10 μm (B and C).
We chose P. mirabilis strain BB2000 as a model because it is genetically tractable (6). We used an agar plate assay to screen 3600 BB2000 mutants, generated by random transposon mutagenesis, in a format where each mutant swarm had two, three or four adjacent neighbors (2). We discovered a single mutant that formed a boundary with every adjacent mutant, and we named the mutant phenotype “identification of self (Ids)” because mutant and parent swarms did not merge with each other. To show that the transposon insertion was responsible for the phenotype, we crossed the insertion in the Ids transposon mutant into the BB2000 parent by homologous recombination and isolated four recombinants, all of which formed boundaries with the parent but not with each other (Fig. S1).
Boundaries between strain BB2000 and the independent isolate HI4320 contained individual cells of both strains at a low density as well as round bodies and debris. Cells of BB2000 and HI4320 made contact with each other within the boundary, but we did not observe cells that penetrated the opposite swarm (Fig. 1B). In boundaries between swarms of the Ids transposon mutant and the BB2000 parent we also observed a low density of cells, but round bodies and debris were not evident. Cells from the BB2000 parent swarm appeared to traverse the boundary and penetrate the Ids transposon mutant swarm (Fig. S2). During the merger of two swarms of strain BB2000, cells from each swarm penetrated the opposite swarm without apparent hindrance (Fig. 1C).
By using the sequenced genome of strain HI4320 (7), we found that the ids mutation mapped to codon 1030 of a 1033-codon open reading frame occurring between base pairs 3282912 to 3286013 and residing in a cluster of six genes (Fig. 2A). A homologous cluster was found by sequencing the parent strain BB2000 (2). We refer to the six-gene cluster as idsABCDEF for identification of self. We constructed an idsA-F deletion mutant of strain BB2000 and found that boundaries formed between swarms of the idsA-F deletion mutant and the BB2000 parent, but not between the deletion mutant and the Ids transposon mutant (Fig. 1D). Complementation of the idsA-F deletion mutation with an idsA-F expression vector (which included the 800-bp region directly upstream of idsA) resulted in a transformant that merged with the BB2000 parent but formed boundaries with the deletion mutant (Fig. 1E). The complementation analysis confirmed that the idsA-F locus encodes self-recognition factors.
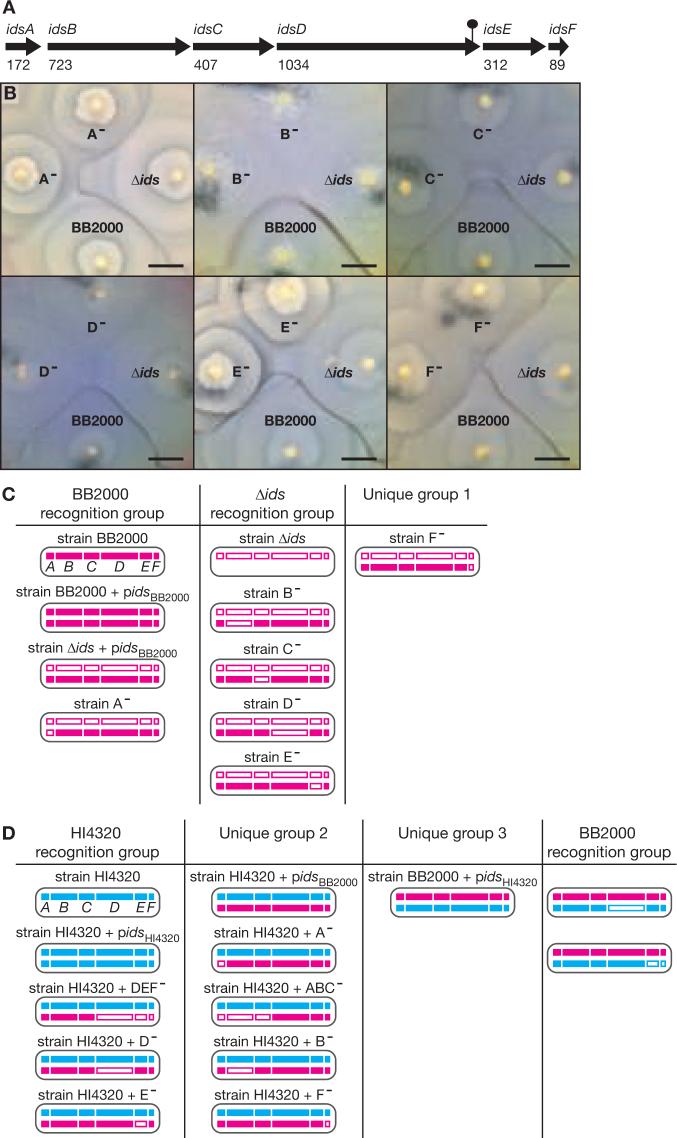
Figure 2. Genetic analysis of the ids gene cluster.
(A) The length of the encoded polypeptides in the six-gene ids cluster of strain BB2000 is shown underneath each gene. The lollipop demarks the site of the transposon insertion in the Ids transposon mutant. (B) Sections of agar plates with the BB2000 parent, the idsA-F deletion mutant (Δids) and the idsA-F deletion mutant carrying a plasmid-borne BB2000 idsA-F gene cluster with single-gene disruptions in idsA (A−), idsB (B−), idsC (C−), idsD (D−), idsE (E−) or idsF (F−). The bars are 1 cm. (C) Recognition groups of strains constructed in the BB2000 and Δids backgrounds. A subset of the strains is shown in panel B. (D) Recognition groups of transgenic diploid derivatives of wildtype HI4320 and wildtype BB2000. Agar plates with swarms of representative strains are shown in Figure S3. For panels C and D, a cell of each strain is represented by an oval in which the chromosomal and plasmid-borne ids gene clusters are on the top and bottom, respectively. A white box denotes a gene disruption. The BB2000 and HI4320 ids gene clusters are in red and blue, respectively. Each strain was tested against every other strain to determine the recognition groups (2). Each recognition group was comprised of strains whose swarms merged; boundaries formed between swarms of strains in different recognition groups.
To assess individual ids gene functions, we introduced plasmids containing idsA-F gene clusters in which individual genes were disrupted into the idsA-F deletion mutant (2). We then tested all of the ids-plasmid-carrying strains to determine whether they merged with each other or formed boundaries on swarm plates (Fig. 2B). We classified the constructs into recognition groups that were comprised of strains whose swarms merged with each other but not with swarms of strains in different recognition groups (Fig. 2C). An idsA-deficient strain merged with swarms of wildtype BB2000 but formed boundaries with the idsA-F deletion mutant (Fig. 2B-C). In contrast, idsB, idsC, idsD or idsE-deficient strains merged with the idsA-F deletion mutant but formed boundaries with wildtype BB2000. The idsF-deficient mutant likewise formed boundaries with wildtype BB2000 but had the additional property of forming boundaries with the idsA-F deletion mutant and in fact, swarms of the idsF-deficient mutant formed boundaries with swarms of any construct but itself. We conclude that idsA is not required for recognition of the BB2000 parent as self, but idsB, idsC, idsD, idsE and idsF are required for self-recognition. The idsF gene appears to encode a recognition factor distinct in function from idsB, idsC, idsD or idsE-encoded factors as indicated by the fact that idsF mutants merged only with themselves.
To further investigate the function of the ids genes in self-recognition, DNA containing either the complete BB2000 idsA-F gene cluster or a combination of disruptions in the BB2000 idsA-F gene cluster were introduced into wildtype HI4320 by conjugation to create transgenic diploids (2). The diploid HI4320 strains partitioned into those that merged with wildtype HI4320 or those that formed boundaries with wildtype HI4320 (Fig. 2D and Fig. S3). A diploid HI4320 strain carrying the complete BB2000 idsA-F gene cluster formed boundaries with wildtype HI4320, but merged with swarms of diploid HI4320 strains carrying the BB2000 idsA-F gene cluster with disruptions of idsA, idsB, idsC or idsF (Fig. 2D). In contrast, swarms of diploid HI4320 strains carrying the BB2000 idsA-F gene cluster with disruptions in either idsD or idsE merged with wildtype HI4320 (Fig. 2D). Therefore idsB, idsC and idsF encode essential self-recognition functions and the idsB, idsC and idsF alleles can be complemented by alleles from a different strain. However, idsD and idsE are essential for self-recognition and appear to encode identity determinants.
To confirm that idsD and idsE encode identity determinants, DNA containing the HI4320 idsA-F gene cluster with gene disruptions in idsD and separately in idsEF were conjugated into wildtype BB2000 (2). Swarms of both the idsD-deficient and idsEF-deficient diploid BB2000 strains merged with wildtype BB2000 but formed boundaries with a diploid BB2000 strain carrying the complete HI4320 idsA-F gene cluster (Fig. 2D). Thus idsD and idsE encode identity determinants, which we refer to as molecular identifiers.
We note that a diploid BB2000 strain carrying the complete HI4320 idsA-F gene cluster formed boundaries with all other strains including the diploid HI4320 strain carrying the complete BB2000 idsA-F gene cluster (Fig. 2D). Therefore the idsA-F gene cluster is probably not the sole determinant of boundary formation between different strains. Consistent with the presence of additional unidentified determinants, boundaries formed even in situations where one of the swarming strains did not carry any of the idsA-F genes (i.e. the idsA-F deletion mutant).
The idsA and idsB genes encode polypeptides with significant sequence similarity to the conserved bacterial proteins Hcp and VgrG, respectively. Recently, hcp and vgrG were shown to form the first two genes in the type VI protein secretion system of Vibrio cholerae (8) and both hcp and vgrG homologs occur in multiple copies in many bacterial species including P. mirabilis (7, 8, 9). We have included idsA as part of the ids cluster even though it is not required for self-recognition, because it is linked to idsB homologs in other bacteria and because it is possible that another hcp homolog may be recruited to replace idsA in idsA-deficient P. mirabilis strains. The idsC, idsD and idsE gene products do not show significant similarity to other known polypeptides. The idsF gene encodes a conserved hypothetical bacterial protein.
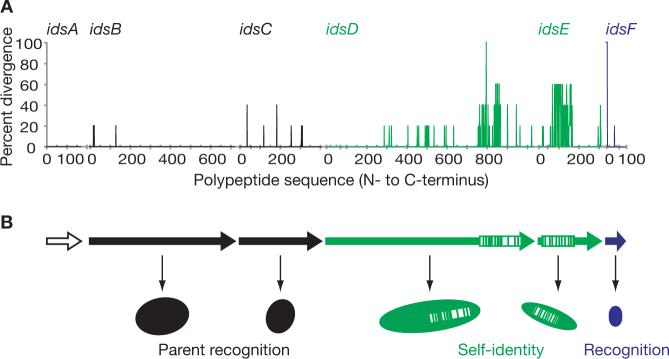
We sequenced the ids loci from five additional isolates of P. mirabilis: CW677, CW977, G151, I5/5 and S4/3 (2). Swarms of the five strains formed boundaries with BB2000, HI4320, and each other. All strains had the six-gene ids locus, except strain CW677, which had a seven-gene ids locus that contained an additional gene with similarity to idsE (Fig. S4). In all strains, idsA, idsB and idsC were identical in length, and each polypeptide encoded by idsA, idsB, idsC or idsF had over 96% identity with its homologs from the other strains (Fig. 3A). Both IdsD and IdsE could be separated into two distinct subfamilies with 30% pair-wise identity. Within a single IdsD or IdsE subfamily, there was 97−99% pair-wise identity across the majority of the sequence. However within a subfamily, there was a C-terminal region in IdsD with only 72−84% pair-wise identity and a similar region of only 32−80% pair-wise identity in IdsE (between amino acids 80 and 169). The variable regions of idsD and idsE are reminiscent of alleles encoding antigenic variation in some bacterial pathogens (10).
Figure 3. Organization and model of the ids gene cluster.
(A) A plot showing the percent divergence of the encoded polypeptides in the idsABCDEF gene cluster amongst P. mirabilis strains BB2000, HI4320, CW977, G151 and S4/3. (B) A model for self-recognition. The idsB, idsC, idsD, idsE and idsF genes are required for recognition of the BB2000 parent as self. IdsA is not required for self-recognition. IdsF has a function in self-non-self recognition that is distinct from that of IdsB and IdsC. The idsD and idsE genes encode specific molecular identifiers of self.
The DNA immediately downstream of the idsA-F locus in strain BB2000 contains a gene coding for a polypeptide with sequence similarity to IdsF and two genes coding for polypeptides with similarity to IdsE (Fig. S4). We do not know if there are additional IdsE or F family members coded in the BB2000 genome, but the sequenced HI4320 genome contains a six-gene repeat between base pairs 84801 and 91381 coding for polypeptides with similarity to IdsE (7, Fig. S5). It is possible that the putative IdsE homologs could act as additional molecular identifiers.
We have not yet succeeded in detecting the products of any of the ids genes in P. mirabilis cells, and so we do not know their cellular locations or how they might function to allow swarms to discriminate themselves from other encroaching swarms. It is unlikely that this is a toxin-antitoxin system because we do not see evidence of dead cells in the boundaries between the Ids transposon mutant and its parent (Fig. S2) and because the idsA-F deletion mutant and the BB2000 parent grew equally well in mixed cultures. When inoculated at a 1:1 ratio, the ratio of the parent and the idsA-F deletion mutant in stationary phase remained 1:1. Instead, our data are consistent with a model for self-recognition in which idsD and idsE encode specific molecular identifiers of self. The idsB, idsC and idsF products are devices necessary for self-non-self recognition and the idsF product has a function distinct from those of idsB and idsC (Fig. 3B).
Self-recognition may play a role in maintaining clonal Proteus infections (11). It also seems likely that other species of bacteria have genes encoding self-recognition. In fact, there is a report of swarm boundary formation between strains of the opportunistic pathogen Pseudomonas aeruginosa (12). The P. mirabilis genetic model of swarm identity provides a simplified system to further examine the molecular mechanisms of self-non-self recognition.
Supplementary Material
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. Their manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Supporting Online Material www.sciencemag.org Materials and Methods Figs. S1 to S5 References
References and Notes
- 1.Dienes L. Proceedings of the Society for Experimental Biology and Medicine. 1946;63:265. doi: 10.3181/00379727-63-15570. [DOI] [PubMed] [Google Scholar]
- 2.Materials and methods are available as supporting material on Science Online.
- 3.Sabbuba NA, Mahenthiralingam E, Stickler DJ. J Clin Microbiol. 2003;41:4961. doi: 10.1128/JCM.41.11.4961-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senior BW. J Gen Microbiol. 1977;102:235. doi: 10.1099/00221287-102-2-235. [DOI] [PubMed] [Google Scholar]
- 5.Senior BW. J Med Microbiol. 1983;16:323. doi: 10.1099/00222615-16-3-323. [DOI] [PubMed] [Google Scholar]
- 6.Belas R, Erskine D, Flaherty D. J Bacteriol. 1991;173:6289. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson MM, et al. J Bacteriol. 2008;190:4027. doi: 10.1128/JB.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pukatzki S, et al. Proc Natl Acad Sci U S A. 2006;103:1528. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mougous JD, et al. Science. 2006;312:1526. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Woude MW, Baumler AJ. Clin. Microbiol. Rev. 2004;17:581. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbuba NA, et al. J Urol. 2004;171:1925. doi: 10.1097/01.ju.0000123062.26461.f9. [DOI] [PubMed] [Google Scholar]
- 12.Munson EL, Pfaller MA, Doern GV. J Clin Microbiol. 2002;40:4285. doi: 10.1128/JCM.40.11.4285-4288.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.We thank Bernard Senior for his generous sharing of many P. mirabilis strains and helpful comments on the swarm assay. We also thank Robert Belas for providing strain BB2000, Harry Mobley for providing strain HI4320, and Melissa Visalli for providing strain G151. We thank Sudha Chugani, Breck Duerkop and Amy Schaefer for thoughtful scientific discussions and the W. M. Keck Foundation for support. K.A.G. was supported by training grant AI55396 from the National Institutes of Health. The sequences of the ids loci and flanking regions from strains BB2000, CW677, CW977, G151, I5/5 and S4/3 were deposited at GenBank, and the accession numbers are EU635876, EU635877, EU635878, EU635879, EU635880 and EU635881, respectively.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.