Abstract
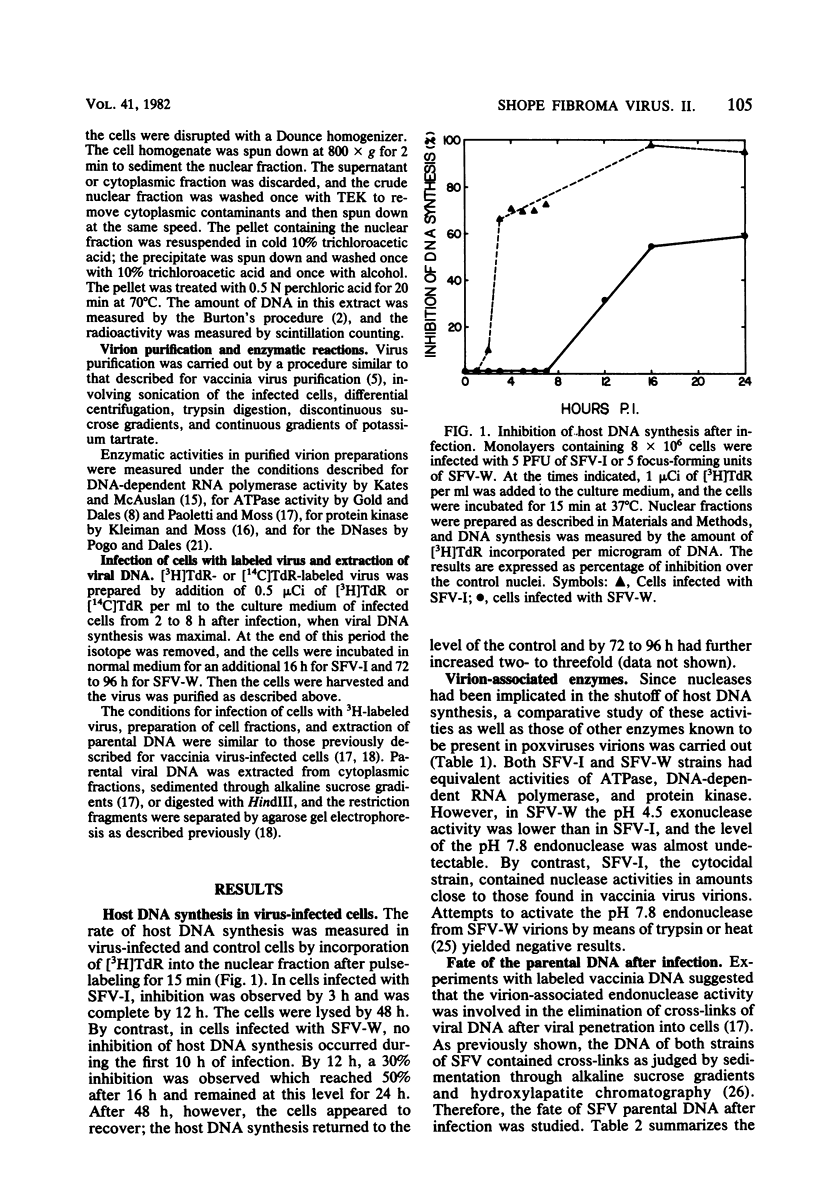
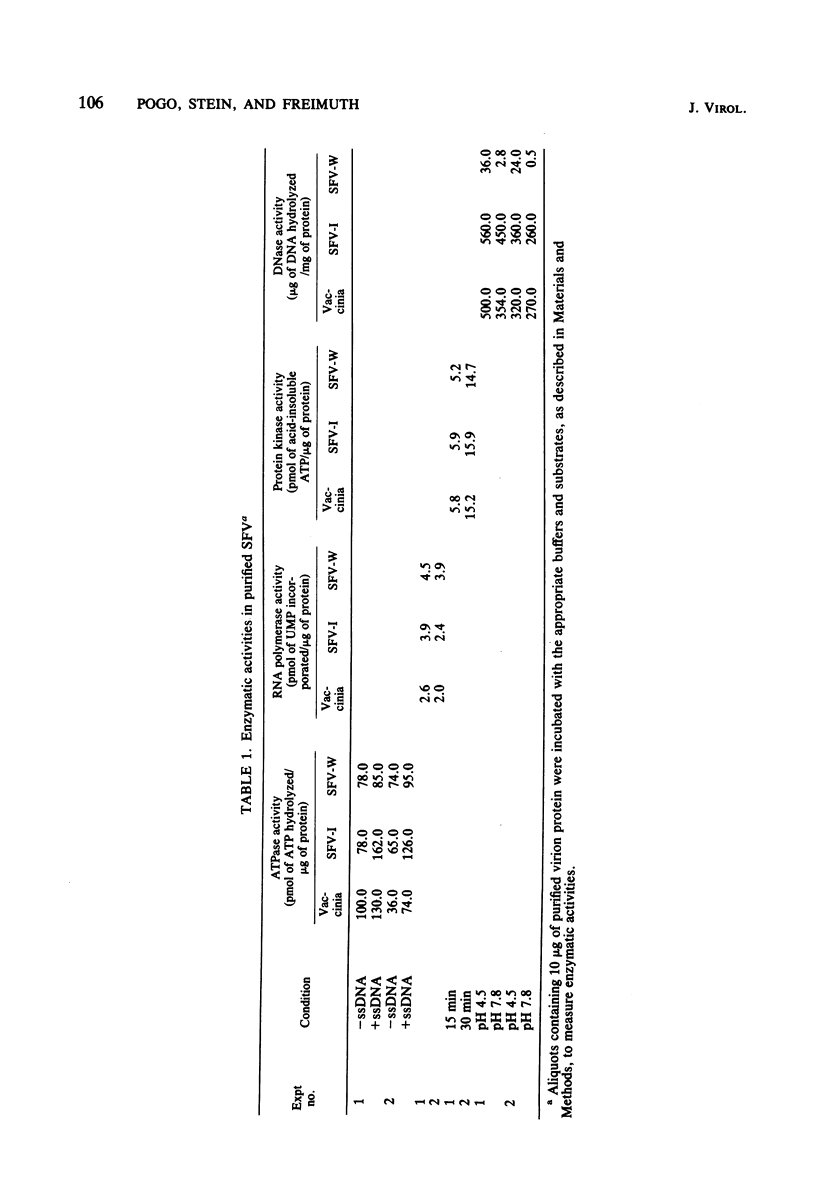
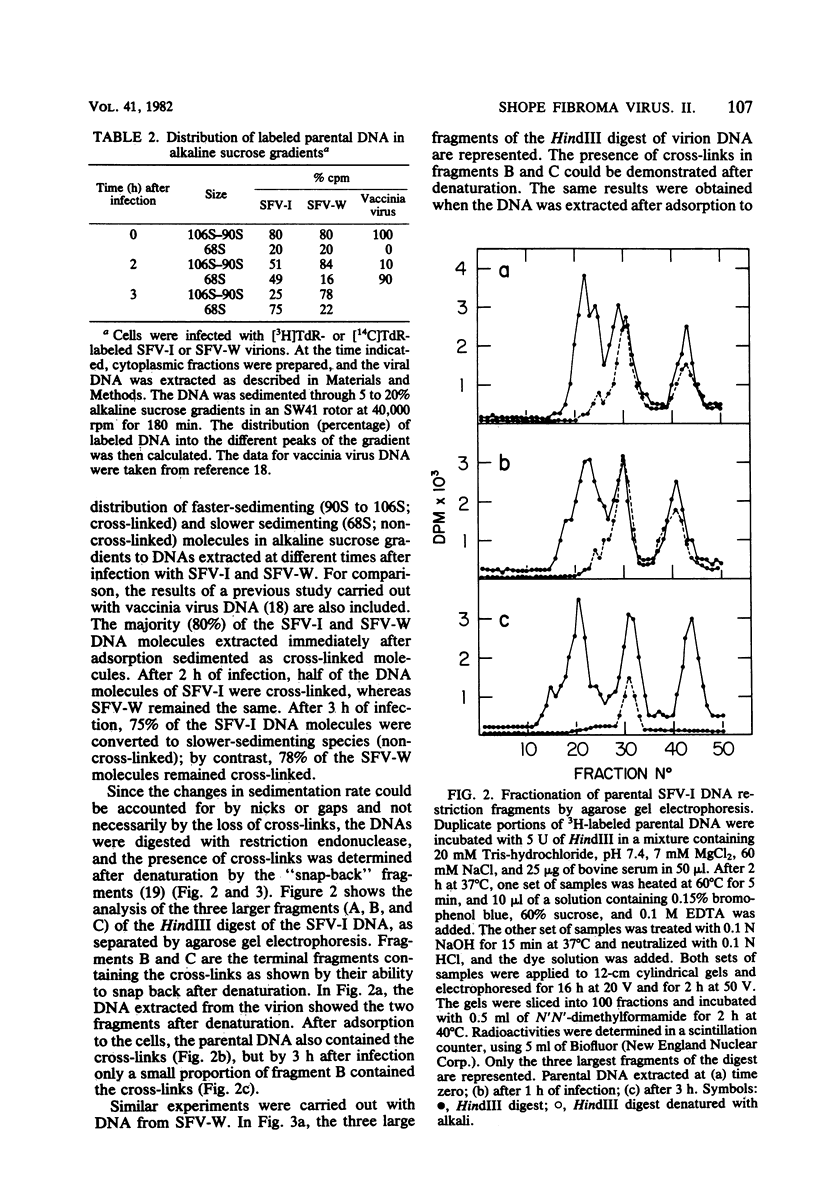
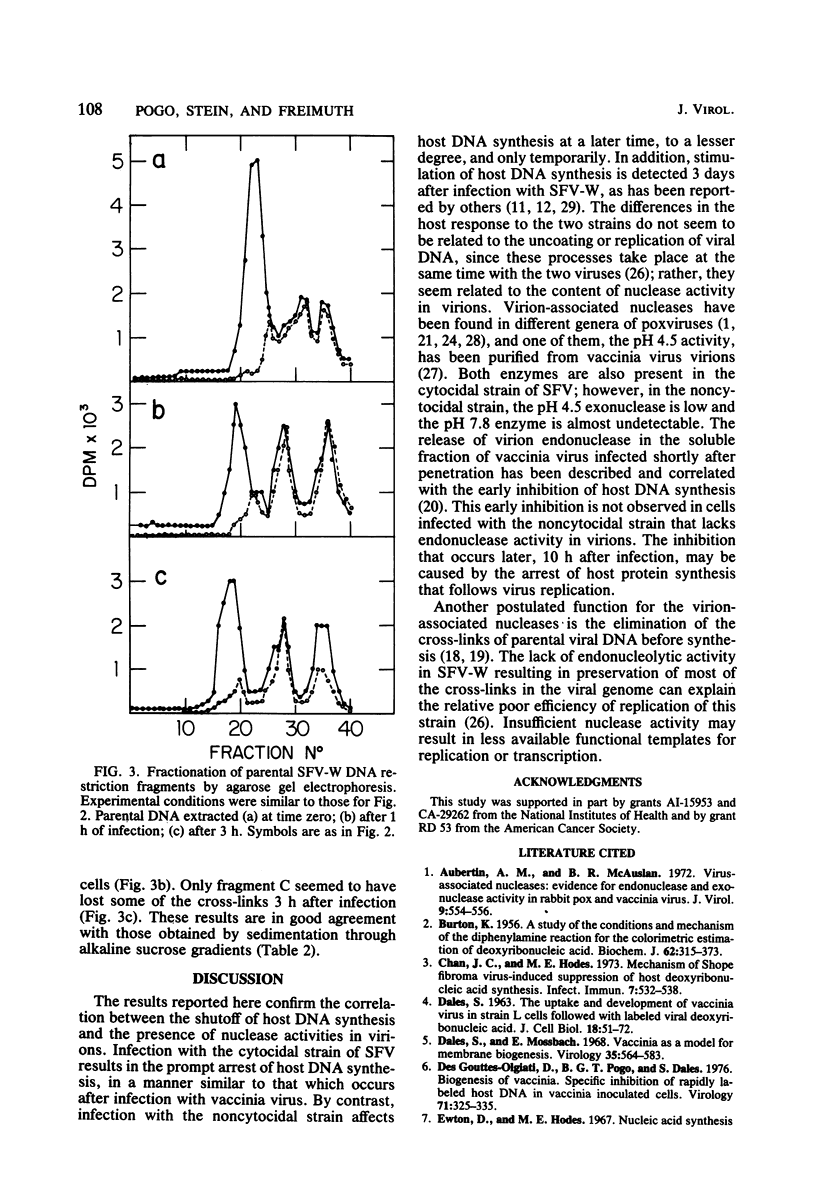
The effect of Shope fibroma virus (SFV) infection on host DNA synthesis was investigated. The cytocidal strain, SFV-I, inhibited the incorporation of [3H]thymidine into nuclear DNA very shortly (2 h) after infection, whereas the noncytocidal strain, SFV-W, did so later (10 h postinfection) and to a lesser extent. Furthermore, a two- to threefold stimulation of host DNA synthesis was recorded in SFV-W-infected cells 3 to 4 h after infection. Since virion-associated nucleases have been implicated in the shutoff of host synthesis, these and other enzymatic activities were measured in purified virion preparations. The SFV strains and vaccinia virus contained equivalent amounts of DNA-dependent RNA polymerase, ATPase, and protein kinase activities. However, in SFV-W the pH 4.5 exonuclease activity was lower than in SFV-I and vaccinia virus, and the level of pH 7.8 endonuclease was almost undetectable. To test whether the lack of endonucleolytic activity had some effect on the removal of the cross-links in the parental DNA that occurs after viral penetration, the fate of the virion SFV DNA was followed. The majority (80%) of the SFV-I and SFV-W DNA molecules extracted after viral adsorption sedimented in alkaline sucrose gradients as cross-linked. After 3 h of infection, 75% of the SFV-I DNA molecules lacked cross-links, whereas 78% of the SFV-W DNA still remained cross-linked. The same results were obtained when the presence of cross-links was tested in restriction fragments. Taken together, these results indicate that virion-associated nucleases are involved in the early shutoff of host DNA synthesis and in the elimination of cross-links from the parental viral DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. C., Hodes M. E. Mechanism of Shope fibroma virus-induced suppression of host deoxyribonucleic acid synthesis. Infect Immun. 1973 Apr;7(4):532–538. doi: 10.1128/iai.7.4.532-538.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. RESPONSE OF CULTURED RABBIT CELLS TO INFECTION WITH THE SHOPE FIBROMA VIRUS. I. PROLIFERATION AND MORPHOLOGICAL ALTERATION OF THE INFECTED CELLS. J Bacteriol. 1964 Oct;88:1185–1194. doi: 10.1128/jb.88.4.1185-1194.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jacquemont B., Gazzolo L. Existence d'une synth'ese de DNA cellulaire liée à l'infection virale dans les cellules infectées par le virus du fibrome de Shope. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jul 12;273(2):253–256. [PubMed] [Google Scholar]
- Jungwirth C., Launer J. Effect of poxvirus infection on host cell deoxyribonucleic acid synthesis. J Virol. 1968 May;2(5):401–408. doi: 10.1128/jvi.2.5.401-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Pogo B. G. Changes in parental vaccinia virus DNA after viral penetration into cells. Virology. 1980 Mar;101(2):520–524. doi: 10.1016/0042-6822(80)90466-3. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S., Bergoin M., Roberts D. W. Enzymes associated with an insect poxvirus. Virology. 1971 Jan;43(1):306–309. doi: 10.1016/0042-6822(71)90249-2. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of poxviruses: further evidence for inhibition of host and virus DNA synthesis by a component of the invading inoculum particle. Virology. 1974 Apr;58(2):377–386. doi: 10.1016/0042-6822(74)90073-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of poxviruses: inactivation of host DNA polymerase by a component of the invading inoculum particle. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1726–1729. doi: 10.1073/pnas.70.6.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Regulation of the synthesis of nucleotide phosphohydrolase and neutral deoxyribonuclease: two activities present within purified vaccina virus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1297–1303. doi: 10.1073/pnas.63.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Elimination of naturally occurring crosslinks in vaccinia virus DNA after viral penetration into cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1739–1742. doi: 10.1073/pnas.74.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Freimuth P., Stein A. Shope fibroma virus. I. Biological and molecular properties of a cytocidal and a noncytocidal strain. J Virol. 1982 Jan;41(1):97–103. doi: 10.1128/jvi.41.1.97-103.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., O'shea M. T. Further characterization of deoxyribonucleases from vaccinia virus. Virology. 1977 Mar;77(1):56–66. doi: 10.1016/0042-6822(77)90405-6. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- Schwartz J., Dales S. Biogenesis of poxviruses: identification of four enzyme activities within purified Yaba tumor virus. Virology. 1971 Sep;45(3):797–801. doi: 10.1016/0042-6822(71)90198-x. [DOI] [PubMed] [Google Scholar]
- Tompkins W. A., Walker D. L., Hinze H. C. Cellular deoxyribonucleic acid synthesis and loss of contact inhibition in irradiated and contact-inhibited cell cultures infected with fibroma virus. J Virol. 1969 Nov;4(5):603–609. doi: 10.1128/jvi.4.5.603-609.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Gouttes Olgiati D., Pogo B. G., Dales S. Biogenesis of vaccinia: specific inhibition of rapidly labeled host DNA in vaccinia inoculated cells. Virology. 1976 May;71(1):325–335. doi: 10.1016/0042-6822(76)90116-1. [DOI] [PubMed] [Google Scholar]


