Abstract
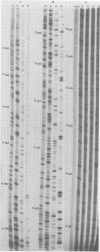
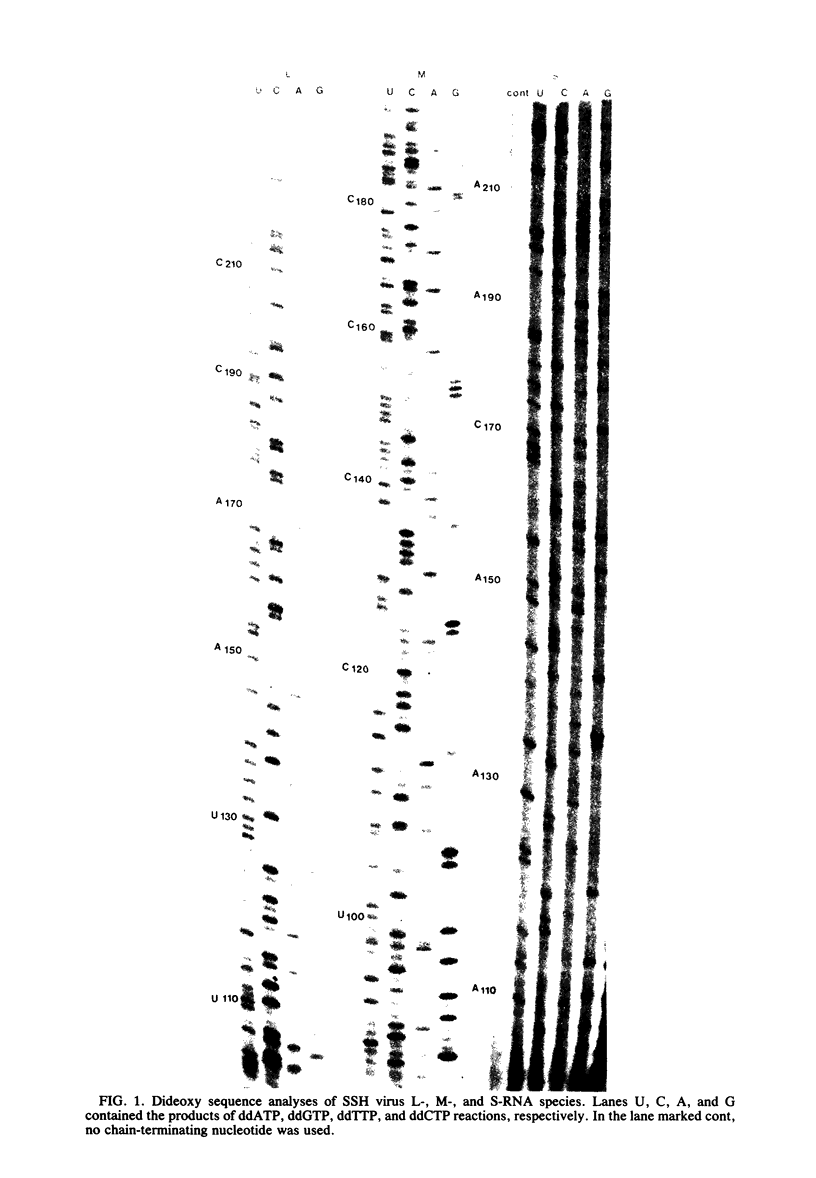
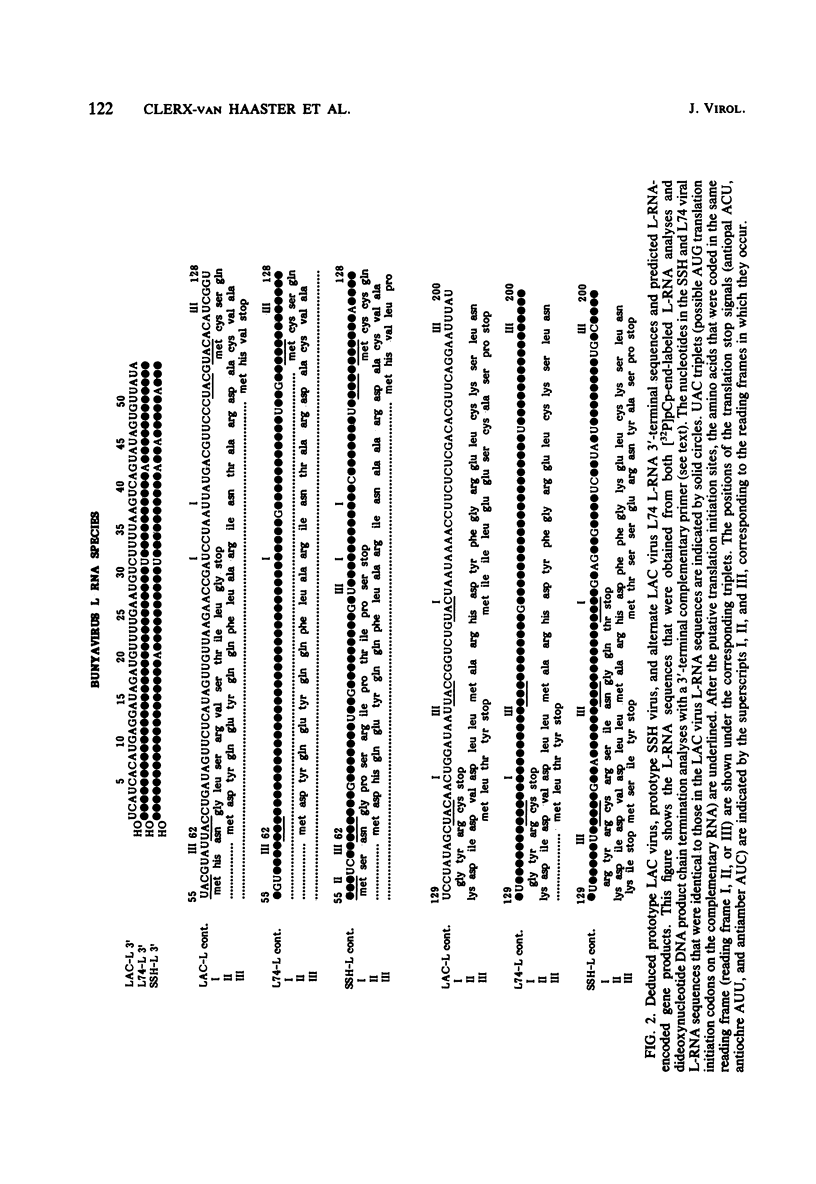
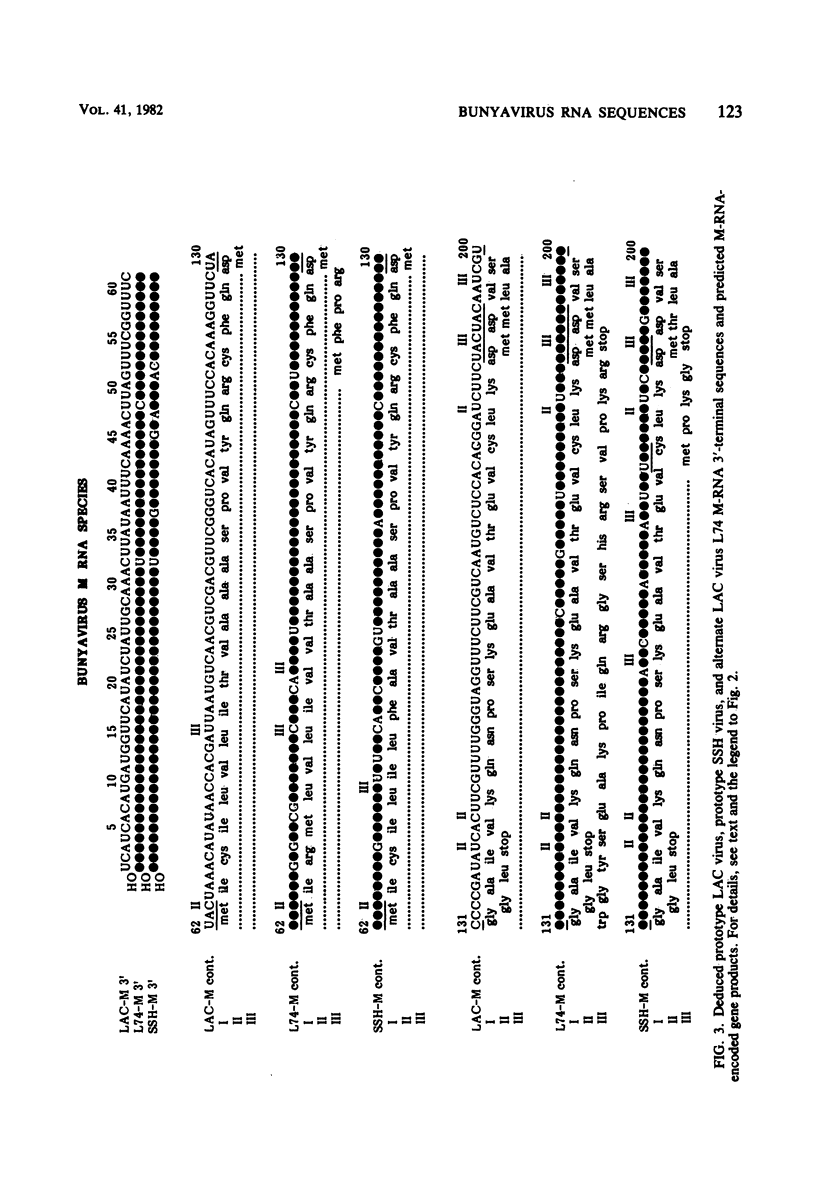
We performed 3′ RNA sequence analyses of [32P]pCp-end-labeled La Crosse (LAC) virus, alternate LAC virus isolate L74, and snowshoe hare bunyavirus large (L), medium (M), and small (S) negative-stranded viral RNA species to determine the coding capabilities of these species. These analyses were confirmed by dideoxy primer extension studies in which we used a synthetic oligodeoxynucleotide primer complementary to the conserved 3′-terminal decanucleotide of the three viral RNA species (Clerx-van Haaster and Bishop, Virology 105:564-574, 1980). The deduced sequences predicted translation of two S-RNA gene products that were read in overlapping reading frames. So far, only single contiguous open reading frames have been identified for the viral M- and L-RNA species. For the negative-stranded M-RNA species of all three viruses, the single reading frame developed from the first 3′-proximal UAC triplet. Likewise, for the L-RNA of the alternate LAC isolate, a single open reading frame developed from the first 3′-proximal UAC triplet. The corresponding L-RNA sequences of prototype LAC and snowshoe hare viruses initiated open reading frames; however, for both viral L-RNA species there was a preceding 3′-proximal UAC triplet in another reading frame that was followed shortly afterward by a termination codon. A comparison of the sequence data obtained for snowshoe hare virus, LAC virus, and the alternate LAC virus isolate showed that the identified nucleotide substitutions were sufficient to account for some of the fingerprint differences in the L-, M-, and S-RNA species of the three viruses. Unlike the distribution of the L- and M-RNA substitutions, significantly fewer nucleotide substitutions occurred after the initial UAC triplet of the S-RNA species than before this triplet, implying that the overlapping genes of the S RNA provided a constraint against evolution by point mutation. The comparative sequence analyses predicted amino acid differences among the corresponding L-, M-, and S-RNA gene products of snowshoe hare virus and the two LAC virus isolates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Cash P., Vezza A. C., Gentsch J. R., Bishop D. H. Genome complexities of the three mRNA species of snowshoe hare bunyavirus and in vitro translation of S mRNA to viral N polypeptide. J Virol. 1979 Sep;31(3):685–694. doi: 10.1128/jvi.31.3.685-694.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerx J. P., Bishop D. H. Qalyub virus, a member of the newly proposed Nairovirus genus (Bunyavividae). Virology. 1981 Jan 30;108(2):361–372. doi: 10.1016/0042-6822(81)90444-x. [DOI] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Said L. H., Vorndam V., Gentsch J. R., Clewley J. P., Calisher C. H., Klimas R. A., Thompson W. H., Grayson M., Trent D. W., Bishop D. H. A comparison of La Crosse virus isolated obtained from different ecological niches and an analysis of the structural components of California encephalitis serogroup viruses and other bunyaviruses. Am J Trop Med Hyg. 1979 Mar;28(2):364–386. doi: 10.4269/ajtmh.1979.28.364. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. H. Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol. 1978 Oct;28(1):417–419. doi: 10.1128/jvi.28.1.417-419.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. L. M viral RNA segment of bunyaviruses codes for two glycoproteins, G1 and G2. J Virol. 1979 Jun;30(3):767–770. doi: 10.1128/jvi.30.3.767-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Bishop D. H. Recombination and complementation between temperature-sensitive mutants of a Bunyavirus, snowshoe hare virus. J Virol. 1976 Oct;20(1):351–354. doi: 10.1128/jvi.20.1.351-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaster C. C., Bishop D. H. Analyses of the 3'-terminal sequences of snowshoe hare and La Crosse Bunyaviruses. Virology. 1980 Sep;105(2):564–574. doi: 10.1016/0042-6822(80)90056-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976 Dec;20(3):664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., McCauley J., Skehel J. J. Nucleotide sequences at the terminal of La Crosse virus RNAs. Nucleic Acids Res. 1980 Jun 11;8(11):2431–2438. doi: 10.1093/nar/8.11.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon E. J., Gensemer P., Shope R. E., Bishop D. H. Attenuation of virulence of a bunyavirus involving an L RNA defect and isolation of LAC/SSH/LAC and LAC/SSH/SSH reassortants. Virology. 1981 May;111(1):125–138. doi: 10.1016/0042-6822(81)90659-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H., Clerx-van Haaster M., Bishop D. H. Analyses of Patois group Bunyaviruses: evidence for naturally occurring recombinant Bunyaviruses and existence of immune precipitable and nonprecipitable nonvirion proteins induced in Burnyavirus-infected cells. Virology. 1981 Apr 30;110(2):318–332. [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]