Abstract
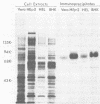
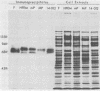
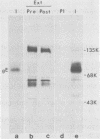
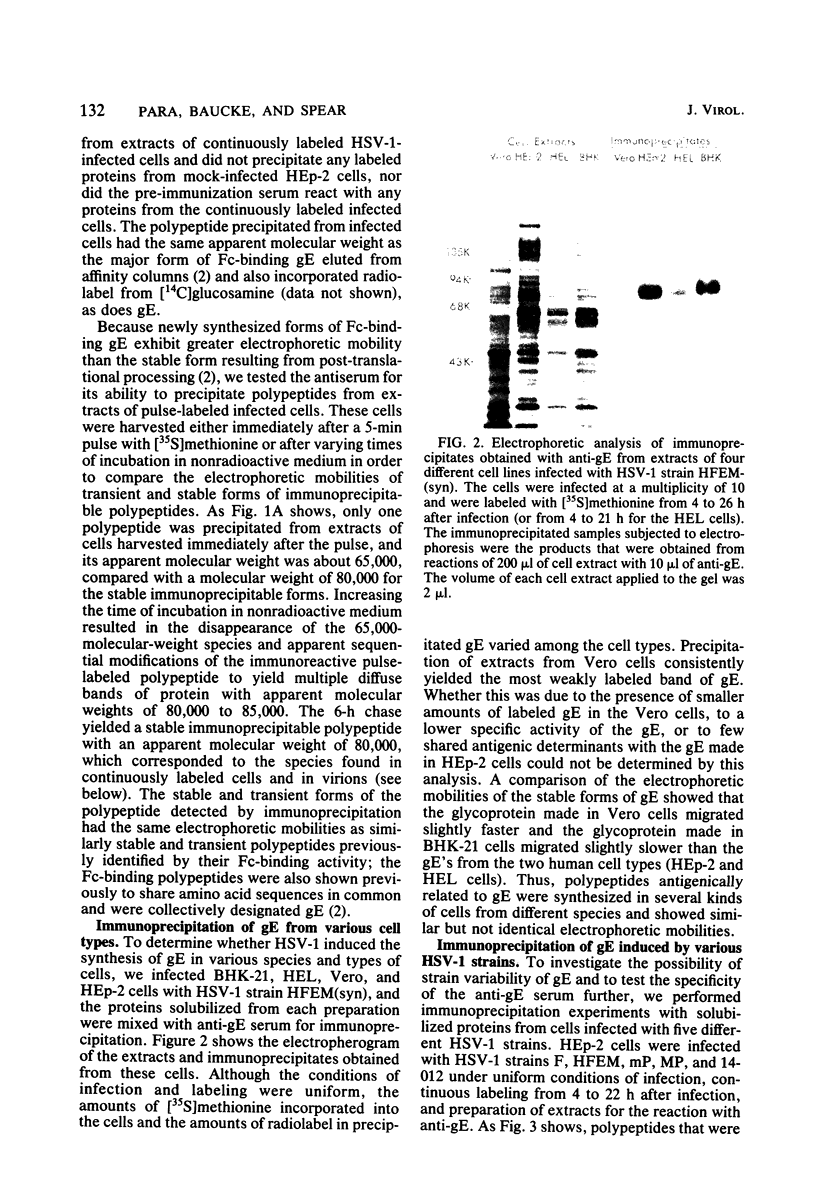
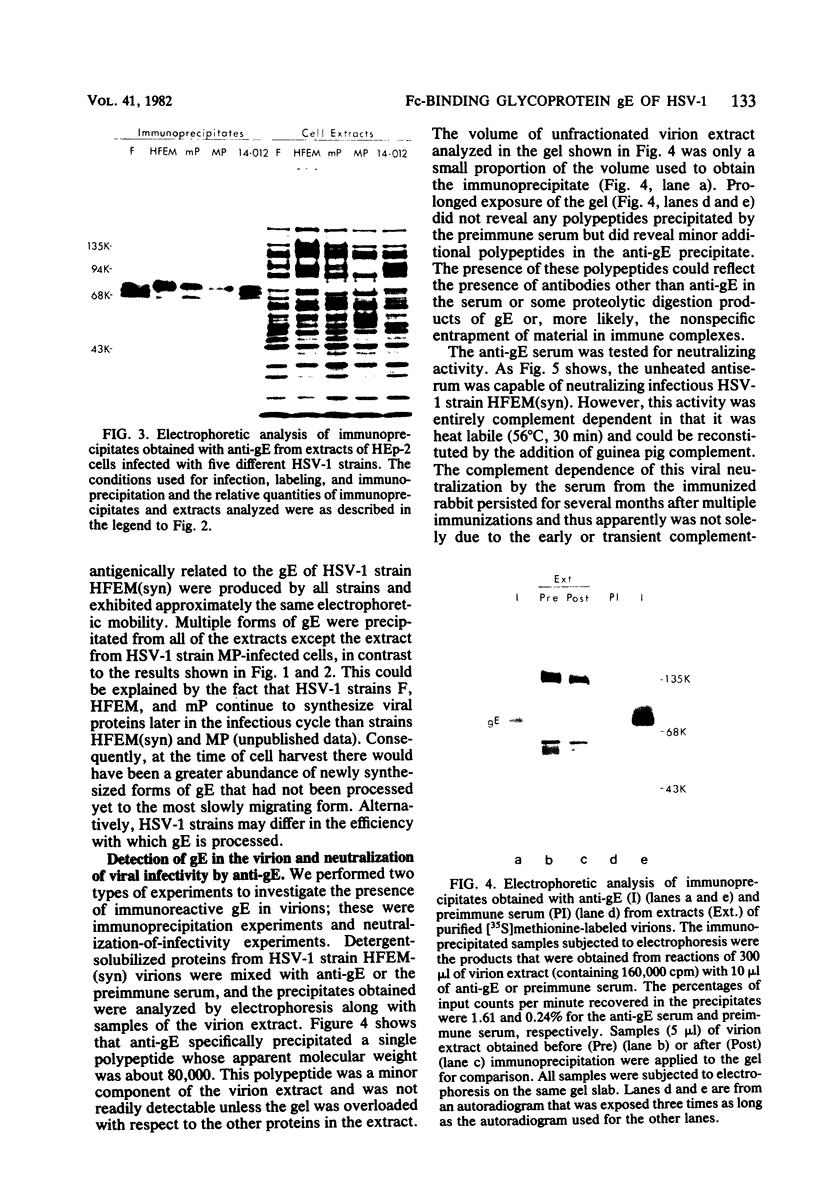
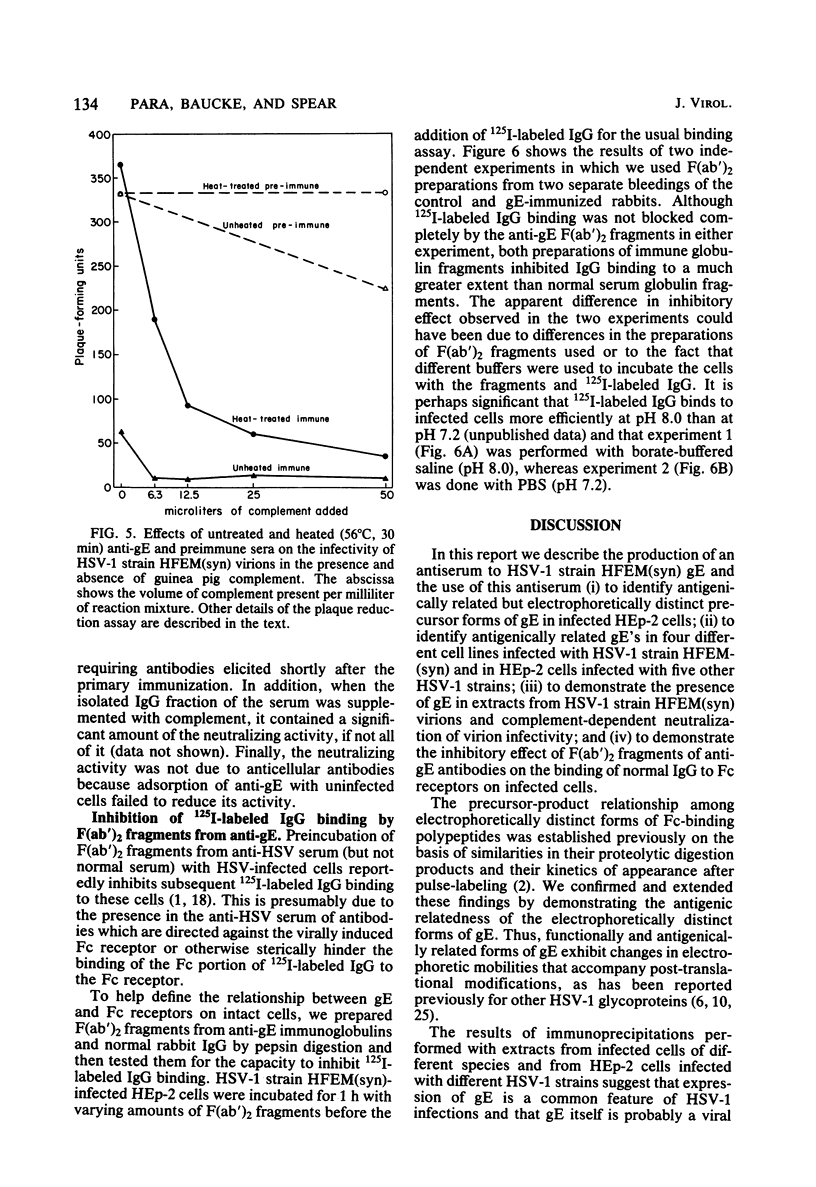
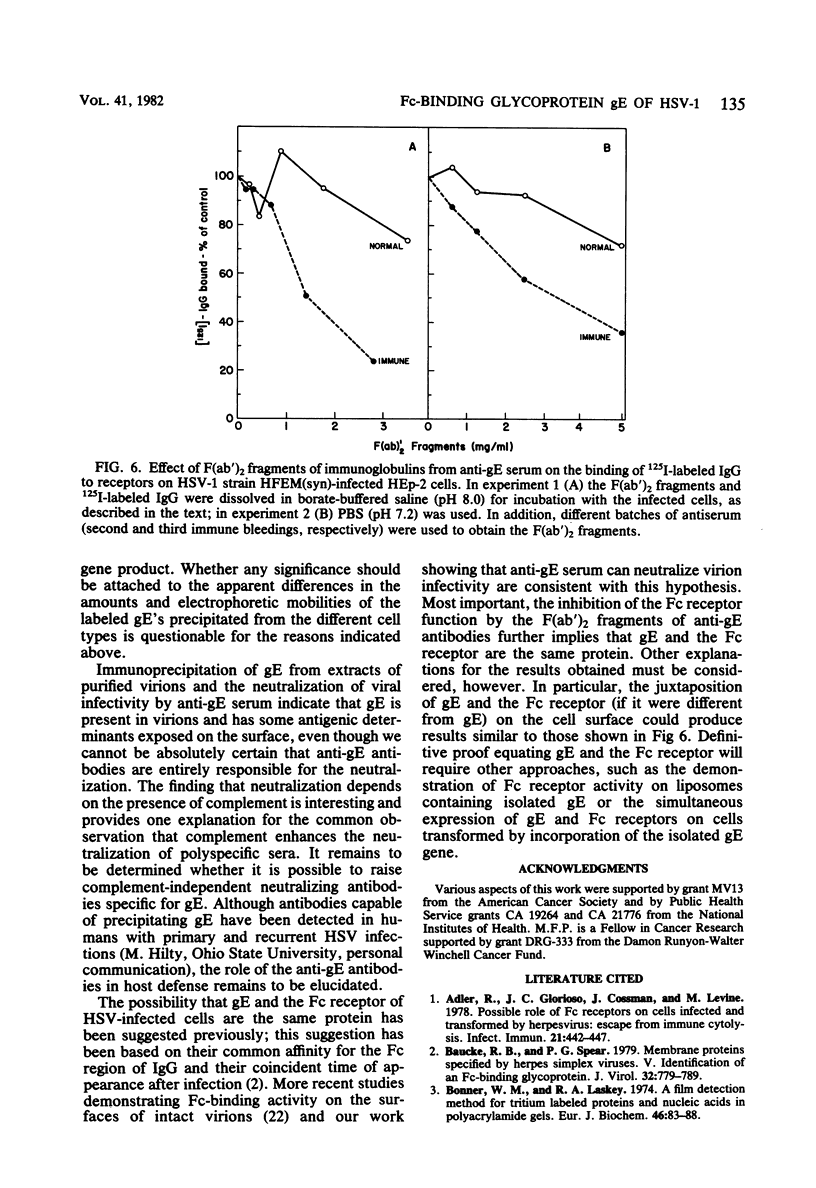
An Fc-binding glycoprotein, designated gE, was detected previously in cells infected with herpes simplex virus type 1 (HSV-1) and in virion preparations isolated from infected cells. For the studies reported here, we purified gE from HSV-1 strain HFEM(syn) by affinity chromatography and preparative electrophoresis and then immunized a rabbit to produce an antiserum to glycoprotein gE. We found that this antiserum selectively precipitated gE and its precursors from detergent-solubilized extracts of HSV-1 strain HFEM(syn)-infected HEp-2 cells, from extracts of other cell lines infected with the same virus, and from extracts of HEp-2 cells infected with several other HSV-1 strains. The antiserum did not precipitate any proteins from uninfected cells. The several forms of gE detected by immunoprecipitation accumulated in variable quantities in different cells infected with the different virus strains and also varied slightly with respect to electrophoretic mobility, suggesting some differences in the gE's from different HSV-1 strains and some effects of the host cell on the nature and extent of post-translational processing. One of the electrophoretic forms of gE previously detected in purified preparations of virions could be precipitated by anti-gE from extracts of purified HSV-1 strain HFEM(syn) virions. Moreover, anti-gE neutralized HSV-1 infectivity, but only in the presence of complement. Finally, F(ab')2 fragments of the anti-gE immunoglobulin partially inhibited the binding of 125I-labeled immunoglobulin G to the Fc receptors on HSV-1-infected cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Yee C., Nakamura Y., Rabson A. Characteristics of the Fc receptor induced by herpes simplex virus. Intervirology. 1978;10(1):32–39. doi: 10.1159/000148965. [DOI] [PubMed] [Google Scholar]
- Dorval G., Bourkas A. E., Menezes J. Comparative studies on the expression of Fc-receptors, Ia antigens, and beta 2-microglobulin following in vitro herpes simplex virus infection of human lymphoid cells. Clin Exp Immunol. 1979 Sep;37(3):477–485. [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Shore S. L., Reimer C. B. Detection by indirect immunofluorescence of Fc receptors in cells acutely infected with Herpes simplex virus. Int Arch Allergy Appl Immunol. 1977;53(3):222–233. doi: 10.1159/000231756. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Peitchel R., Goldman J. N., Goldman M. An IgG-Fc receptor induced in cytomegalovirus-infected human fibroblasts. J Immunol. 1976 Mar;116(3):772–777. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- McTaggart S. P., Burns W. H., White D. O., Jackson D. C. Fc receptors induced by herpes simplex virus. I. Biologic and biochemical properties. J Immunol. 1978 Aug;121(2):726–730. [PubMed] [Google Scholar]
- NISONOFF A. ENZYMATIC DIGESTION OF RABBIT GAMMA GLOBULIN AND ANTIBODY AND CHROMATOGRAPHY OF DIGESTION PRODUCTS. Methods Med Res. 1964;10:134–141. [PubMed] [Google Scholar]
- Nakamura Y., Costa J., Tralka T. S., Yee C. L., Rabson A. S. Properties of the cell surface Fc-receptor induced by herpes simplex virus. J Immunol. 1978 Sep;121(3):1128–1131. [PubMed] [Google Scholar]
- Ogata M., Shigeta S. Appearance of immunoglobulin G Fc receptor in cultured human cells infected with varicella-zoster virus. Infect Immun. 1979 Nov;26(2):770–774. doi: 10.1128/iai.26.2.770-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Studies of the determinant antigens of viable cells. I. A method, and its application in tissue culture studies, for enumeration of killed cells, based on the failure of virus multiplication following injury by cytotoxic antibody and complement. J Immunol. 1961 Dec;87:714–727. [PubMed] [Google Scholar]
- Rahman A. A., Teschner M., Sethi K. K., Brandis H. Appearance of IgG (Fc) receptor(s) on cultured human fibroblasts infected with human cytomegalovirus. J Immunol. 1976 Jul;117(1):253–258. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J. F. ADSORPTION OF SENSITIZED SHEEP ERYTHROCYTES TO HELA CELLS INFECTED WITH HERPES SIMPLEX VIRUS. Nature. 1964 Jun 27;202:1364–1365. doi: 10.1038/2021364a0. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., St Jeor S., Rapp F. The development by cytomegalovirus-infected cells of binding affinity for normal human immunoglobulin. J Immunol. 1976 Jun;116(6):1566–1570. [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F. The IgG receptor induced by herpes simplex virus: studies using radioiodinated IgG. J Gen Virol. 1974 Jul;24(1):167–178. doi: 10.1099/0022-1317-24-1-167. [DOI] [PubMed] [Google Scholar]
- Yasuda J., Milgrom F. Hemadsorption by herpes simplex-infected cell cultures. Int Arch Allergy Appl Immunol. 1968;33(2):151–170. doi: 10.1159/000229985. [DOI] [PubMed] [Google Scholar]