Abstract
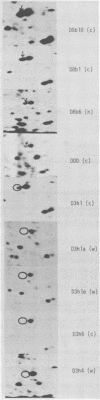
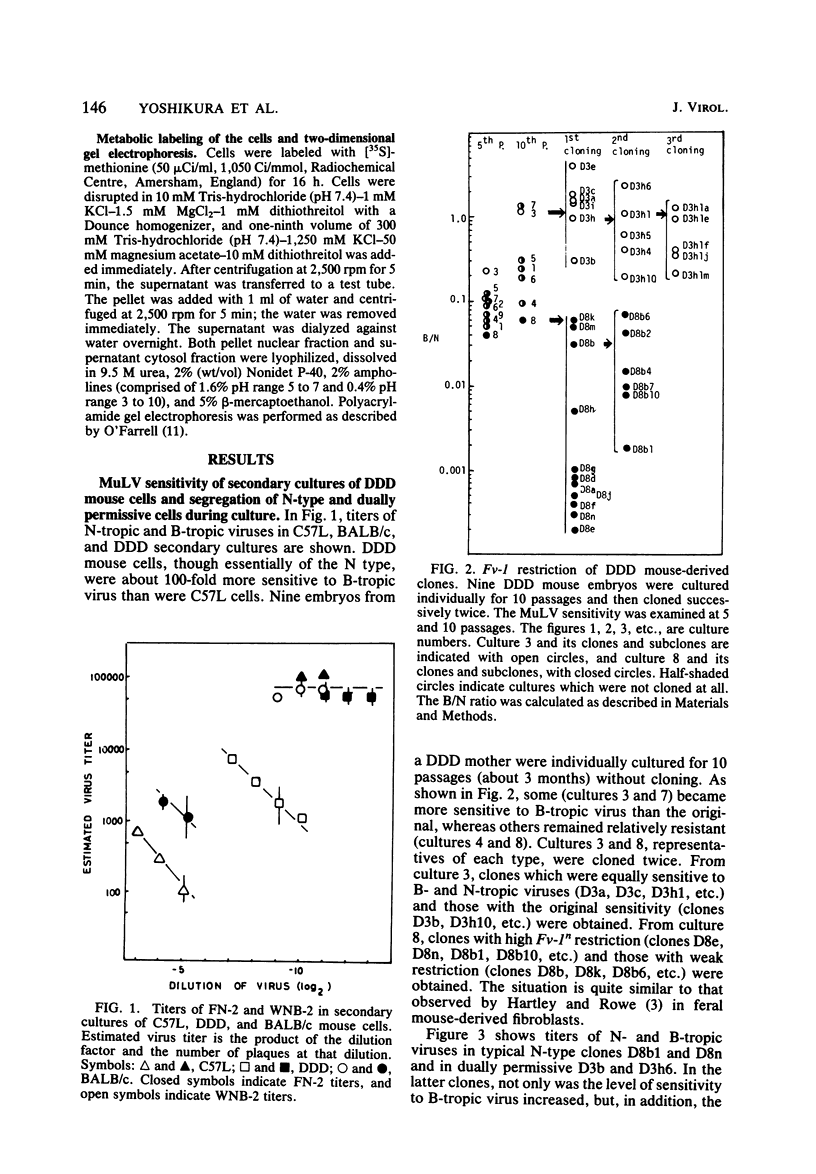
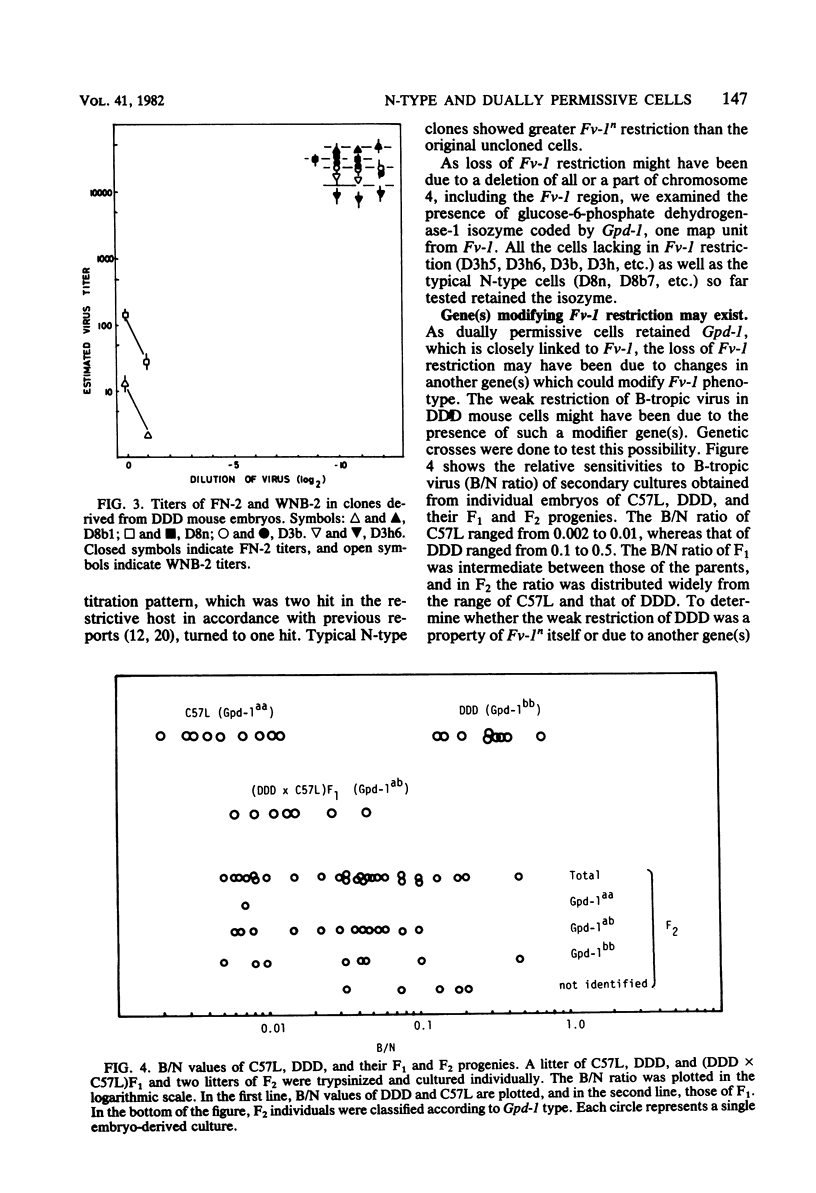
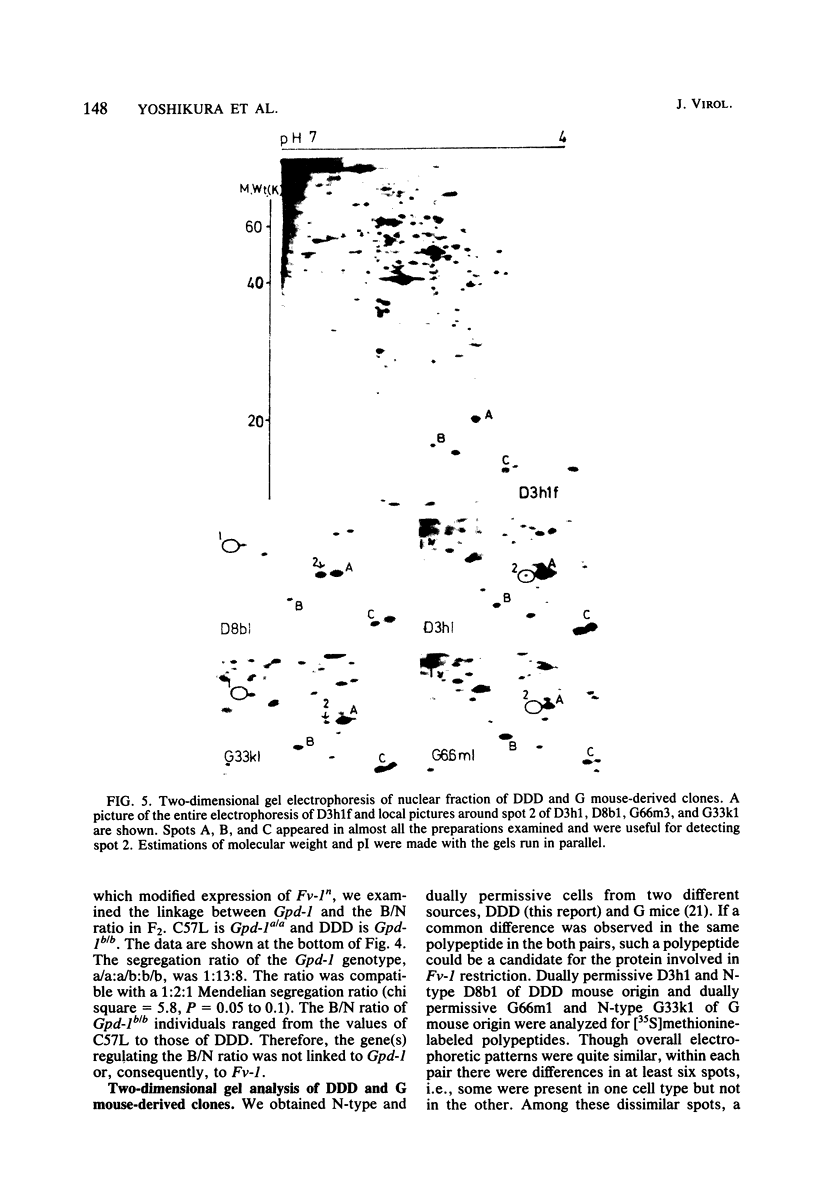
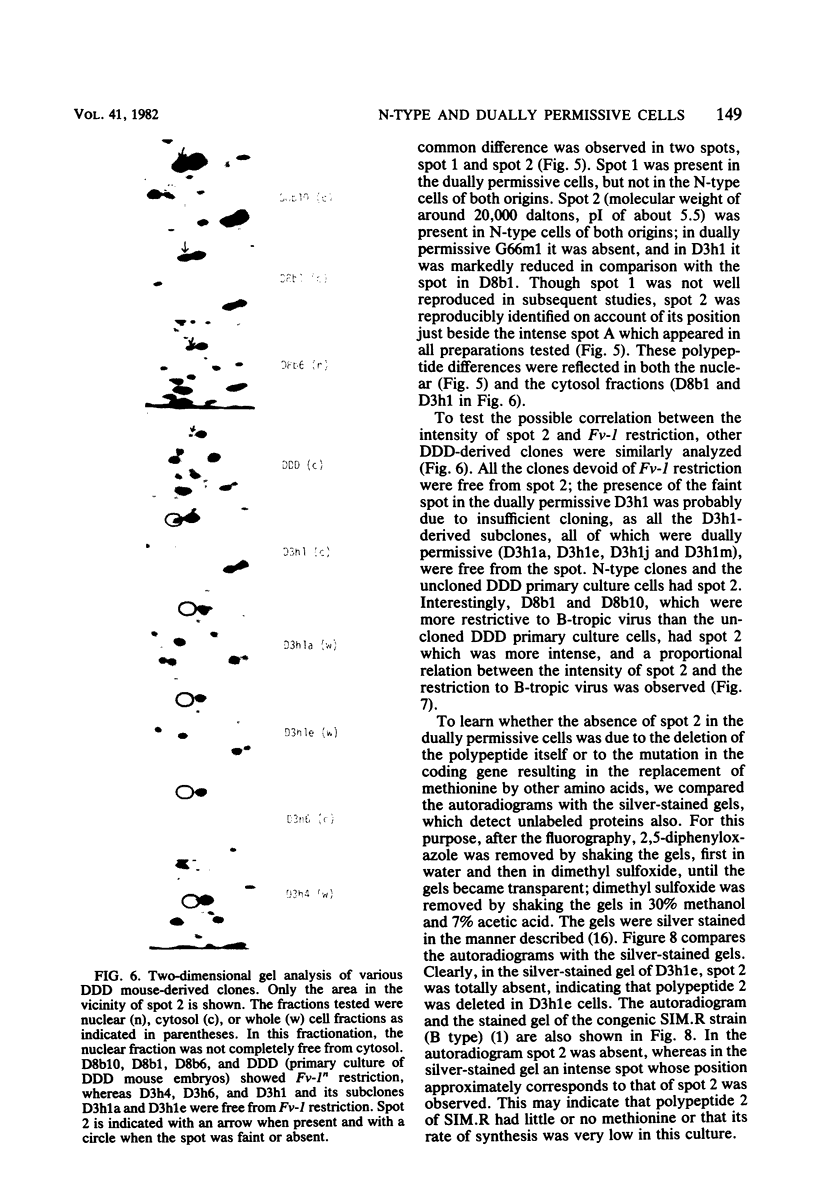
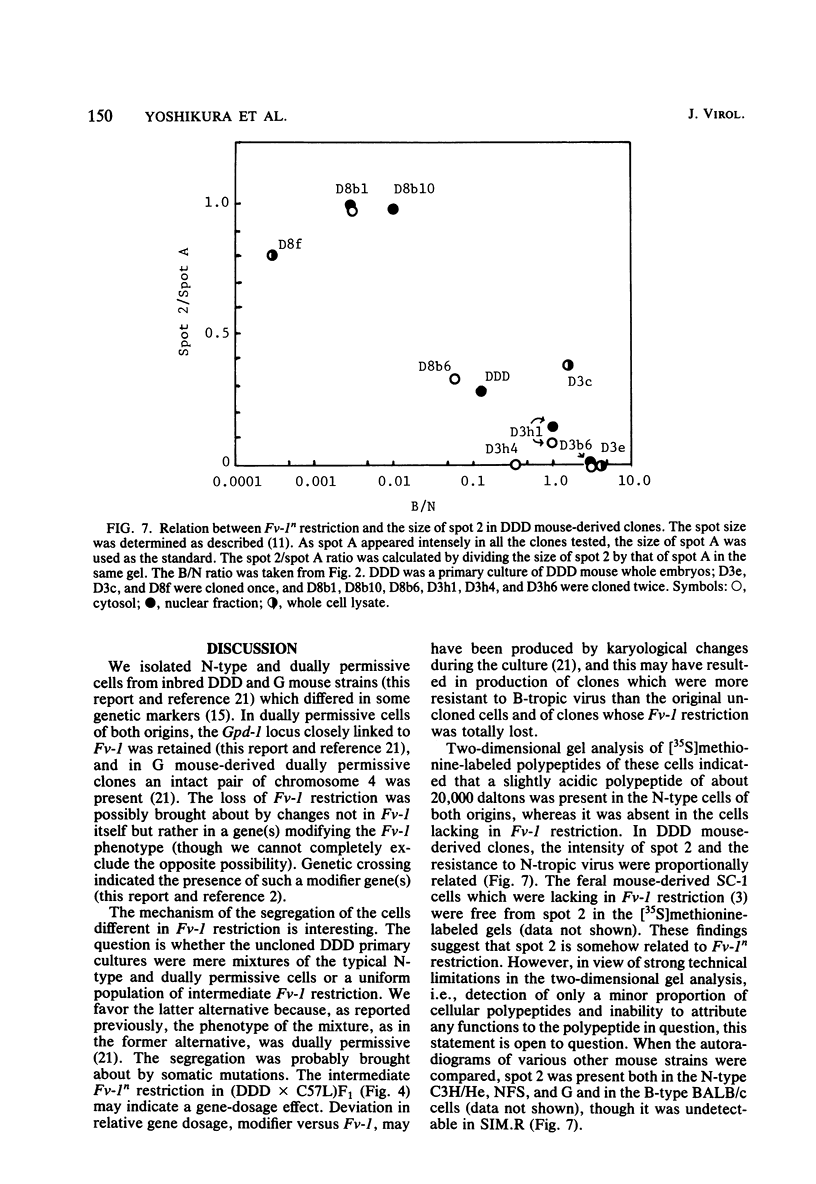
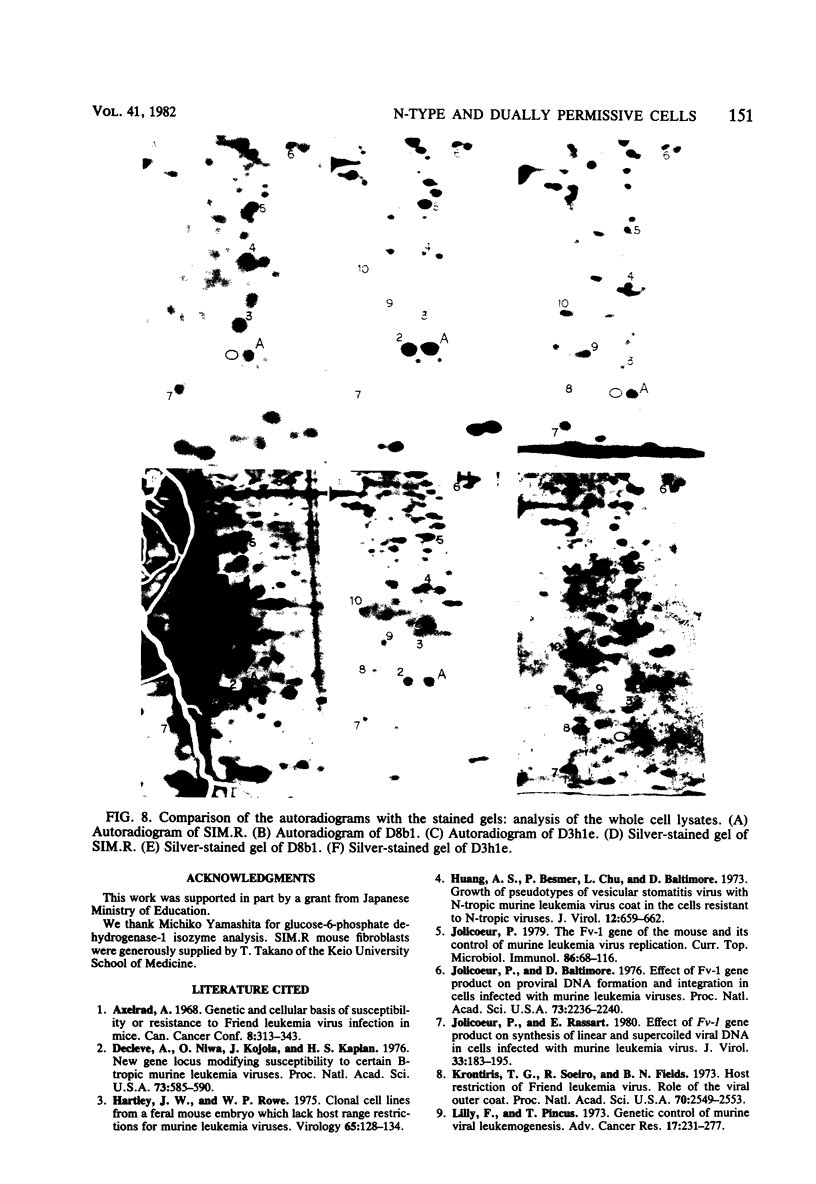
Though the inbred DDD mouse strain is essentially of the N type, the primary culture of this strain was about 100-fold more sensitive to B-tropic WN1802B virus than were the typical N-type strains (C3H/He, C57L, etc.). After cloning, DDD mouse cells segregated two types of cells, typical N-type cells and cells lacking in Fv-1 restriction. As both types of cells so far tested retained glucose-6-phosphatase-1 coded by a locus closely linked to Fv-1 and genetic cross experiments indicated the presence of a gene(s) modifying the Fv-1 phenotype, variation in Fv-1 restriction could presumably be brought about by genetic changes in a gene(s) other than Fv-1 itself. N-type and dually permissive cell clones were similarly established from the inbred G mouse. Compositions of polypeptides labeled with [35S]methionine in the N-type and dually permissive cells of DDD and G mouse origins were compared by two-dimensional gel electrophoresis. The polypeptide maps of these cells were similar except for a few spots. Among these dissimilar spots, a spot of about 20,000 daltons with a pI of about 5.5 was always present in N-type cells, whereas it was absent in dually permissive cells. In DDD mouse-derived clones, a proportional relation was observed between the intensity of the spot and the restriction to the B-tropic virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. Genetic and cellular basis of susceptibility or resistance to Friend leukemia virus infection in mice. Proc Can Cancer Conf. 1969;8:313–343. [PubMed] [Google Scholar]
- Declève A., Niwa O., Kojola J., Kaplan H. S. New gene locus modifying susceptibility to certain B-tropic murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Feb;73(2):585–590. doi: 10.1073/pnas.73.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Sato H. Genetic mapping of the Fv-1 lcous of the mouse. Science. 1973 May 11;180(4086):640–641. doi: 10.1126/science.180.4086.640. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Matsubara S. Isolation of Friend leukemia virus resistant line from non-inbred mouse colony. Jpn J Exp Med. 1975 Dec;45(6):467–471. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Schluter B., Myer F. E., Otten J. A., Yang W. K., Brown A. Genetic evidence for a product of the Fv-1 locus that transfers resistance to mouse leukemia viruses. J Virol. 1976 Dec;20(3):589–596. doi: 10.1128/jvi.20.3.589-596.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. Host range conversion of the murine sarcoma-leukaemia complex. J Gen Virol. 1973 Jun;19(3):321–327. doi: 10.1099/0022-1317-19-3-321. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Naito Y., Moriwaki K. Unstable resistance of G mouse fibroblasts to ecotropic murine leukemia virus infection. J Virol. 1979 Mar;29(3):1078–1086. doi: 10.1128/jvi.29.3.1078-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. Ultraviolet inactivation of murine leukemia virus and its assay in permissive and non-permissive cells. Int J Cancer. 1973 May;11(3):739–746. doi: 10.1002/ijc.2910110325. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Yoshida M. Intracellular restriction of ecotropic murine leukemia virus in rat NRK cells and its abolishment by adaptation. J Virol. 1978 Sep;27(3):612–618. doi: 10.1128/jvi.27.3.612-618.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]