Abstract
The brahma gene encodes the catalytic subunit of the Drosophila melanogaster BRM chromatin-remodeling complexes. Screening for mutations that interact with brahma, we isolated the dominant-negative Pearl-2 allele of γTub23C. γTub23C encodes one of the two γ-tubulin isoforms in Drosophila and is essential for zygotic viability and normal adult patterning. γ-Tubulin is a subunit of microtubule organizer complexes. We show that mutations in lethal (1) discs degenerate 4, which encodes the Grip91 subunit of microtubule organizer complexes, suppress the recessive lethality and the imaginal phenotypes caused by γTub23C mutations. The genetic interactions between γTub23C and chromatin-remodeling mutations suggest that γ-tubulin might have a role in regulating gene expression.
THE trithorax and Polycomb group genes encode positive and negative factors required for the proper function of homeotic genes. Kennison and Tamkun (1988) identified brahma (brm) as a trithorax group gene required for the maintenance of homeotic gene expression, but brm also regulates the expression of many developmental regulators and facilitates global transcription from RNA polymerase II (Armstrong et al. 2002). The Brm protein is a SWI2/SNF2 family ATPase and is the catalytic subunit of BRM chromatin-remodeling complexes. These complexes modify nucleosome structure; they can also act to generate Z-DNA structures (reviewed in Flaus and Owen-Hughes 2004).
Drosophila BRM complexes and related mouse and human SWI/SNF complexes have roles in a variety of processes, including cell proliferation, differentiation, viral infection, and cancer (reviewed by Roberts and Orkin 2004). Targeting of the BRM complexes for transcriptional regulation involves contact with members of the basal transcription machinery and gene-specific transcriptional activators (for examples, see Sharma et al. 2003; Armstrong et al. 2005). To identify proteins that are required for proper function of homeotic genes, we screened for mutations that showed genetic interactions with brm mutations to cause a held-out-wings phenotype. This approach allowed us to isolate mutations in the trithorax group genes osa, tonalli, and taranis (Vázquez et al. 1999; Gutiérrez et al. 2003). In this work, we describe the characterization of another mutation isolated in this genetic screen, the Pearl-2 allele of γTub23C. Some γTub23C mutant phenotypes are modified (enhanced or suppressed) by mutations in genes encoding subunits of the BRM complexes and by mutations in Grip91, a γ-tubulin ring complex subunit. These data suggest a role for γ-tubulin in transcription.
MATERIALS AND METHODS
Fly strains:
Flies were raised at 25° on a yeast–sucrose–agar medium with either Nipagin or propionic acid or on a cornmeal–molasses–yeast–agar medium with Tegosept. Unless otherwise noted, all mutations and chromosome aberrations are described in Lindsley and Zimm (1992). Mutant stocks carrying l(1)dd4G0122, l(1)dd42, l(2)23CeA6-2, l(2)23CeA14-9, l(2)23CeA15-2, and γTub23Cbmps1 were provided by the Bloomington stock center.
Mutant phenotypes:
The viability (in percentage) of homozygous or heteroallelic combinations of alleles was determined by dividing the observed number of flies by the expected number and multiplying by 100%. The expected numbers were calculated by counting the numbers of progeny in the crosses that received the balancer chromosomes and dividing by half.
Genetic mapping:
The γTub23CPl-2 mutant was first mapped meiotically between the visible markers al and dp.
The dd4su(Pl) mutation was first mapped by meiotic recombination using visible markers. Individual recombinant sons from females heterozygous for dd4su(Pl) and a y2 wa ct6 g2 f mutant chromosome were recovered and tested for the survival of γTub23Cbmps1/γTub23CA6-2 trans-heterozygotes. After the initial mapping, 28 recombinants between ct6 and g2 and 13 recombinants between g2 and f were recovered and tested. None of these recombinants separated the suppressor from g+.
P-induced male recombination mapping of γTub23CPl-2:
Females with the P-element insertions shown in Table 1 were crossed with males of the genotype al γTub23CPl-2 KrIf/+; TMS, P{ry+t7.2=Delta2-3}99B/+. Sons that were P{X}/al γTub23CPl-2 KrIf; TMS/+ were crossed to al dp b pr c px sp females and the progeny scored for recombinants between al and KrIf. Recombinants were recovered and balanced for further testing. To determine which recombinants carried flanking deletions that removed essential genes, each recombinant chromosome was crossed to deletions in 23CD and to known mutants in 23C [lilli, l(2)23Cb, l(2)23Cd, γTub23CA6-2, and okra]. Although l(2)23Cb has been renamed l(2)23Dd by FlyBase, our deletion mapping places the gene between Rpb9 and γTub23C, consistent with the original mapping to 23C and the original gene name.
TABLE 1.
P-induced male recombination
| P-transposon insertion | Polytene location | No. of recombinants |
|---|---|---|
| P{lacW}lilli05431 | 23C1-3 | 4 |
| P{SUPor-P}Rrp1KG01159 | 23C3 | 9 |
| P{EP}Rrp11020 | 23C3-4 | 3 |
| P{EPgy2} CG9643EY07345 | 23C4 | 31 |
| P{SUPor-P}CG3558KG02323 | 23C4 | 7 |
| P{lacW}v(2)k05816 | 23C5 | 3 |
| P{PZ}toc01361 | 23D2 | 3 |
| P{lacW}Madk00237 | 23D3 | 3 |
Molecular analyses:
After γTub23CPl-2 was mapped between P{EP}Rrp11020 and P{EPgy2}CG9643EY07345, the DNA sequences of the open reading frames of all four predicted genes in the region (CG9641, CG3165, CG9643, and γTub23C) were determined from DNA isolated from homozygotes of γTub23CPl-2 (and the parental w;red e strain in which it was induced) and of γTub23CA6-2 (and the parental cn bw chromosome in which it was induced). As the only nonsynonomous changes found between the two mutants and their parental chromosomes were in the γTub23C open reading frame (Figure 2C), we then determined the DNA sequence of γTub23C from γTub23CA14-9 and γTub23CA15-2 homozygotes. Sequencing was done from PCR-amplified genomic fragments. Mutant homozygotes were identified using a GFP-expressing balancer chromosome [CyO, P{w+mC=ActGFP}JMR1].
Figure 2.—
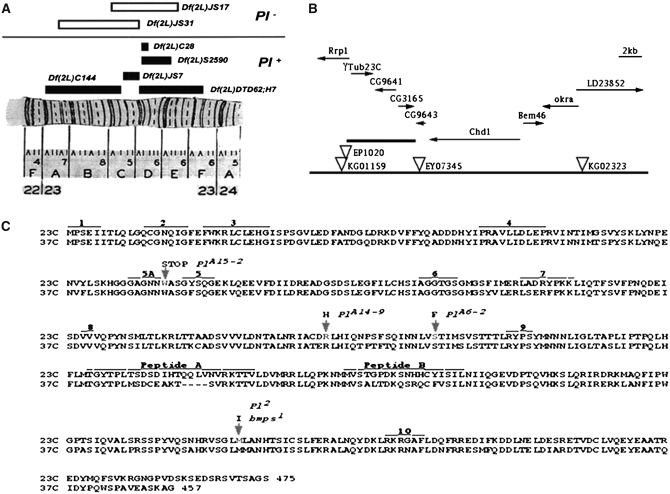
Genetic and molecular characterization of the Pearl region in 23C. (A) Deficiency mapping of the Pearl region. (B) Genomic map of region 23C. Predicted transcriptional units in the region are shown by arrows indicating their transcriptional direction. P-element transposons in the region are indicated by inverted triangles. EP1020 is P{EP}Rrp11020, KG01159 is P{SUPor-P}Rrp1KG01159, and EY07345 is P{EPgy2}CG9643EY07345. The thickest line marks the 7-kbp region where γTub23CPl-2 was localized by P-induced male recombination. (C) The amino acid sequences for the two γ-tubulin proteins (labeled 23C and 37C) in Drosophila (Wilson et al. 1997) and the changes found in the γTub23C mutant alleles. The arrows indicate the changes in the γTub23C mutant alleles. At the top of each arrow are the allele name and the amino acid substitution. The numbered areas indicate peptides with known or presumed functions (taken from Burns 1995 and references therein): 1 is a peptide implicated in the autoregulation of β-tubulin translation; peptides 2–10 are implicated in the binding or the hydrolysis of GTP by β-tubulin. Peptide 10 is also implicated in the release of α-, β-, and γ-tubulin from the TCP1α chaperonine. Note that none of the γTub23C mutations are in any of the peptides with known or presumed functions and that all the changed residues in the γTub23C mutants are identical in both γTub23C and γTub37C wild-type genes.
RESULTS
The γTub23CPl-2 mutation enhances brahma mutants:
Flies heterozygous for some combinations of mutations in trithorax group genes have held-out wings (Vázquez et al. 1999). On the basis of this phenotype we isolated several dominant enhancers of brm, including alleles of the trithorax group genes osa (osa), tonalli (tna), and taranis (tara) (Vázquez et al. 1999; Gutiérrez et al. 2003). From that same genetic screen, we also isolated the γTub23CPl-2 mutation. In addition to its dominant enhancement of brm (Table 2 and Figure 1C), γTub23CPl-2 has additional dominant phenotypes in the wing blade (Figure 1B), including pearl-like structures [predominantly in the second (L2) and/or third (L3) wing vein(s)], blisters in the wing blade, and notches or gaps in the ventral and dorsal margins in one or both wings (Figure 1B). γTub23CPl-2 heterozygotes also have small round eyes. We mapped γTub23CPl-2 to the same chromosomal region as Pearl (Pl) (Rosin 1951, 1952), a dominant mutation that had the same unique combination of phenotypes. The original Pl mutant is no longer extant. We originally called our mutation Pl2, but since it is allelic to γTub23C (see below), we have renamed it γTub23CPl-2.
TABLE 2.
γTub23CPl-2 interactions with general transcription machinery and trithorax-group mutants
| Genotype | No. of flies with held-out wings/total | Penetrance (%) |
|---|---|---|
| brm2/+ | 9/498 | 2 |
| mor1/+ | 0/120 | 0 |
| osa1/+ | 6/208 | 3 |
| osa2/+ | 0/41 | 0 |
| tna1/+ | 19/115 | 17 |
| tara2/+ | 0/129 | 0 |
| tara20/+ | 0/151 | 0 |
| γTub23CPl-2/+ | 5/727 | 1 |
| γTub23CPl-2/+; brm1/+ | 16/46 | 35 |
| γTub23CPl-2/+; brm2/+ | 71/94 | 76 |
| γTub23CPl-2/+; mor1/+ | 10/105 | 10 |
| γTub23CPl-2/+; mor2/+ | 18/43 | 42 |
| γTub23CPl-2/+; mor6/+ | 20/107 | 19 |
| γTub23CPl-2/+; osa1/+ | 307/314 | 98 |
| γTub23CPl-2/+; osa2/+ | 17/44 | 39 |
| γTub23CPl-2/+; tna1/+ | 111/148 | 75 |
| γTub23CPl-2/+; tara2/+ | 19/88 | 22 |
| γTub23CPl-2/+; tara20/+ | 19/91 | 21 |
When trans-heterozygous with γTub23CPl-2, the following mutations gave no more than 3% penetrance for the held-out-wings phenotype: Taf11, Taf41, Taf4S466, Taf61, Taf62, trxE2, trxB11, ash16, ash1B1, ash21, ash22, kis1, kis2, kto1, kto3, vtd3, vtd14, sls1, dev1, dev2, Vha5512, Vha5516, urd2, Trl3, Trl62, and skd3.
Figure 1.—
γTub23CPl-2-dependent phenotypes. (A) Wild-type fly with the wings held back parallel to the body axis. (B) γTub23CPl-2/+ flies; note the notches and the pearl-like structures in the wings (indicated by arrows, one of them pointing to the area shown in the inset). (C) γTub23CPl-2/+; brm2/+ (shown in C) and γTub23CPl-2/+; osa1/+ double heterozygous flies have held-out wings in addition to the wing notches and pearl-like structures. (D) γTub23CPl-2/+; brm2/osa1 triple heterozygous fly with twisted wings (in addition to held-out and notched wings with pearl-like structures). (E) Wild-type wing from Oregon-R stock. (F) Wing from γTub23CPl-2/+; osa1/+ double heterozygous fly. The arrow indicates a pearl-like structure along the third wing vein shown in the inset (G).
Mapping of γTub23CPl-2:
We first mapped γTub23CPl-2 by meiotic recombination and then by complementation with available chromosome deletions to polytene chromosome subdivisions 23CD (Figure 2A). We next used the P-element insertion lines in the 23CD region to map γTub23CPl-2 by P-induced male recombination (Chen et al. 1998). Of 63 recombinants recovered, all except 1 appear to have resulted from recombination at the P-element insertion site. These 62 recombinants all place γTub23CPl-2 in the 7-kbp region between P{EP}Rrp11020 and P{EPgy2} CG9643EY07345 (Figure 2B). Inverse PCR with recombinants that still retained the P insertion and genetic complementation with mutants in the region were both used to identify recombinants with flanking deletions and duplications in the 23BD region. The flanking deletions were useful for determining the order of the essential genes in the region. One of the flanking deletions recovered by P-induced male recombination from P{EPgy2}CG9643EY07345 is Df(2L)3G, which behaves as a deletion of γTub23CPl-2 (Table 3), but does not delete any of the other essential genes in 23C that have been identified [lilli, Rpb9, or l(2)23Cb].
TABLE 3.
Survival to eclosion (%) of γTub23C mutant trans-heterozygotes with dd4+ or dd4su(Pl)
|
dd4su(Pl) suppressible (class I)
|
dd4su(Pl) nonsuppressible (class II)
|
||||
|---|---|---|---|---|---|
| γTub23C genotype | dd4+ | dd4su(Pl) | γTub23C genotype | dd4+ | dd4su(Pl) |
| γTub23CA14-9/Df(2L)3G | 83 | 100 | γTub23CA6-2/γTub23CA15-2 | 3a | 0 |
| γTub23CA14-9/Df(2L)JS17 | 70 | 100 | γTub23CA6-2/Df(2L)3G | 2a | 0 |
| γTub23CA14-9/γTub23C A6-2 | 65 | 74 | γTub23CA6-2/Df(2L)JS17 | 0 | 0 |
| γTub23CA14-9/γTub23CA15-2 | 25 | 94 | γTub23CA14-9/γTub23Cbmps1 | 0 | 2 |
| γTub23CPl-2/γTub23CA6-2 | 6a | 74 | γTub23CA14-9/γTub23CPl-2 | 0 | 1 |
| γTub23Cbmps1/γTub23CA6-2 | 0 | 73 | γTub23CA15-2/Df(2L)JS17 | 0 | 2 |
| γTub23CPl-2/Df(2L)JS17 | 0 | 24 | γTub23CA15-2/Df(2L)3G | 0 | 0 |
| γTub23CPl-2/Df(2L)3G | 0 | 19 | Df(2L)3G/Df(2L)JS17 | 0 | 0 |
| γTub23Cbmps1/Df(2L)3G | 0 | 19 | γTub23CPl-2/γTub23Cbmps1 | 0 | 0 |
| γTub23Cbmps1/Df(2L)JS17 | 0 | 16 | |||
| γTub23CA15-2/γTub23Cbmps1 | 0 | 13 | |||
| γTub23CA15-2/γTub23CPl-2 | 0 | 7 | |||
Eclosed, but all quickly became stuck in the medium.
l(2)23Ce is allelic to γTub23CPl-2:
We mapped all of the phenotypes associated with γTub23CPl-2 (the held-out-wings phenotype in the presence of brm alleles and the dominant phenotypes in the wing blade) to polytene chromosome band 23CD. We then tested mutants previously mapped to 23CD. We found that all alleles of l(2)23Ce that we tested [l(2)23CeA6-2, l(2)23CeA14-9, and l(2)23CeA15-2] failed to complement γTub23CPl-2 for viability (Table 3). We have renamed the l(2)23Ce alleles as γTub23C A6-2, γTub23CA14-9, and γTub23CA15-2. When trans-heterozygous to other alleles, γTub23CA15-2 is similar to the two deficiencies tested [Df(2L)JS17 and Df(2L)3G] and is probably a null allele. The γTub23CA14-9 allele behaves as a hypomorph, often eclosing when heterozygous to the deficiencies and most other alleles. All of the eclosing mutant flies (regardless of which combination of alleles) have the same phenotypes observed for γTub23CPl-2 heterozygotes (small round eyes and held-out and blistered wings with pearl-like structures), but with far fewer notches in the wing margins. While flies hemizygous for γTub23CA14-9 eclosed at 70–83% of the expected numbers, no γTub23CA14-9/γTub23CPl-2 flies eclosed. γTub23CPl-2 is an antimorphic, or dominant-negative, allele and the phenotypes observed in γTub23CPl-2 heterozygotes are loss-of-function phenotypes for γTub23C caused by interference of the γTub23CPl-2 mutant protein with the wild-type γTub23C protein. Consistent with this interpretation is the suppression of the dominant phenotypes of γTub23CPl-2 by an additional wild-type copy of γTub23C [observed with both Dp(2;1)JS13 and with tandem duplications recovered from the P-induced male recombination]. These tests allowed us to establish a γTub23C allelic series in order of decreasing activity: γTub23CA14-9>γTub23CA6-2>γTub23CA15-2=Df(2L)JS17=Df(2L)3G>γTub23CPl-2.
Molecular characterizations:
We mapped the γTub23CPl-2 mutation to a 7-kbp genomic region that includes four predicted genes, γTub23C, CG9641, CG3165, and CG9643 (Figure 2B). Our analyses showed changes in the γTub23C open reading frame for all four alleles that we sequenced (Figure 2C). The γTub23C gene encodes a protein of 475 residues. The γTub23CA15-2 mutation changes tryptophan 104 to a stop codon, predicting the formation of a truncated protein. This is in agreement with our complementation analyses that suggested that γTub23CA15-2 behaves as a null mutation. γTub23CA14-9 changes arginine 217 to histidine (R217H), γTub23CA6-2 changes serine 233 to phenylalanine (S233F), and γTub23CPl-2 changes methionine 382 to isoleucine (M382I).
Differential suppression of γTub23C lethality by an X-linked suppressor:
Subsequent to our molecular analyses, we obtained the γTub23Cbmps1 mutant (Mahoney et al. 2006). While both γTub23Cbmps1 and γTub23CPl-2 change the same methionine to isoleucine (M382I), the description of γTub23Cbmps1 differed from our observations for γTub23CPl-2 in two important aspects. The first difference was the much lower penetrance of the dominant phenotypes for γTub23Cbmps1. The second important difference was the eclosion of many γTub23Cbmps1 hemizygotes. We observed these same differences when we began experiments with γTub23Cbmps1, but noted striking differences in the results of the reciprocal crosses with the γTub23Cbmps1 mutant flies. For example, when γTub23CA6-2 heterozygous females were mated to γTub23Cbmps1 heterozygous males, no γTub23Cbmps1/γTub23CA6-2 mutant progeny eclosed. In the reciprocal cross in which γTub23Cbmps1 heterozygous females were mated to γTub23CA6-2 heterozygous males, 73% of the expected γTub23Cbmps1/γTub23CA6-2 sons eclosed (Tables 3 and 4), while <2% of the expected γTub23Cbmps1/γTub23CA6-2 daughters eclosed (Table 4). The eclosion of mutant sons only when the mothers were from the γTub23Cbmps1 stock suggested the presence of an X-linked suppressor in this stock. We replaced the X chromosome in the γTub23Cbmps1 stock with an X chromosome marked with w1 and repeated the crosses. The penetrance of the dominant phenotypes in γTub23Cbmps1 heterozygotes was much greater and the differential eclosion of mutant sons in reciprocal crosses disappeared. When we replaced the X chromosomes in the other γTub23C mutant and deficiency stocks with the X chromosome from the original γTub23Cbmps1 stock, we found that more mutant flies eclosed. The γTub23Cbmps1 stock contains a recessive X-linked suppressor that we named l(1)dd4su(Pl) [see the following section for a discussion of the allelism to l(1)dd4]. We will refer to the suppressor as dd4su(Pl) for the remainder of this work. The survival of γTub23C mutant genotypes with the suppressor [dd4su(Pl)] and without [dd4+] are given in Table 3.
TABLE 4.
Suppression of γTub23Cbmps1/γTub23CA6-2 lethality by dd4 mutations
| Survival of γTub23Cbmps1/γTub23CA6-2 flies
|
||
|---|---|---|
| dd4 genotype | No. observed/expected | % |
| +/Y | 0/351 | 0 |
| +/+ | 0/218 | 0 |
| +/dd4su(Pl) | 8/478 | 2 |
| +/dd42 | 0/38 | 0 |
| +/dd4G0122 | 2/109 | 2 |
| dd4su(Pl)/Y | 186/255.5 | 73 |
| dd4su(Pl)/dd4su(Pl) | 58/75.5 | 77 |
| dd4su(Pl)/dd42 | 102/101 | 101 |
| dd4su(Pl)/dd4G0122 | 105/105 | 100 |
The expected numbers of γTub23Cbmps1/γTub23CA6-2 flies is half the number of CyO individuals in the same crosses. For +/Y or dd4su(Pl)/Y genotypes, the expected frequency was calculated with the CyO sons and for the rest of the genotypes it was calculated with the CyO daughters.
While most heteroallelic γTub23C combinations were lethal, dd4su(Pl) rescued some genotypes to eclosion (Table 3). On the basis of the ability to be rescued by dd4su(Pl), we separated heteroallelic γTub23C combinations into two classes (class I and class II, Table 3). For example, for the class I genotype γTub23Cbmps1/γTub23CA6-2, no flies eclosed when they were dd4+ but 73% eclosed when they were dd4su(Pl). The same effect was observed for γTub23CPl-2/γTub23CA6-2 and γTub23CA14-9/γTub23CA15-2 flies, where 6 and 25% eclosed, respectively, with dd4+, but 74 and 94% eclosed, respectively, with dd4su(Pl). For class II genotypes, there were almost no differences in the eclosion rates between dd4+ and dd4su(Pl).
The class I genotypes include one, and only one, of the following alleles: γTub23CA14-9, γTub23CPl-2, and γTub23Cbmps1. Genotypes that do not include one of these three suppressible alleles (or which include two of the suppressible alleles) are class II. The failure of suppression in flies with two suppressible alleles is interesting. For example, dd4+ flies heterozygous for one of the suppressible alleles, γTub23CPl-2 or γTub23Cbmps1, do not eclose when also heterozygous for a deficiency or for the weak hypomorphic allele γTub23CA14-9. The dd4su(Pl) flies that are heterozygous for γTub23CPl-2 or γTub23Cbmps1, however, eclose at 16–24% of the expected frequencies if also heterozygous for one of the deficiencies, but at only 1–2% of the expected frequency if also heterozygous for the hypomorphic (and suppressible) γTub23CA14-9 allele.
The X-linked suppressor of γTub23C lethality is in the gene encoding the Grip91 γ-tubulin-interacting protein:
We used meiotic recombination to map the X-linked suppressor in the γTub23Cbmps1 stock to a region that includes the garnet gene. Because we recovered no recombinants between the suppressor and garnet in our mapping experiments, the suppressor is very close to garnet. The gene next to garnet in the genome is lethal (1) discs degenerate 4 [l(1)dd4], which we refer to as dd4. dd4 encodes the Grip91 protein, a subunit of the γ-tubulin γTuSC and γTuRC complexes (Barbosa et al. 2000). Some dd4 mutant alleles cause zygotic lethality, while flies with other mutant alleles survive in dry media and have held-up wings, absence of some bristles, defects in abdominal segments, and male sterility (Barbosa et al. 2000). Although the suppressor had no mutant phenotype in either sex, we decided to test for allelism to dd4 because of the biochemical data. We used two lethal alleles, dd42 and dd4G0122, and the test genotype γTub23Cbmps1/γTub23CA6-2 (see Table 4). Rescue of γTub23Cbmps1/γTub23CA6-2 lethality by the suppressor is almost completely recessive (only 2% of the expected flies eclosed if heterozygous for the suppressor, while 77% survived if homozygous for the suppressor). Both lethal alleles of dd4 (dd42 and dd4G0122) complemented the suppressor [dd4su(Pl)] for viability, with the mutant females eclosing at the expected numbers. However, neither of the lethal alleles complemented dd4su(Pl) for the suppression of γTub23Cbmps1/γTub23CA6-2 lethality; i.e., both lethal alleles of dd4 also suppressed the γTub23C lethality. We conclude that the suppressor dd4su(Pl) is an allele of dd4. The suppression is a loss-of-function phenotype, but the suppressor must retain some functions necessary for zygotic viability and fertility.
γTub23CPl-2 genetically interacts with BRM complex mutations and with tna and tara:
γTub23CPl-2 was isolated because it interacts with brm2 to cause a held-out wings phenotype (Table 2). Since several trithorax group mutants were isolated in this same genetic screen, we tested γTub23CPl-2 for genetic interactions with a collection of trithorax group mutants and with mutants in some general transcription factors. From the general transcription factors, we chose to test Taf1, Taf4, and Taf6 and the Mediator complex subunits Med12 and Med13 encoded by the trithorax-group genes kohtalo (kto) and skuld (skd), respectively. We tested mutants in subunits of nucleosome-remodeling or histone-modification complexes, including brm, mor, snr1, osa, kismet, ash1, ash2, and trithorax. We also tested the trithorax group genes Trithorax-like (Trl), tna, tara, verthandi (vtd), sallimus (sls), devenir (dev), Vha55, and urdur (urd). The mutations tested and the results are summarized in Table 2. We found that only alleles of brm, osa, and tna showed strong genetic interactions with γTub23CPl-2 to cause the held-out wings phenotype. The genetic interactions between osa1 and γTub23CPl-2 (Table 2 and Figure 1, F and G) are even stronger than the genetic interactions between brm2 and γTub23CPl-2 (Table 2 and Figure 1C). Flies heterozygous for all three mutations (γTub23CPl-2, brm2, and osa1) have poor survival and twisted and blistered wings even more severe than the phenotypes of the γTub23CPl-2/+; osa1/+ double heterozygotes (Figure 1, C and D). Other mutations that showed weaker but significant interactions with γTub23CPl-2 included brm1, osa2, mor1, mor2, mor6, tna1, tara2, and tara20 (Table 2). We did not find significant interactions with any of the other mutations tested. We considered the possibility that the interactions between the BRM complex mutations and γTub23CPl-2 could be explained by reduced transcription of γTub23C. If heterozygous brm mutants have reduced transcription of γTub23C, then all loss-of-function γTub23C phenotypes should be enhanced in all mutant genotypes. To test this prediction, we chose several combinations of hypomorphic γTub23C alleles to examine in brm2 heterozygotes. CyO/γTub23CA14-9;TM6C/brm2 females were crossed to males with mutations or deletions of γTub23C. A reduction in transcription would be expected to reduce viability and enhance all of the γTub23C mutant phenotypes in progeny that receive the brm2 mutant when compared to their TM6C siblings. While γTub23CA14-9/γTub23CA6-2, γTub23CA14-9/γTub23CA15-2, and γTub23CA14-9/ Df(2L)3G trans-heterozygous flies all have reduced viability, the viability was not further reduced in brm2 heterozygous flies. As expected, the held-out wings phenotype of γTub23CA14-9/Df(2L)3G flies was enhanced in brm2 heterozygotes, but the expressivity of pearl-like structures in the wings was not enhanced. Finally, we examined interactions among brm2, dd4su(Pl), and γTub23C. The rescue of γTub23CPl-2/γTub23CA6-2 by dd4su(Pl) was reduced by about half in brm2 heterozygotes. The enhancement of the held-out-wings phenotype in γTub23CPl-2/+; brm2/+ double heterozygotes was reduced from 76 to 14% in dd4su(Pl) males.
DISCUSSION
Proteins identified as part of the eukaryotic cytoskeleton may have more direct roles in transcriptional regulation than originally thought (reviewed in Olave et al. 2002). Actin and actin-related proteins (ARPs) are found in BRM complexes from yeast to humans, including the BRM complexes in Drosophila (Papoulas et al. 1998). The function of actin and ARPs in these complexes is not well understood. Some ARPs interact with DNA-bending proteins and with histones and it was proposed that they facilitate chromatin architecture and interactions between complexes or function as histone chaperones (Shen et al. 2003; Szerlong et al. 2003). Actin is also part of preinitiation complexes and is necessary for transcription by RNA polymerases I, II, and III (Hofmann et al. 2004; Hu et al. 2004; Philimonenko et al. 2004). The α- and/or β-tubulins are also found with a subset of trithorax-group proteins in the mammalian ASCOM complex (Activating signal cointegrator 2, Asc2 complex), which is required for transactivation by nuclear receptors (Goo et al. 2003; Lee et al. 2006), and in a histone H2A deubiquitinase complex (Zhu et al. 2007). γ-Tubulin is essential for microtubule function, but unlike α- and β-tubulin, it is not a component of microtubules. Rather, it is located at microtubule-organizing centers (MTOCs) and functions in the initiation of microtubule nucleation and in the establishment of microtubule polarity (Oakley 1992; Luders and Stearns 2007). γ-Tubulin contributes to the proper formation of mitotic spindles and cytoplasmic microtubular arrays. There are critical cytoskeletal and nuclear envelope connections, linking, for example, MTOCs to the nuclear lamina (reviewed in Taddei et al. 2004). In addtion, γ-tubulin has been proposed to have microtubule- and/or centrosome-independent function(s) in mitosis (Prigozhina et al. 2004) or spindle assembly checkpoints (Muller et al. 2006).
Drosophila embryonic γ-tubulin exists in two related complexes: a large complex similar to the Xenopus γTuRC (γ-tubulin ring complex) (36.9S, ∼2000 kDa) and a small soluble complex called γTuSC (γ-tubulin small complex) (8.5S ∼240 kDa) (Oegema et al. 1999). The Drosophila γTuRC consists of approximately eight polypeptides, including γ-tubulin, Grip163, Grip128, Grip91, Grip84, Grip75, and GP71WD (Oegema et al. 1999; Gunawardane et al. 2000, 2003). The γTuRC has a lockwasher-like structure and a cap at one of the ends of the complex. The Drosophila γTuSC is a tetramer of two γ-tubulin molecules and one molecule each of Grip91 and Grip84. Several γTuSCs form the γTuRC lockwasher region. The other Grips (Grip163, 128, and 75) form the cap (Moritz et al. 2000).
Drosophila is the only metazoan in which the genes encoding subunits of the γTuSC and γTuRC complexes have been functionally studied using genetic approaches (for recent examples, see Barbosa et al. 2003; Gunawardane et al. 2003; Colombié et al. 2006; Vogt et al. 2006). Null mutations in dd4 (which encodes Grip91) and in Grip84 are lethal and display defects in spindle assembly (Barbosa et al. 2003; Colombié et al. 2006), while null mutations in Grip128 and Grip75 are viable, but sterile (Schnorrer et al. 2002; Vogt et al. 2006).
In Drosophila there are two γ-tubulin genes, γTub23C and γTub37C. They encode very similar (but not identical) proteins, but they have different expression patterns and mutant phenotypes. γTub37C is largely restricted to the female germline and early stages of embryogenesis. It is required for bicoid (bcd) mRNA localization at mid-oogenesis (Schnorrer et al. 2002), female meiosis, and nuclear proliferation (Tavosanis et al. 1997; Wilson et al. 1997). In syncytial embryos, γTub23C is in the soluble small γTuSC fraction (Raynaud-Messina et al. 2001) and is absent at the centrosome (Wilson et al. 1997). At this stage, γTub37C is found in both γTuSC and γTuRC fractions (Raynaud-Messina et al. 2001). It is localized at the centrosome (Tavosanis et al. 1997; Wilson et al. 1997) and over the spindle regions (Tavosanis et al. 1997). γTub37C mutants are female sterile (Tavosanis et al. 1997; Tavosanis and González 2003).
The γTub23C isoform is expressed in a variety of tissues in both sexes (Wilson et al. 1997), including larval brains and imaginal discs, and it is required for somatic mitotic divisions. It is also expressed in ovaries and is the only isoform expressed in testes. γTub23C is required for meiosis in males and for spermatogenesis (Sampaio et al. 2001).
γTub23C is essential for zygotic viability and for development of imaginal tissues:
We isolated the γTub23CPl-2 mutation in a mutant screen designed to identify genes that interact with brm in wing development. In addition to showing genetic interactions with brm, γTub23CPl-2 mutants are homozygous lethal, while the heterozygotes have defects in imaginal eye and wing development. We showed that γTub23CPl-2 is a dominant-negative mutation and that l(2)23Ce alleles are loss-of-function mutations in γTub23C with recessive phenotypes similar to the dominant phenotypes of γTub23CPl-2. γTub23C has 30% identity to α- and β-tubulins, which are structural components of microtubules. It is known which parts of the β-tubulin protein are involved in autoregulation for translation and for binding and hydrolysis of GTP. The γ-tubulin protein shares some of these regions with β-tubulin. The γTub23C mutations characterized in this work do not map to any of these known regions, with the exception of the truncated form in the γTub23CA15-2 allele (Figure 2C). This suggests that the proteins synthesized from the γTub23CA14-9, γTub23CA6-2, γTub23CPl-2, and γTub23Cbmps1 alleles might affect other γ-tubulin functions (see below).
We were surprised during the course of this work to identify a dd4 allele with no discernible phenotype except the suppression of some γTub23C mutant phenotypes (including zygotic lethality). Since dd4 encodes Grip91, a protein that physically interacts with γ-tubulin, we believe that the genetic interactions have important implications.
Implications of the genetic interactions between γTub23C and dd4 (Grip91) mutations on current structural models of γTuRC and γTuSC complexes:
Grip91, Grip84, and γ-tubulin form the lockwasher region of γTuRC and γTuSC complexes (Moritz et al. 2000). Grip91 and Grip84 (or their orthologs in yeast and humans) interact with each other and with γ-tubulin (Moritz et al. 2000; Aldaz et al. 2005; Wiese 2008). The interactions between Grip91 and γ-tubulin facilitate binding of GTP to γ-tubulin (Gunawardane et al. 2003). Grip91 is required for correct bipolar spindle assembly during mitosis and male meiosis and it helps to locate γ-tubulin to the centrosome (Barbosa et al. 2000, 2003).
Grip91 is an essential protein encoded by the dd4 gene (Barbosa et al. 2000, 2003). Semilethal alleles have held-up wings and other imaginal defects and are male sterile (Barbosa et al. 2000, 2003). The dd4su(Pl) allele that we identified is unusual in that it has no defects in viability, fertility, or developmental patterning. Its only phenotype is the suppression of class I (but not class II) genotypes of γTub23C.
What is the significance of the two types of γTub23C alleles from the functional point of view? The defects produced by suppressible alleles may involve γTuSC and/or γTuRC functions, while the defects produced by nonsuppressible alleles may involve γTub23C functions independent of the γTuSC and γTuRC complexes. It is also possible that different mutant proteins, although in some cases retaining partial activity, may affect other different functions of γTub23C. Some of these other functions may require Grip91 (and possibly the integrity of γTuRC and/or γTuSC complexes) and some may not. Such functions could affect the assembly of the γTuSC and/or γTuRC complexes, the transport of the complex(es) to subcellular compartments, and/or the relationships of γTub23C with other proteins involved in microtubule-independent processes. We believe that the new alleles of γTub23C and dd4 that we have characterized can help to test the current structural models of γTuRC and γTuSC complexes proposed in biochemical and crystallographic studies (Erickson 2000; Moritz et al. 2000).
Recent work shows that γ-tubulin has a microtubule-independent role in establishing or maintaining a mitotic checkpoint block (Prigozhina et al. 2004) and that γTuRCs proteins may have a centrosome-independent role in the spindle assembly checkpoint. For this latter function, γ-tubulin is probably in a complex associated with Cdc20 and BubR1 kinases (Muller et al. 2006). We have found that the genetic interactions between γTub23C and Brm are caused not by reduced γTub23C transcription, but more probably by the presence of defective γ-tubulin proteins. This suggests roles for γ-tubulin in transcription and/or chromatin remodeling. This is further supported by the recent description of interactions between Pericentrin (an integral centrosomal component) and CHD3, a Brm-related protein in the NuRD chromatin-remodeling complex (Sillibourne et al. 2007).
Acknowledgments
This work was supported by grants from the Consejo Nacional de Ciencia y Technológia (M.V.), the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica–Universidad Nacional Autónoma de México (M.V. and M.Z.), and the Howard Hughes Medical Institute (M.Z.). This research was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development of the National Institutes of Health.
References
- Aldaz, H., L. M. Rice, T. Stearns and D. A. Agard, 2005. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature 435 523–527. [DOI] [PubMed] [Google Scholar]
- Armstrong, J. A., O. Papoulas, G. Daubresse, A. S. Sperling, J. T. Lis et al., 2002. The Drosophila BRM complex facilitates global transcription by RNA polymerase II. EMBO J. 21 5245–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. A., A. S. Sperling, R. Deuring, L. Manning, S. L. Moseley et al., 2005. Genetic screens for enhancers of brahma reveal functional interactions between the BRM chromatin-remodeling complex and the Delta-Notch signal transduction pathway in Drosophila. Genetics 170 1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, V., R. R. Yamamoto, D. S. Henderson and D. Glover, 2000. Mutation of a Drosophila γ-tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 14 3126–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, V., M. Gatt, E. Rebollo, C. González and D. Glover, 2003. Drosophila dd4 mutants reveal that γTuRC is required to maintain juxtaposed half spindles in spermatocytes. J. Cell Sci. 116 929–941. [DOI] [PubMed] [Google Scholar]
- Burns, R. G., 1995. Analysis of the γ-tubulin sequences: implications for the functional properties of γ-tubulin. J. Cell Sci. 108 2123–2130. [DOI] [PubMed] [Google Scholar]
- Chen, B., T. Chu, E. Harms, J. P. Gergen and S. Strickland, 1998. Mapping of Drosophila mutations using site-specific male recombination. Genetics 149 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombié, N., C. Vérollet, P. Sampaio, A. Moisand, C. E. Sunkel et al., 2006. The Drosophila γ-tubulin small complex subunit Dgrip84 is required for structural and functional integrity of the spindle apparatus. Mol. Biol. Cell 17 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, H. P., 2000. γ-Tubulin nucleation: Template or protofilament? Nat. Cell Biol. 2 E93–E96. [DOI] [PubMed] [Google Scholar]
- Flaus, A., and T. Owen-Hughes, 2004. Mechanisms for ATP-dependent chromatin remodelling: Farewell to the tuna-can octamer? Curr. Opin. Genet. Dev. 14 165–173. [DOI] [PubMed] [Google Scholar]
- Goo, Y. H., Y. C. Sohn, D. H. Kim, S. W. Kim, M. J. Kang et al., 2003. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol. Cell. Biol. 23 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R. N., O. C. Martin, K. Cao, L. Zhang, K. Dej et al., 2000. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 151 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R. N., O. C. Martin and Y. Zheng, 2003. Characterization of a new γTuRC subunit with WD repeats. Mol. Biol. Cell 14 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez, L., M. Zurita, J. A. Kennison and M. Vázquez, 2003. The Drosophila trithorax group gene tonalli (tna) interacts genetically with the Brahma remodeling complex and encodes an SP-RING finger protein. Development 130 343–354. [DOI] [PubMed] [Google Scholar]
- Hofmann, W. A., L. Stojiljkovic, B. Fuchsova, G. M. Vargas, E. Mavrommatis et al., 2004. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 6 1094–1101. [DOI] [PubMed] [Google Scholar]
- Hu, P., S. Wu and N. Hernández, 2004. A role for β-actin in RNA polymerase III transcription. Genes Dev. 18 3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison, J. A., and J. W. Tamkun, 1988. Dosage-dependent modifiers of Polycomb and Antennapedia mutations in Drosophila. Proc. Natl. Acad. Sci. USA 85 8136–8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., D. K. Lee, Y. Dou, J. Lee, B. Lee et al., 2006. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc. Natl. Acad. Sci. USA 103 15392–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Luders, J., and T. Stearns, 2007. Microtubule-organizing centers: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8 161–167. [DOI] [PubMed] [Google Scholar]
- Mahoney, M. B., A. L. Parks, D. A. Ruddy, S. Y. K. Tiong, H. Esengil et al., 2006. Presenilin-based genetic screens in Drosophila melanogaster identify novel Notch pathway modifiers. Genetics 172 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, M., M. B. Braunfeld, V. Guénebaut, J. Heuser and D. A. Agard, 2000. Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2 365–370. [DOI] [PubMed] [Google Scholar]
- Muller, H., M. L. Fogeron, V. Lehmann, H. Lehrach and B. M. H. Lange, 2006. A centrosome-independent role for γ-TuRC proteins in the spindle assembly checkpoint. Science 314 654–657. [DOI] [PubMed] [Google Scholar]
- Oakley, B. R., 1992. γ-tubulin: The microtubule organizer? Trends Cell Biol. 2 1–5. [DOI] [PubMed] [Google Scholar]
- Oegema, K., C. Wiese, O. C. Martin, R. A. Milligan, A. Iwamatsu et al., 1999. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olave, I. A., G. R. Reck-Peterson and G. R. Crabtree, 2002. Nuclear actin and actin-related proteins in chromatin remodeling. Annu. Rev. Biochem. 71 755–781. [DOI] [PubMed] [Google Scholar]
- Papoulas, O., S. J. Beek, S. L. Moseley, C. M. McCallum, M. Sarte et al., 1998. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125 3955–3966. [DOI] [PubMed] [Google Scholar]
- Philimonenko, V. V., J. Zhao, S. Iben, H. Dingova, K. Kysela et al., 2004. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 6 1165–1172. [DOI] [PubMed] [Google Scholar]
- Prigozhina, N. L., C. E. Oakley, A. M. Lewis, T. Nayak, S. A. Osmani et al., 2004. γ-Tubulin plays an essential role in the coordination of mitotic events. Mol. Biol. Cell 15 1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud-Messina, B., A. Debec, Tollon, M. Gares and M. Wright, 2001. Differential properties of the two Drosophila γ-tubulin isotypes. Eur. J. Cell Biol. 80 643–649. [DOI] [PubMed] [Google Scholar]
- Roberts, C. W., and S. H. Orkin, 2004. The SWI/SNF complex-chromatin and cancer. Nat. Rev. Cancer 4 133–142. [DOI] [PubMed] [Google Scholar]
- Rosin, S., 1951. Zur Entwicklungsphysiologie der mutante Pearl (Pl) von Drosophila melanogaster. Developmental physiology of the mutant Pearl (Pl) of Drosophila melanogaster. Rev. Suisse Zool. 58 398–403. [Google Scholar]
- Rosin, S., 1952. Veränderungen des borstenmusters bei der mutante Pearl von Drosophila melanogaster. Alterations of bristle patterns in the Pearl mutant of Drosophila melanogaster. Rev. Suisse Zool. 59 261–268. [Google Scholar]
- Sampaio, P., E. Rebollo, H. Varmark, C. E. Sunkel and C. González, 2001. Organized microtubule arrays in γ-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 11 1788–1793. [DOI] [PubMed] [Google Scholar]
- Schnorrer, F., S. Luschnig, I. Koch and C. Nüsslein-Volhard, 2002. γ-Tubulin37C and γ-tubulin ring complex protein 75 are essential for bicoid RNA localization during Drosophila oogenesis. Dev. Cell 3 685–696. [DOI] [PubMed] [Google Scholar]
- Sharma, V. M., B. Li and J. C. Reese, 2003. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAF(II) and the general transcription machinery. Genes Dev. 17 502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X., H. Xiao, R. Ranallo, W. H. Wu and C. Wu, 2003 Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science 299 112–114. [DOI] [PubMed]
- Sillibourne, J. E., B. Delaval, S. Redick, M. Sinha and S. J. Doxsey, 2007. Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol. Biol. Cell 18 3667–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerlong, H., A. Saha and B. R. Cairns, 2003. The nuclear actin-related proteins Arp7 and Arp9: a dimeric module that cooperates with architectural proteins for chromatin remodeling. EMBO J. 22 3175–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei, A., F. Hediger, F. R. Neumann and S. M. Gasser, 2004. The function of nuclear architecture: a genetic approach. Annu. Rev. Genet. 38 305–345. [DOI] [PubMed] [Google Scholar]
- Tavosanis, G., and C. González, 2003. γ-Tubulin function during female germ cell development and oogenesis in Drosophila. Proc. Natl. Acad. Sci. USA 100 10263–10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis, G., S. Llamazares, G. Goulielmos and C. González, 1997. Essential role for γ-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J. 16 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez, M., L. Moore and J. A. Kennison, 1999. The trithorax-group gene osa encodes an ARID-domain protein that interacts with the Brahma chromatin-remodeling factor to regulate transcription. Development 126 733–742. [DOI] [PubMed] [Google Scholar]
- Vogt, N., I. Koch, H. Schwarz, F. Schnorrer and C. Nüsslein-Volhard, 2006. The γTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development 133 3963–3972. [DOI] [PubMed] [Google Scholar]
- Wiese, C., 2008. Distinct Dgrip84 isoforms correlate with distinct γ-tubulins in Drosophila. Mol. Biol. Cell 19 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, P. G., Y. Zheng, C. E. Oakley, B. R. Oakley, G. G. Borisy et al., 1997. Differential expression of two γ-tubulin isoforms during gametogenesis and development in Drosophila. Dev. Biol. 184 207–221. [DOI] [PubMed] [Google Scholar]
- Zhu, P., W. Zhou, J. Wang, J. Puc, K. A. Ohgi et al., 2007. A histone H2A deubiquitinase complex coordinating histone acetylation and H1 dissociation in transcriptional regulation. Mol. Cell 27 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]