Abstract
Dinitroanilines (oryzalin, trifluralin, ethafluralin) disrupt microtubules in protozoa but not in vertebrate cells, causing selective death of intracellular Toxoplasma gondii parasites without affecting host cells. Parasites containing α1-tubulin point mutations are dinitroaniline resistant but show increased rates of aberrant replication relative to wild-type parasites. T. gondii parasites bearing the F52Y mutation were previously demonstrated to spontaneously acquire two intragenic mutations that decrease both resistance levels and replication defects. Parasites bearing the G142S mutation are largely dependent on oryzalin for viable growth in culture. We isolated 46 T. gondii lines that have suppressed microtubule defects associated with the G142S or the F52Y mutations by acquiring secondary mutations. These compensatory mutations were α1-tubulin pseudorevertants or extragenic suppressors (the majority alter the β1-tubulin gene). Many secondary mutations were located in tubulin domains that suggest that they function by destabilizing microtubules. Most strikingly, we identified seven novel mutations that localize to an eight-amino-acid insert that stabilizes the α1-tubulin M loop, including one (P364R) that acts as a compensatory mutation in both F52Y and G142S lines. These lines have reduced dinitroaniline resistance but most perform better than parental lines in competition assays, indicating that there is a trade-off between resistance and replication fitness.
TOXOPLASMA gondii is a human pathogen grouped within the Apicomplexa, a phylum of protozoa that comprises all obligate intracellular parasites (Levene 1988). Infection with this parasite can be life threatening in immunocompromised individuals and cause birth defects or miscarriage during fetal infection (Black and Boothroyd 2000). Other apicomplexans such as Plasmodium spp. and Cryptosporidium spp. have considerable medical importance or are animal pathogens that affect livestock. Apicomplexans have a specialized apex that contains unique organelles that coordinate invasion of host cells (Morrissette and Sibley 2002a). These parasites are delimited by a pellicle, a composite structure formed by the association of the plasma membrane with the inner membrane complex, an assemblage of flattened vesicles. The invasive stages of apicomplexan parasites have two microtubule populations: subpellicular microtubules and spindle microtubules. Subpellicular microtubules are nondynamic; they maintain both apical polarity and the characteristic crescent shape of the parasite by interacting with the cytoplasmic face of the pellicle. Spindle microtubules form an intranuclear spindle to coordinate chromosome segregation. Both populations are critically important to parasite survival and replication.
Microtubules are built by the polymerization of α-β-tubulin heterodimers and typically contain 13 protofilaments. Protofilaments are formed by longitudinal head-to-tail association of heterodimers and laterally associate to assemble the microtubule lattice (Downing and Nogales 1998). Biochemical and structural studies have elucidated the roles of tubulin domains, which are important to the regulation of microtubule polymerization and depolymerization. Both α- and β-tubulins have H1-S2 (N) and M loops, which coordinate contacts between adjacent protofilaments (Nogales et al. 1997, 1999; Downing and Nogales 1998; Lowe et al. 2001; Li et al. 2002). α-Tubulin contains an eight-amino-acid insert missing from the analogous region of β-tubulin, which stabilizes the α-tubulin M loop to promote protofilament lateral association. Both α- and β-tubulins bind to GTP; however, only β-tubulin GTP can be exchanged or hydrolyzed while α-tubulin GTP plays a structural role in this subunit (David-Pfeuty et al. 1977; Sage et al. 1995a,b; Nogales et al. 1998; Anders and Botstein 2001). The β-tubulin subunits are stimulated to hydrolyze GTP in a polymerization-dependent fashion by association of a GTPase-activating domain from the α-tubulin subunit of the adjacent dimer within the protofilament. Structural evidence indicates that GTP-bound dimers have a conformation that facilitates protofilament contacts by both α- and β-subunits. After hydrolysis, GDP-tubulin dimers have a kinked conformation that is believed to weaken lateral contacts to promote microtubule disassembly (Nogales et al. 2003; Wang et al. 2005; Wang and Nogales 2005; Nogales and Wang 2006a,b).
Protozoan parasites are sensitive to dinitroaniline compounds, which disrupt microtubules to inhibit replication (Stokkermans et al. 1996; Bogitsh et al. 1999; Makioka et al. 2000; Traub-Cseko et al. 2001; Bhattacharya et al. 2002). Given that these compounds are inactive against vertebrate or fungal tubulins, the mechanism of dinitroaniline action is of particular interest for development of new antimicrobial therapies. Our assays, as well as data from others, indicate that wild-type T. gondii parasites have an IC50 value of 0.25 μm for the dinitroaniline oryzalin and display aberrant phenotypes in culture at 0.5 μm oryzalin (Stokkermans et al. 1996; Morrissette and Sibley 2002b). Although extracellular parasites are refractory to the effects of microtubule-disrupting drugs, during intracellular growth, parasite microtubules are dynamic and sensitive to disruption by submicromolar concentrations of oryzalin or other dinitroanilines.
Dinitroaniline selectivity is associated with restricted binding to sensitive (plants and protozoa) but not to resistant (vertebrates and fungi) tubulin (Hess and Bayer 1977; Morejohn et al. 1987; Chan and Fong 1990; Hugdahl and Morejohn 1993). Studies using molecular dynamics analysis and compound docking indicate that dinitroanilines interact with a consistent site on multiple conformations of α-tubulin derived from independent molecular dynamics models of T. gondii, Leishmania spp., and Plasmodium spp. tubulins (Morrissette et al. 2004; Mitra and Sept 2006). Parallel analysis of vertebrate (bovine) α-tubulin reveals that dinitroanilines have nonspecific, low-affinity interactions and no consensus binding site, consistent with in vivo and in vitro observations that these compounds do not bind to vertebrate tubulin or disrupt vertebrate microtubules. This work predicts a binding site on parasite α-tubulin that is located beneath the H1-S2 (N) loop. Since this loop participates in protofilament interactions, the binding site location suggests that dinitroanilines disrupt lateral contacts in the microtubule lattice. Consistent with this notion, a comparison of α-tubulin molecular dynamics simulations in the presence and absence of a bound drug suggests that dinitroaniline binding profoundly limits flexibility of the α-tubulin H1-S2 loop, which is drawn in toward the core of the tubulin dimer (Mitra and Sept 2006).
T. gondii is a haploid organism for most of its life cycle, although it is capable of sexual recombination during a transient diploid zygote stage. There are three α- and three β-tubulin genes in the genome: the α2 gene appears to be gamete stage specific and the divergent α3 gene may be used to construct the conoid, an unusual structure built of tubulin sheets rather than microtubules (Hu et al. 2002). The α1- and β1-tubulin genes are the dominantly expressed forms in the proliferative (tachyzoite) stage of the parasite studied here. We have previously identified a number of α1-tubulin point mutations associated with oryzalin resistance in T. gondii (Morrissette et al. 2004; Ma et al. 2007). The mutations are distributed throughout the linear sequence of α1-tubulin. However, when mapped onto a model of T. gondii α1-tubulin based on the structure of the vertebrate tubulin dimer, many of the mutations cluster in specific domains of the protein. For example, many mutations are located in or near domains that are associated with microtubule stability or in the core of α-tubulin, adjacent to or within the dinitroaniline-binding site identified by computational methods. Our working model suggests that most tubulin mutations confer dinitroaniline resistance by one of two possible mechanisms: (1) by increasing subunit affinity within the microtubule to compensate for the action of a bound drug and (2) by reducing the affinity of the tubulin for dinitroanilines by changes to the compound binding site.
Our previous observations that diverse mutations to α1-tubulin are sufficient for conferring resistance to dinitroanilines such as oryzalin in the protozoan parasite T. gondii might suggest that dinitroaniline-binding site ligands are not appropriate for development of antiparasitic agents. However, in many cases, drug resistance mutations isolated under laboratory conditions are not observed in clinical settings since parasites are incapable of robust growth in natural reservoirs. For example, a subset of laboratory-selected dihydrofolate reductase (DHFR) mutations that confer pyrimethamine resistance in Plasmodium falciparum are either rare or not observed in the field (Hankins et al. 2001; Hastings et al. 2002; Bates et al. 2004). Moreover, studies that have exploited growth competition assays to assess the fitness of T. gondii strains bearing wild-type or pyrimethamine-resistant DHFR genes have concluded that even mutant strains that behave similarly to wild-type parasites in vitro can display growth defects in vivo (Fohl and Roos 2003). We show here that dinitroaniline-resistant parasites have reduced fitness reflected by increased rates of replication defects and poor performance in growth competition assays. These lines rapidly and spontaneously acquire compensatory mutations that correct the fitness defects and reduce resistance to dinitroanilines. We conclude that drugs that selectively target parasite microtubules remain a realistic option for the development of new antiparasitic therapies.
MATERIALS AND METHODS
Culture of Toxoplasma lines:
T. gondii tachyzoites (the standard RH strain and mutants derived from the RH strain) were propagated in human foreskin fibroblast (HFF) cells in DMEM with 10% FBS as previously described (Roos et al. 1994). Oryzalin (Riedel-deHaen, Germany) stock solutions were made up in DMSO. Lines bearing oryzalin-resistance mutations were propagated in 0.5 μm oryzalin to suppress selection of secondary mutations.
Selection of suppressors:
T. gondii lines bearing allelic replacements of the F52Y or G142S mutations to the α1-tubulin gene were generated as previously described (Morrissette et al. 2004; Ma et al. 2007). These lines were serially passed for 3–4 weeks in the absence of oryzalin to select for spontaneous mutants with improved growth. After single-cell cloning, the clones displayed robust growth and each of the lines analyzed represents an independently derived (nonsibling) lineage. Clonal lines were analyzed for changes to the α1- or β1-tubulin genes.
Analysis of tubulin point mutations:
The α1- and β1-tubulin genes were amplified from genomic DNA isolated from individual parasite lines as previously described (Morrissette et al. 2004; Ma et al. 2007). The α1-tubulin gene was amplified using thermal cycling with primers GAGTCTCGTAGAGAACAAGC (5′ UTRA) and CGTTTATACCTTCACCTTTTC (3′ UTRA), and the purified fragment was sequenced as previously described. The β1-tubulin gene was amplified with primers GTGGTGTTGCGCCTTC (5′ UTRB) and CGAGTGTTTAGGACAGTGAC (3′ UTRB) and was sequenced with the following primers: (BT3E1) CATTCTCCGCGATTCTC; (BT5E2) GTCCGGGTGTTCCTAC; (BTF2a) CATCATGGAGACTTTCTCC; (BTR2a) GGAGAAAGTCTCCATGATG; (BTF2b) CAAAGAACATGATGTGCG; (BTR2b) CGCACATCATGTTCTTTG; (BT3E2) CTCGTCCATACC TTCACC; (BT5E3) GAGATGGCACATTTAGTGTG; (BT3E3) CTCCCTCTTCCTCTGC; and (BT5E4) CCGAGTATCAGCAGTACC. DNA sequences were analyzed using Sequencher software (GeneCodes) to identify point mutations in the coding sequence of the α1-tubulin or β1-tubulin genes. We also checked lines for α2-, α3-, β2-, and β3-tubulin mutations but did not identify any changes to these genes.
Structural models of T. gondii tubulin:
Point mutations were mapped onto models of T. gondii tubulin using PyMol (Delano 2002). The models (containing a rebuilt H1-S2 loop) were previously generated for computational studies (Morrissette et al. 2004; Mitra and Sept 2006).
Immunofluorescence staining:
Extracellular parasites were fixed, permeablized, and stained in suspension as previously described (Morrissette and Sibley 2002b). The T. gondii plasma membrane was labeled with DG52 antibody (Burg et al. 1988) and detected with an Alexa 594 secondary antibody (Invitrogen). DNA was visualized with DAPI. Suspension samples were allowed to settle onto 35-mm dishes with glass coverslip insets (MatTek). Images were collected on a Zeiss Axioskop using the Axiovision camera and software. Images were exported and manipulated in Photoshop 8.0.
Quantification of replication defects:
T. gondii were passed into HFF cells without oryzalin selection and allowed to grow until complete host cell lysis. Extracellular parasites were viewed in suspension in MatTek dishes with a coverslip inset using a ×63 phase-contrast lens on a Zeiss Axioskop microscope. Images were captured as tif files and scored by counting as previously described (Ma et al. 2007). To avoid underrepresenting aberrant forms, we counted the number of apical regions to establish total parasites lost from the parasite population through replication defects.
Competition assays:
T25 flasks with confluent HFF cells were inoculated with a 1:1 ratio of RH-strain-derived lines described here and wild-type (RH strain) parasites expressing cytosolic GFP (1 × 107 parasites from each line) (Kim et al. 2001). After host monolayer lysis, a new T25 flask with confluent HFF cells was inoculated with 1.0 ml of the lysed culture and the remaining material (5 ml) was used for flow cytometry analysis. Extracellular parasites were purified away from host cell debris by passing lysate through a 3-μm filter. After parasites were collected by centrifugation (all spins performed at 6000 rpm for 6 min), they were fixed in 1.0% formalin (Sigma) for 1 min at RT. After a PBS wash, parasites were blocked in 10% (w/v) BSA/PBS (Fisher Scientific) for 30 min at 4° and surface labeled with DG52 anti-P30/SAG1 mouse antibody [30 min at 4°, 1:1000 in 10% (w/v) BSA/PBS] and a PE-Cy5.5-conjugated goat anti-mouse secondary antibody (Invitrogen) for 30 min at 4°, 1:1000 in 10% (w/v) BSA/PBS. The total number of parasites (PE-Cy5.5 labeling) and the number of parasites with GFP fluorescence were enumerated by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with Flowjo software (Tree Star). The trend lines represent the percentage of total mutant parasites at each time point, and the analysis was carried out for 7 and 15 serial parasite passages.
RESULTS
Toxoplasma α1-tubulin mutations are associated with oryzalin resistance but have fitness defects:
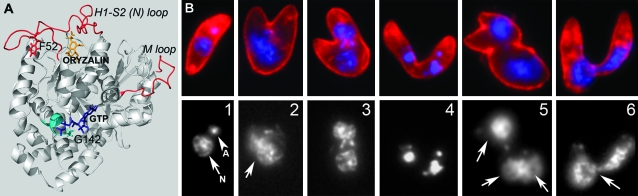
We previously isolated a number of T. gondii lines that have mutations to α1-tubulin that are sufficient for conferring resistance to dinitroanilines such as oryzalin (Morrissette et al. 2004; Ma et al. 2007). Among the lines isolated in our work are two that are oryzalin resistant due to point mutations F52Y or G142S in the α1-tubulin gene (Figure 1). Parasites bearing the G142S mutation are resistant to 1.0 μm oryzalin. G142 participates in binding the α-phosphate portion of GTP (Gigant et al. 2000; Lowe et al. 2001), and parasites with the G142S mutation are largely dependent on the presence of oryzalin for viable growth in culture. F52 is located in the H1-S2 loop, a domain that is critically important for protofilament–protofilament associations in the microtubule lattice (Li et al. 2002). Parasites bearing the F52Y mutation have high rates of defective replication and have been shown to spontaneously acquire compensatory mutations. We previously reported that parasites with the F52Y mutation were resistant to 7 μm oryzalin (Ma et al. 2007). In the course of carrying out the experiments reported here, we discovered that parasites bearing this mutation rapidly acquire secondary mutations (even when under 0.5 μm oryzalin selection) and that the true resistance of the parental line is 13 μm. Relative to wild-type T. gondii, both F52Y and G142S parasite lines have high rates of overt replication defects (Figure 1B). We previously quantified the total percentage of aberrant extracellular parasites for wild type (∼4%) and the F52Y (∼13%) line (Ma et al. 2007). Using a similar analysis, the G142S line has ∼19% replication defects when grown in the absence of oryzalin (not shown).
Figure 1.—
(A) The F52Y and G142S α-tubulin point mutations confer 13 and 1 μm oryzalin resistance when integrated into wild-type (sensitive) T. gondii parasites. The F52Y mutation (red) is located in the H1-S2 (N) loop (red) of α1-tubulin. The computationally determined dinitroaniline-binding site is below this loop (oryzalin in orange). Interactions between the H1-S2 (N) loop and the M loop of adjacent dimers coordinate lateral associations between protofilaments in the microtubule lattice. The G142S mutation is located in the core of α1-tubulin and the G142 residue represents the initial glycine in the GGGTGSG motif (teal), which is characteristic of tubulins. This motif is part of the GTP-binding site, which is essential for correct folding of the tubulin dimer and interacts with the phosphate portion of GTP (purple). (B) (Top) Immunofluorescence images of extracellular parasites of the G142S line labeled with DG52 (red labels the parasite surface) and DAPI (blue labels the parasite DNA). (Bottom) DNA distribution only. (1) A normal-appearing parasite has a crescent shape and DNA staining of the nucleus (arrow) and apicoplast DNA (arrowhead). (2–6) Parasite “monsters” result from replication defects due to improper coordination of spindle microtubule (nuclear division) and subpellicular microtubule (cytokinesis) functions. Although the extracellular parasites in 3 and 4 are similar to intracellular replicating stages (see diagram in Ma et al. 2007), parasites cannot complete division outside of host cells and their abnormal shape prevents invasion of new host cells. In addition, 2, 5, and 6 illustrate parasites with diffuse (2), improperly segregated (three nuclei) (5), and nonsegregated (6) nuclear staining (compare to 1).
Defects are corrected by compensatory mutations in lines derived from the two parental parasite strains:
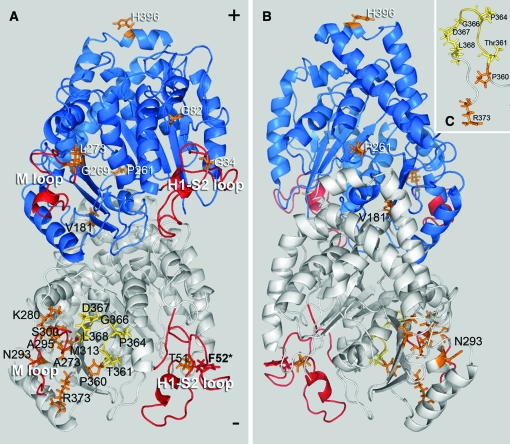
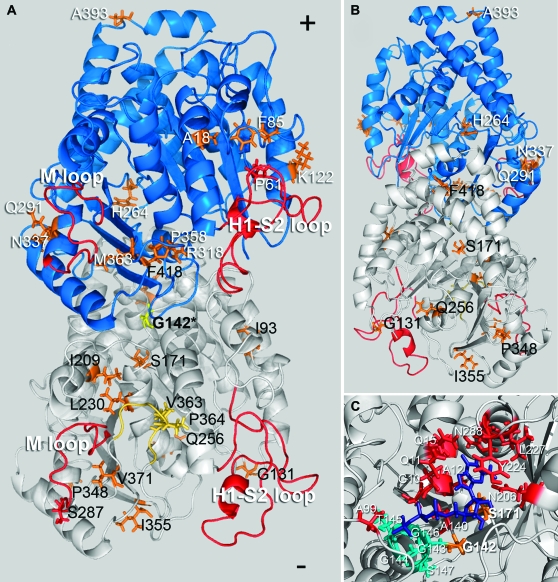
When T. gondii parasites bearing an allelic integration of the F52Y or G142S mutation are passed for several generations in the absence of oryzalin selection, they spontaneously acquire mutations that allow them to grow with increased robustness. After single-cell cloning independently selected lines with improved growth, we analyzed the sequences of T. gondii α1- and β1-tubulin genes for mutations associated with suppression of defects. We identified both α1-tubulin pseudorevertants and extragenic mutations in β1-tubulin and other (unidentified) genes. We isolated 27 independent lines from the F52Y line and identified mutations that localize to α1-tubulin or β1-tubulin in 22 of these lines (Figure 2 and Table 1). Two of the α1-tubulin mutations are located in the M or H1-S2 (N) loops and seven mutations are located in the eight-amino-acid α-tubulin-specific insert. Six of the lines have mutations to the β1-tubulin gene; two of these are in the M or H1-S2 (N) loops. The G142S resistance mutation alters the GGGTGSG tubulin motif, which is associated with binding the phosphate portion of GTP. Of the 24 G142S-derived lines characterized here, 13 acquired a secondary mutation in the α1-tubulin gene (Figure 3 and Table 2). One of these mutations is associated with GTP binding (S171A), two localized in the eight-amino-acid α-tubulin-specific insert, and one is in the M loop. We also isolated 13 lines with extragenic suppressors, with 11 of these occurring in the β1-tubulin gene and 2 being in other, unidentified loci. During the course of these studies we did not identify any true revertants of F52Y or G142S parasites.
Figure 2.—
Location of compensatory mutations obtained from the F52Y line mapped on a model of the Toxoplasma tubulin dimer. The α1-subunit is white and the β1-subunit is blue. (A) Most mutations fall between the M and H1-S2 loops (red) facing the inner lumen of the microtubule. The F52Y mutation is located in the H1-S2 loop of α1-tubulin (red) and secondary mutations in α1-tubulin (black text) occur at T51A, V181I, A273V, K280N, N293D, A295G, S300T, M313T, P360A, T361P, P364A, P364R, G366R, D367V, L368F, and R373C (orange/yellow). Mutations in yellow localize to the α-tubulin-specific insert. Extragenic suppressors in the β1-tubulin subunit (white text) occur at G34S, G82D, P261S, G269V, L273V, and H396Q. (B) The β1-tubulin mutation P261S is the only mutation that faces the outer surface of the dimer within the microtubule lattice, while the β-subunit mutation H396Q is located at the dimer–dimer interface within the protofilament. (C) The mutations T361P, P364A, P364R, G366R, D367V, and L368F occur in the α-tubulin-specific insert (yellow) and the mutations P360A and R373C are adjacent to this insert (orange).
TABLE 1.
F52Y secondary mutations
| Mutant | Codon change | Location | Other nearby mutations |
|---|---|---|---|
| T51A | ACC to GCC | AT H1-S2 loop | F52I, F52L, F52Y: Tg dinitroanilineR (Morrissette et al. 2004) |
| V181I | GTT to ATT | AT β-5 to α-5 loop | S180P, A180T: At twisting (monomer interface) (Ishida et al. 2007a) |
| A273V | GCG to GTG | AT M loop | I275T: Tg dinitroanilineR (Morrissette et al. 2004) |
| K280N | AAG to AAC | AT M loop | K279A-K281A: Sc benomylss (Richards et al. 2000), A281T: At twisting (Ishida et al. 2007a) |
| N293D | AAC to GAC | AT α-helix 9 | A295V: Tg dinitroanilineR (Morrissette et al. 2004) |
| A295G | GCT to GGT | AT α-helix 9 | A295V: Tg dinitroanilineR (Morrissette et al. 2004) |
| S300T | AGC to ACC | AT α-9 to β-8 loop | S300T: Ant. fish coldR (Detrich et al. 2000), M301T: Tg dinitroanilineR (Morrissette et al. 2004) |
| M313T | ATG to ACG | AT β-sheet 8 | D310A-K312A: Sc benomylss (Richards et al. 2000) |
| P360A | CCC to GCC | AT near insert | G354E: Ce mec-12 (Fukushige et al. 1999) |
| T361P | ACT to CCT | AT insert | |
| P364A | CCT to GCT | AT insert | |
| P364R | CCT to CGT | AT insert | Also suppresses G142S |
| G366R | GGT to CGT | AT insert | |
| D367V | GAC to GTC | AT insert | |
| L368F | TTG to TTC | AT insert | |
| R373C | CGC to TGC | AT β-sheet 10 | D373A-R374A: Sc benomylss (Richards et al. 2000) |
| G34S | GGT to AGT | BT H1-S2 loop | G34S: Ce mec-7 (Savage et al. 1989) |
| G82D | GGC to GAC | BT α-2 to β-3 loop | F85L: Tg suppressor of G142S |
| P261S | CCT to TCT | BT α-8 to β-7 loop | F261V: Hs paclitaxelR (Monzo et al. 1999) |
| G269V | GGG to GTG | BT β-sheet 7 | G269D: Ce mec-7 (Savage et al. 1989) |
| L273V | CTC to GTC | BT M loop | T274I: Hs epothilone/taxaneR (Giannakakou et al. 2000) |
| H396Q | CAC to CAG | BT α-helix 11 | A393T: Ce mec-7 (Savage et al. 1989); A394T: At twisting (Ishida et al. 2007a) |
At, Arabidopsis thaliana; Ant. fish, Antarctic fish; Ce, Caenorhabditis elegans; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae; Tg, Toxoplasma gondii; R, resistant; ss, supersensitive.
Figure 3.—
Location of compensatory mutations obtained from the G142S line mapped on a model of the Toxoplasma tubulin dimer (the α1-subunit is white and the β1-subunit is blue). The G142S mutation (yellow) is located in the GTP-binding domain of α-tubulin. (A) Most mutations (orange/yellow) are on the surface of the dimer that faces the inner lumen of the microtubule. Secondary mutations in α1-tubulin (black text) occur at I93V, G131R, S171A, I209V, L230V, Q256H, S287P, P348L, I355E, V363A, P364R, V371G, and F418I. Suppressors in the β1-tubulin subunit (white text) occur at residues A18P, P61A, F85L, K122E, H264Q, Q291E, R318C, N337T, P358R, M363V, and A393V. G142S α1-tubulin mutations at V363A and P364R occur in the α-tubulin-specific insert (yellow). (B) The α1-tubulin mutation at Q256 and the β1-tubulin mutation at H264 are the only mutations that face the outer surface of the dimer within the microtubule lattice. The β-subunit mutation A393V is located at the dimer–dimer interface within the protofilament. (C) The primary resistance mutation at G142 is located within the tubulin motif (teal) of the α-tubulin GTP-binding site. The GTP moiety is purple and residues contributing to GTP binding outside of the GGGTGS tubulin motif are red. The primary mutation G142S is located in the binding site and interacts with the α-phosphate. The S171A mutation also locates to the binding site; S171 interacts with the ribose portion of GTP (Gigant et al. 2000; Lowe et al. 2001).
TABLE 2.
G142S secondary mutations
| Mutant | Codon change | Location | Other nearby mutations |
|---|---|---|---|
| I93V | ATC to GTC | AT β-sheet 3 | H89A-E91A: Sc benomylss (Richards et al. 2000) |
| G131R | GGT to CGT | AT α-3 to β-4 loop | D128A-D131A: Sc colds, benomylss (Richards et al. 2000) |
| S171A | TCG to GCG | AT β-5 to α-5 loop | K167A-E169A: Sc benomylss (Richards et al. 2000) |
| I209V | ATC to GTC | AT α-helix 6 | A208F: At lefty pseudorevertant (Thitamadee et al. 2002) |
| L230V | CTG to GTG | AT α-helix 7 | I231T: Tg dinitroanilineR (Morrissette et al. 2004) |
| Q256H | CAG to CAC | AT α-helix 8 | E255A: Sc dominant lethal (Richards et al. 2000) |
| S287P | TCT to CCT | AT M-loop | S287P: Antarctic fish coldR (Detrich et al. 2000) |
| P348L | CCC to CTC | AT α-10 to β-9 loop | T349I: At twisting (Ishida et al. 2007a) |
| I355E | GAA to ATC | AT β-sheet 9 | G354E: Ce mec-12 (Fukushige et al. 1999); C356Y: Sp lethalts (Radcliffe et al. 1998) |
| V363A | GTT to GCT | AT insert | |
| P364R | CCT to CGT | AT insert | Also suppresses F52Y |
| V371G | GTC to GGC | AT β-9 to β-10 loop | D373A-R374A: Sc benomylss (Richards et al. 2000) |
| F418I | TTC to ATC | AT α-helix 12 | E415K: Ce mec-12 (Fukushige et al. 1999); E416A-E418A: Sc slow growth, benomylss (Richards et al. 2000) |
| A18P | GCC to CCC | BT α-helix 1 | A19K Sc required for taxol binding (Gupta et al. 2003) |
| P61A | CCG to GCG | BT H1-S2 loop | V60A: CHO D45Y supp. (Wang et al. 2004); P61L, P61S: Ce mec-7 (Savage et al. 1989) |
| F85L | TTC to TTG | BT α-2 to β-2 loop | G82D: Tg compensatory for F52Y |
| K122E | AAG to GAG | BT α-helix 3 | R121A-R122A: Sc benomylss (Reijo et al. 1994) |
| H264Q | CAC to CAG | BT α-8 to β-7 loop | L263Q: Dm E194K suppr. (Fackenthal et al. 1995); G269D: Ce mec-7 (Savage et al. 1989) |
| Q291E | CAG to GAG | BT α-helix 9 | Q292G: Hs epothiloneR (He et al. 2001); Q292P: Ce mec-7 (Savage et al. 1989); Q292H: D45Y supp. (Wang et al. 2004) |
| R318C | CGT to TGT | BT β-sheet 8 | F317I, R318G: Ce mec-7 (Savage et al. 1989); R318A-K320A:Sc benomylR (Reijo et al. 1994) |
| N337T | AAC to ACC | BT α-10 to β-9 loop | K336A-D339A: Sc recessive lethal (Reijo et al. 1994) |
| P358R | CCG to CGG | BT β-9 to β-10 loop | C354S, C354A: Sc benomylR, cold stable (Reijo et al. 1994) |
| M363V | ATG to GTG | BT β-9 to β-10 loop | A364T: Hs paclitaxelR (Giannakakou et al. 2000) |
| A393V | GCT to GTT | BT α-helix 11 | A393T: Ce mec-7 (Savage et al. 1989); A394T: At twisting (Ishida et al. 2007a) |
At, A. thaliana; Ce, C. elegans; CHO, Chinese hamster ovary cells; Dm, Drosophila melanogaster; Hs, H. sapiens; Sc, S. cerevisiae; Sp, Schizzosacharomyces pombe; R, resistant; s, sensitive; ss, supersensitive; ts, temperature sensitive.
Growth competition assays indicate that the derived lines have increased fitness relative to the parental strains:
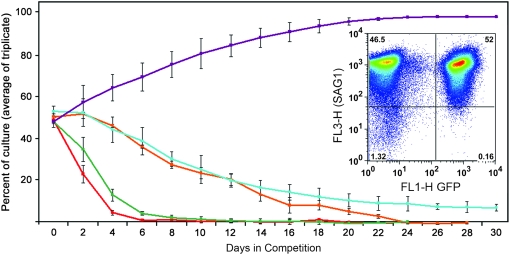
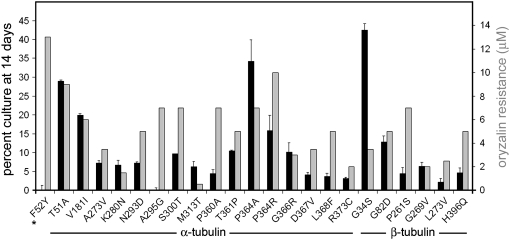
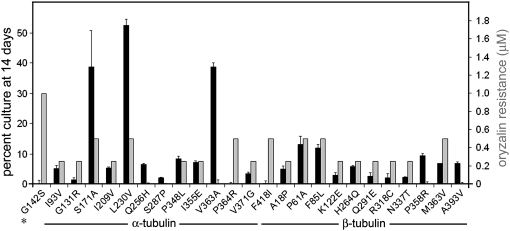
We have exploited an established GFP-expressing T. gondii line to assess the relative fitness of the F52Y and G142S parasite lines and the derived progeny (Kim et al. 2001). Both the GFP-expressing line and the tubulin mutant lines are derived from RH strain T. gondii. We co-infected HFF cells with an equivalent number of GFP-expressing parasites and parasites of each tubulin mutant line and exploited green fluorescence to follow the relative rates of replication and growth over serial passage (Figure 4). Parasites from lysed flasks were used to inoculate new flasks such that they would lyse the host cell monolayer in 2 days. The remainder of each sample was analyzed by flow cytometry. In initial experiments, the parasites were labeled with the DG52 (anti-SAG1) antibody to ensure that the entire parasite population was visualized for analysis (Figure 4 inset). Once we were confident that our gating had captured the parasite population, we omitted the DG52 labeling, with consistent results. At each time point, the total number of parasites and the number of GFP-labeled parasites were determined. Nonfluorescing parasites represent the fraction of the population expressing the specific tubulin mutation(s). These growth competition assays indicate that the F52Y and G142S lines compete poorly with wild-type GFP parasites (Figure 4). After 6 days, the G142S line is not detectable and at 8 days the F52Y line cannot be detected. In contrast, the β1-P61A and α1-P364R lines that are derived from the G142S and F52Y parasites are still present at this time, although their declining percentage in the population indicates that they are less fit than the GFP-RH line. As a control, we also carried out growth competition for wild-type RH parasites vs. the GFP-expressing parasites. Over time, we reproducibly observed that the GFP-expressing parasites were at a disadvantage relative to the wild-type RH T. gondii. Therefore, the growth competition using GFP-RH parasites underestimates the fitness defects associated with the dinitroaniline resistance mutations and the associated suppressed lines. We analyzed all of the F52Y- and G142S-derived lines after 2 weeks in serial passage with GFP-RH parasites to assess relative fitness relationships in competition with wild-type parasites (Figures 5 and 6). In most cases, lines with compensatory mutations do not fully recover robust growth. The most effective F52Y-derived lines with respect to fitness are point mutations at T51A (the α-tubulin H1-S2 loop), P364A (the α-tubulin insert), and G34S (the β-tubulin H1-S2 loop), which were still all <50% of the culture after 2 weeks (Figure 5 and Table 3). The most effective G142S-derived lines are S171A (an α-tubulin GTP-binding site residue), L230V (located in helix 7 of α-tubulin), and V363A (in the α-tubulin insert). The L230V line is present in ∼50% of the culture after 2 weeks (Figure 6 and Table 3). However, since the GFP-RH parasites have a fitness defect relative to unlabeled RH strain wild-type parasites, it is still likely that the L230V line would compete unfavorably with wild-type T. gondii.
Figure 4.—
To assess relative fitness of the F52Y and G142S lines as well as the strains derived from these lines, equivalent numbers of parasites from a specific mutant line and wild-type RH strain parasites expressing GFP were inoculated into culture and the relative percentage of each population was quantified over time during serial passage using flow cytometry. The relative parasite concentrations were quantified at the time of complete host cell lysis when the lysate was passed into a new flask of host cells containing parasites. The red line indicates the behavior of G142S parasites in competition and the orange line is the G142S suppressor line β-P61A (located in the β-tubulin H1-S2 loop). The green line follows a competition assay containing F52Y and the teal line represents behavior of the F52Y secondary mutation α-P364R (located in the α-tubulin-specific insert). The G142S parasites are out-competed by GFP-RH parasites by day 6 whereas the β-P61A suppressor line persists until day 24. The F52Y parasites are eliminated at day 12 but, in the presence of the α-P364R secondary mutation, they were still present, albeit at reduced levels, when the competition was terminated at 30 days. The purple line traces the control competition between wild-type (untransfected RH strain parasites) and the GFP-transfected “wild-type” RH strain line. Unlabeled parasites have a growth advantage over GFP-transfected parasites. (Inset) A representative FACS dot plot of DG52-labeled (red) parasites showing relative numbers of GFP-expressing wild-type Toxoplasma and a mutant line after a single passage.
Figure 5.—
Replication fitness and oryzalin resistance in F52Y parasites and the derived lines. Solid bars: the percentage of mutant parasites relative to GFP-RH parasites after seven passages (14 days). The assays were carried out in triplicate and error bars indicate standard error of the mean. Shaded bars: oryzalin resistance levels observed for F52Y parasites and the derived lines.
Figure 6.—
Replication fitness and oryzalin resistance in G142S parasites and the 24 derived lines. Solid bars: the percentage of mutant parasites relative to GFP-RH parasites after seven passages (14 days). The assays were carried out in triplicate and error bars indicate standard error of the mean. Shaded bars: oryzalin resistance levels observed in G142S parasites and the derived lines.
TABLE 3.
Resistance and fitness levels
| Mutation | % decrease resistance | % decrease population (day 14) | Mutation | % decrease resistance | % decrease population (day 14) |
|---|---|---|---|---|---|
| F52Y-parental | 0 | 100 | G142S-parental | 0 | 100 |
| F52Y-αT51A | 31 | 43 | G142S-αI93V | 75 | 89 |
| F52Y-αV181I | 54 | 60 | G142S-αG131R | 75 | 97 |
| F52Y-αA273V | 73 | 86 | G142S-αS171A | 50 | 24 |
| F52Y-αK280N | 88 | 87 | G142S-αI209V | 75 | 89 |
| F52Y-αN293D | 62 | 84 | G142S-αL230V | 50 | 0 |
| F52Y-αA295G | 46 | 100 | G142S-αQ256H | 100 | 87 |
| F52Y-αS300T | 46 | 80 | G142S-αS287P | 100 | 96 |
| F52Y-αM313T | 96 | 86 | G142S-αP348L | 75 | 84 |
| F52Y-αP360A | 46 | 92 | G142S-αI355E | 75 | 85 |
| F52Y-αT361P | 62 | 80 | G142S-αV363A | 100 | 25 |
| F52Y-αP364A | 46 | 30 | G142S-αP364R | 50 | 100 |
| F52Y-αP364R | 23 | 69 | G142S-αV371G | 75 | 93 |
| F52Y-αG366R | 77 | 78 | G142S-αF418I | 50 | 84 |
| F52Y-αD367V | 73 | 92 | G142S-βA18P | 75 | 90 |
| F52Y-αL368F | 62 | 92 | G142S-βP61A | 50 | 74 |
| F52Y-αR373C | 85 | 94 | G142S-βF85L | 50 | 76 |
| F52Y-βG34S | 73 | 14 | G142S-βK122E | 75 | 94 |
| F52Y-βG82D | 62 | 75 | G142S-βH264Q | 75 | 88 |
| F52Y-βP261S | 46 | 91 | G142S-βQ291E | 75 | 95 |
| F52Y-βG269V | 85 | 88 | G142S-βR318C | 75 | 96 |
| F52Y-βL273V | 81 | 95 | G142S-βN337T | 75 | 96 |
| F52Y-βH296Q | 62 | 91 | G142S-βP358R | 100 | 81 |
| G142S-βM363V | 50 | 86 | |||
| G142S-βA393V | 100 | 85 |
The derived lines have diminished oryzalin resistance relative to the parental strains:
The F52Y and G142S parasite lines display 13 and 1.0 μm oryzalin resistance, respectively. Lines derived from these parental strains display reduced oryzalin resistance (Figures 5 and 6 and Table 3). With respect to the F52Y-derived lines, the P364R line retains the highest level of oryzalin resistance (7 μm) and competes relatively well with the GFP-RH parasites. Since the G142S line parasites have 1 μm oryzalin resistance, the range of resistance observed in the derived lines is substantially lower. A number of the lines (the Q256H, S287P, and V363A mutations in α-tubulin and the P358R and A393V mutations in β-tubulin) do not have greater resistance to oryzalin than that observed for wild-type parasites (<0.5 μm by morphological criteria). Lines with secondary mutations at S171A and L230V retain the highest levels of oryzalin resistance in the context of the greatest degree of overall fitness in the competition assay. All 46 lines bearing compensatory mutations are associated with diminished oryzalin resistance.
DISCUSSION
Previous genetic studies have identified secondary mutations that correct tubulin-associated defects. In studies of colchicine resistance, intragenic mutations correct defects associated with the D45Y mutation to β-tubulin, which confers colchicine resistance in CHO cells (Wang et al. 2004). This substitution is located in the H1-S2 loop and, since it increases microtubule stability, cells that express it are hypersensitive to taxol. Lines that suppress the taxol hypersensitivity and associated temperature-sensitive defects have point mutations at V60A (the H1-S2 loop) or Q292H (near the M loop) of β-tubulin that alter microtubule stability. When wild-type CHO cells express a tagged β-tubulin construct bearing the D45Y mutation, the microtubule array is strikingly denser relative to the array observed with expression of wild-type tubulin alone. Inclusion of secondary mutations in the β-tubulin constructs reduces the microtubule array, making it similar to that observed with expression of wild-type tubulin. When β-tubulin constructs with the V60A and Q292H secondary mutations alone are expressed, the microtubule arrays are dramatically reduced relative to those observed with wild-type tubulin expression. These observations are strikingly similar to our results described here. The F52Y mutation is predicted to increase microtubule stability to confer dinitroaniline resistance, but also causes increased rates of replication defects. Secondary mutations are located in regions such as the H1-S2 loop, the M loop, and the α-tubulin insert domain, which are predicted to decrease microtubule stability. We attempted to express GFP-tagged vertebrate tubulin with analogous mutations in COS-7 cells to visualize differential assembly of these tubulins. Unfortunately, expression appeared to be somewhat toxic and the patterns were too variable to be used to assess the relative stability of tubulins with these mutations (J. Tran and N. Morrissette, data not shown).
Many of the secondary mutations are identical or quite similar to previously identified mutations that decrease microtubule stability in Caenorhabditis elegans and Arabidopsis thaliana. C. elegans sensory neuron function requires unusual 15-protofilament microtubules (Chalfie and Thomson 1979, 1982; Chalfie 1982; Chalfie et al. 1986; Savage et al. 1989, 1994). These microtubules are built from dimers encoded by distinct α- (mec-12+) and β- (mec-7+) tubulin genes (Savage et al. 1989, 1994; Gu et al. 1996; Fukushige et al. 1999). A number of mec-7 and mec-12 alleles have been identified in screens for sensory neuron defects. In addition, mutations in Arabidopsis that convert linear growth of plant parts (such as roots and stems) to a twisted phenotype have altered cortical microtubule arrays. This twisting morphology can be phenocopied by treatment of wild-type plants with the microtubule inhibitor propyzamide. Many of the twisting mutants have mutations to α- and β-tubulin that decrease microtubule stability and increase plant sensitivity to microtubule-disrupting drugs (Thitamadee et al. 2002; Abe et al. 2004; Nakajima et al. 2004, 2006; Shoji et al. 2004; Abe and Hashimoto 2005; Ishida and Hashimoto 2007; Ishida et al. 2007a,b). The β-tubulin mutation G34S (a F52Y suppressor) was previously identified as a mild, recessive mec-7 allele (Savage et al. 1989). Other mec-7 alleles (P61L/S, G269D, R318G, and A393T) alter equivalent residues to different substitutions in T. gondii β-tubulin (the F52Y suppressor G269V and the G142S suppressors P61A, R318C, and A393V). The G142S secondary mutation I355E in α-tubulin is adjacent to the location of the mec-12 allele G354E. Moreover, the A393 residue is also identified as a threonine substitution causing twisting in Arabidopsis β-tubulin (A394T). The F52Y compensatory mutations V181I and K280N in α-tubulin and H396Q in β-tubulin and the G142S mutation P348L (α-tubulin) are near the twisting mutations S180P, A180T, A281T, and T349I (α-tubulin) and A394T (β-tubulin). Finally, the G142S suppressor Q291E is adjacent to a second glutamine at 292 that is a mec-7 allele (Q292P). This residue also influences microtubule stability in the context of epothilone resistance (Q292G) and suppresses hyperstabilized microtubules (Q292H) in D45Y colchicine-resistant cells (He et al. 2001; Wang et al. 2004).
One of the novel findings presented in this work is the identification of a set of seven mutations that localize to the α-tubulin-specific insert (TVVPGGDL). The compensatory mutations in the F52Y line that localize to the insert are T361P, P364A, P364R, G366R, D367V, and L368F and in the G142S line mutations are V363A and P364R. To our knowledge, the insert substitutions represent the first description of mutations located in this highly conserved domain. We hypothesize that these substitutions impair the ability of the insert to promote M-loop lateral contacts with the adjacent protofilament. Indeed, the D367V mutation eliminates a salt bridge that occurs with R229 (Ma et al. 2007). Strikingly, the P364R mutation functions as a compensatory mutation in both lines, albeit more successfully in the F52Y line (compare competition results in Figures 5 and 6 and Table 3). Although the G142S/P364R line does not compete effectively enough with wild-type parasites to remain in the culture at 14 days, it was isolated as a line with improved growth over the G142S line and at some subtle level is likely to improve fitness of the parental line. This is also likely to be the explanation for the F52Y suppressor A295G in competition at 14 days (Table 3).
The studies presented here show a correlation between acquisition of increased fitness and the appearance of mutations in the α1- or β1-tubulin genes. While the improved fitness observed in parasite lines derived from the F52Y and G142S parental strains is likely due to secondary tubulin mutations, other (nontubulin) mutations may also contribute to the observed phenotypes of decreased resistance and enhanced growth. Indeed, five of the lines derived from the F52Y suppressor screen and two lines derived from the G142S suppressor screen have improved growth but do not have altered α1-, α3-, β1-, or β3-tubulin genes, suggesting that nontubulin compensatory mutations do arise. Moreover, although we have created allelic replacements for two α-tubulin pseudorevertants in wild-type parasites (Ma et al. 2007), we cannot create allelic replacements for the β-tubulin suppressors since we cannot select for these in the absence of dinitroaniline selection. When we analyzed the allelic replacements for the α-tubulin pseudorevertants (F52Y/A273V and F52Y/D367V) in the competition assay, they performed differently from the equivalent lines with these spontaneous mutations. Surprisingly, in both cases the transgene versions of the lines had greater fitness levels than the spontaneous mutant lines (data not shown). The suppressed lines described here were isolated after 4–6 weeks of growth in media without oryzalin. This represents the shortest time in which we could observe parasites with new mutations. In contrast, it takes at least 6 weeks to isolate transgene-bearing lines. We predict that further growth of lines with impaired fitness in the absence of oryzalin selection would select for additional mutations that collectively contribute to increased fitness.
Previous studies have established that there is a tradeoff between carrying genes that confer drug resistance and the fitness cost of these altered alleles, particularly in the absence of drug selection. Recent field studies from Malawi indicate that P. falciparum parasites in this region lost resistance to chloroquine in <10 years after sulfadoxine–pyrimethamine treatment replaced chloroquine treatment for individuals suffering from malaria (Laufer et al. 2006; Vogel 2006). These field observations, as well as the controlled laboratory studies of DHFR mutations in P. falciparum and T. gondii described in the Introduction, indicate that the existence of mutant lines that express resistant forms of target proteins does not rule out the development and use of agents that inhibit these proteins. With respect to the studies presented here, we believe that although dinitroanilines or other microtubule-disrupting compounds do not yet exist in an appropriate form for treatment of parasite infections, selectively targeting protozoan tubulin remains a viable strategy for eliminating parasite infections.
Acknowledgments
We thank Dan Sackett for commenting on this manuscript, David Sibley and Michael Grigg for helpful conversations on competition assays, and Sheryl Tsai for bringing clarity to PyMOL commands. We thank Candice Kwark and Brian Luk, high school student participants in the American Cancer Society summer research program at the University of California at Irvine (UCI), who helped select and analyze the lines described here. Research presented in this article was supported by National Institutes of Health grants AI055790 and AI067981 to N.S.M. L.G. and D.W. are undergraduate students in the campus-wide honors program at UCI and L.G. was supported by a UCI summer undergraduate research program fellowship.
References
- Abe, T., and T. Hashimoto, 2005. Altered microtubule dynamics by expression of modified alpha-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants. Plant J. 43 191–204. [DOI] [PubMed] [Google Scholar]
- Abe, T., S. Thitamadee and T. Hashimoto, 2004. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 45 211–220. [DOI] [PubMed] [Google Scholar]
- Anders, K. R., and D. Botstein, 2001. Dominant-lethal alpha-tubulin mutants defective in microtubule depolymerization in yeast. Mol. Biol. Cell 12 3973–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, S. J., P. A. Winstanley, W. M. Watkins, A. Alloueche, J. Bwika et al., 2004. Rare, highly pyrimethamine-resistant alleles of the Plasmodium falciparum dihydrofolate reductase gene from 5 African sites. J. Infect. Dis. 190 1783–1792. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, G., M. M. Salem and K. A. Werbovetz, 2002. Antileishmanial dinitroaniline sulfonamides with activity against parasite tubulin. Bioorg. Med. Chem. Lett. 12 2395–2398. [DOI] [PubMed] [Google Scholar]
- Black, M. W., and J. C. Boothroyd, 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64 607–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogitsh, B. J., O. L. Middleton and R. Ribeiro-Rodrigues, 1999. Effects of the antitubulin drug trifluralin on the proliferation and metacyclogenesis of Trypanosoma cruzi epimastigotes. Parasitol. Res. 85 475–480. [DOI] [PubMed] [Google Scholar]
- Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware and J. C. Boothroyd, 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141 3584–3591. [PubMed] [Google Scholar]
- Chalfie, M., 1982. Microtubule structure in Caenorhabditis elegans neurons. Cold Spring Harb. Symp. Quant. Biol. 46(Pt 1): 255–261. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., and J. N. Thomson, 1979. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J. Cell Biol. 82 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie, M., and J. N. Thomson, 1982. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell Biol. 93 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie, M., E. Dean, E. Reilly, K. Buck and J. N. Thomson, 1986. Mutations affecting microtubule structure in Caenorhabditis elegans. J. Cell Sci. Suppl. 5 257–271. [DOI] [PubMed] [Google Scholar]
- Chan, M. M., and D. Fong, 1990. Inhibition of leishmanias but not host macrophages by the antitubulin herbicide trifluralin. Science 249 924–926. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty, T., H. P. Erickson and D. Pantaloni, 1977. Guanosinetriphosphatase activity of tubulin associated with microtubule assembly. Proc. Natl. Acad. Sci. USA 74 5372–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano, W. L., 2002. The PyMOL Molecular Graphics System. http://www.pymol.org.
- Detrich, H. W., III, S. K. Parker, R. C. Williams, Jr., E. Nogales and K. H. Downing, 2000. Cold adaptation of microtubule assembly and dynamics. Structural interpretation of primary sequence changes present in the alpha- and beta-tubulins of Antarctic fishes. J. Biol. Chem. 275 37038–37047. [DOI] [PubMed] [Google Scholar]
- Downing, K. H., and E. Nogales, 1998. Tubulin and microtubule structure. Curr. Opin. Cell Biol. 10 16–22. [DOI] [PubMed] [Google Scholar]
- Fackenthal, J. D., J. A. Hutchens, F. R. Turner and E. C. Raff, 1995. Structural analysis of mutations in the Drosophila β2-tubulin isoform reveals regions in the β-tubulin molecular required for general and for tissue-specific microtubule functions. Genetics 139 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohl, L. M., and D. S. Roos, 2003. Fitness effects of DHFR-TS mutations associated with pyrimethamine resistance in apicomplexan parasites. Mol. Microbiol. 50 1319–1327. [DOI] [PubMed] [Google Scholar]
- Fukushige, T., Z. K. Siddiqui, M. Chou, J. G. Culotti, C. B. Gogonea et al., 1999. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell Sci. 112(Pt 3): 395–403. [DOI] [PubMed] [Google Scholar]
- Giannakakou, P., R. Gussio, E. Nogales, K. H. Downing, D. Zaharevitz et al., 2000. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc. Natl. Acad. Sci. USA 97 2904–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigant, B., P. A. Curmi, C. Martin-Barbey, E. Charbaut, S. Lachkar et al., 2000. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell 102 809–816. [DOI] [PubMed] [Google Scholar]
- Gu, G., G. A. Caldwell and M. Chalfie, 1996. Genetic interactions affecting touch sensitivity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93 6577–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, M. L., Jr., C. J. Bode, G. I. Georg and R. H. Himes, 2003. Understanding tubulin-Taxol interactions: mutations that impart Taxol binding to yeast tubulin. Proc. Natl. Acad. Sci. USA 100 6394–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins, E. G., D. C. Warhurst and C. H. Sibley, 2001. Novel alleles of the Plasmodium falciparum dhfr highly resistant to pyrimethamine and chlorcycloguanil, but not WR99210. Mol. Biochem. Parasitol. 117 91–102. [DOI] [PubMed] [Google Scholar]
- Hastings, M. D., S. J. Bates, E. A. Blackstone, S. M. Monks, T. K. Mutabingwa et al., 2002. Highly pyrimethamine-resistant alleles of dihydrofolate reductase in isolates of Plasmodium falciparum from Tanzania. Trans. R. Soc. Trop. Med. Hyg. 96 674–676. [DOI] [PubMed] [Google Scholar]
- He, L., C.-P. Huang Yang and S. B. Horwitz, 2001. Mutations in {beta}-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol. Cancer Ther. 1 3–10. [PubMed] [Google Scholar]
- Hess, F. D., and D. E. Bayer, 1977. Binding of the herbicide trifluralin to Chlamydomonas flagellar tubulin. J. Cell Sci. 24 351–360. [DOI] [PubMed] [Google Scholar]
- Hu, K., D. S. Roos and J. M. Murray, 2002. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J. Cell Biol. 156 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl, J. D., and L. C. Morejohn, 1993. Rapid and reversible high-affinity binding of the dinitroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol. 102 725–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, T., and T. Hashimoto, 2007. An Arabidopsis thaliana tubulin mutant with conditional root-skewing phenotype. J. Plant Res. 120 635–640. [DOI] [PubMed] [Google Scholar]
- Ishida, T., Y. Kaneko, M. Iwano and T. Hashimoto, 2007. a Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104 8544–8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, T., S. Thitamadee and T. Hashimoto, 2007. b Twisted growth and organization of cortical microtubules. J. Plant Res. 120 61–70. [DOI] [PubMed] [Google Scholar]
- Kim, K., M. S. Eaton, W. Schubert, S. Wu and J. Tang, 2001. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 113 309–313. [DOI] [PubMed] [Google Scholar]
- Laufer, M. K., P. C. Thesing, N. D. Eddington, R. Masonga, F. K. Dzinjalamala et al., 2006. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 355 1959–1966. [DOI] [PubMed] [Google Scholar]
- Levene, N. D., 1988. The Protozoan Phylum Apicomplexa. CRC Press, Boca Raton, FL.
- Li, H., D. DeRosier, W. Nicholson, E. Nogales and K. Downing, 2002. Microtubule structure at 8 a resolution. Structure 10 1317. [DOI] [PubMed] [Google Scholar]
- Lowe, J., H. Li, K. H. Downing and E. Nogales, 2001. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 313 1045–1057. [DOI] [PubMed] [Google Scholar]
- Ma, C., C. Li, L. Ganesan, J. Oak, S. Tsai et al., 2007. Mutations in {alpha}-tubulin confer dinitroaniline resistance at a cost to microtubule function. Mol. Biol. Cell 18 4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makioka, A., M. Kumagai, H. Ohtomo, S. Kobayashi and T. Takeuchi, 2000. Effect of dinitroaniline herbicides on the growth of Entamoeba histolytica. J. Parasitol. 86 607–610. [DOI] [PubMed] [Google Scholar]
- Mitra, A., and D. Sept, 2006. Binding and interaction of dinitroanilines with apicomplexan and kinetoplastid alpha-tubulin. J. Med. Chem. 49 5226–5231. [DOI] [PubMed] [Google Scholar]
- Monzo, M., R. Rosell, J. J. Sanchez, J. S. Lee, A. O'Brate et al., 1999. Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J. Clin. Oncol. 17 1786–1793. [DOI] [PubMed] [Google Scholar]
- Morejohn, L. C., T. E. Bureau, J. Mole-Bajer, A. S. Bajer and D. E. Fosket, 1987. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172 252–264. [DOI] [PubMed] [Google Scholar]
- Morrissette, N. S., and L. D. Sibley, 2002. a Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 66 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette, N. S., and L. D. Sibley, 2002. b Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J. Cell Sci. 115 1017–1025. [DOI] [PubMed] [Google Scholar]
- Morrissette, N. S., A. Mitra, D. Sept and L. D. Sibley, 2004. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell 15 1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., I. Furutani, H. Tachimoto, H. Matsubara and T. Hashimoto, 2004. SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., T. Kawamura and T. Hashimoto, 2006. Role of the SPIRAL1 gene family in anisotropic growth of Arabidopsis thaliana. Plant Cell Physiol. 47 513–522. [DOI] [PubMed] [Google Scholar]
- Nogales, E., and H. W. Wang, 2006. a Structural intermediates in microtubule assembly and disassembly: How and why? Curr. Opin. Cell Biol. 18 179–184. [DOI] [PubMed] [Google Scholar]
- Nogales, E., and H. W. Wang, 2006. b Structural mechanisms underlying nucleotide-dependent self-assembly of tubulin and its relatives. Curr. Opin. Struct. Biol. 16 221–229. [DOI] [PubMed] [Google Scholar]
- Nogales, E., S. G. Wolf and K. H. Downing, 1997. Visualizing the secondary structure of tubulin: three-dimensional map at 4 A. J. Struct. Biol. 118 119–127. [DOI] [PubMed] [Google Scholar]
- Nogales, E., K. H. Downing, L. A. Amos and J. Lowe, 1998. Tubulin and FtsZ form a distinct family of GTPases. Nat. Struct. Biol. 5 451–458. [DOI] [PubMed] [Google Scholar]
- Nogales, E., M. Whittaker, R. A. Milligan and K. H. Downing, 1999. High-resolution model of the microtubule. Cell 96 79–88. [DOI] [PubMed] [Google Scholar]
- Nogales, E., H. W. Wang and H. Niederstrasser, 2003. Tubulin rings: Which way do they curve? Curr. Opin. Struct. Biol. 13 256–261. [DOI] [PubMed] [Google Scholar]
- Radcliffe, P., D. Hirata, D. Childs, L. Vardy and T. Toda, 1998. Identification of novel temperature-sensitive lethal alleles in essential beta-tubulin and nonessential alpha 2-tubulin genes as fission yeast polarity mutants. Mol. Biol. Cell 9 1757–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo, R. A., E. M. Cooper, G. J. Beagle and T. C. Huffaker, 1994. Systematic mutational analysis of the yeast beta-tubulin gene. Mol. Biol. Cell 5 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, K. L., K. R. Anders, E. Nogales, K. Schwartz, K. H. Downing et al., 2000 Structure-function relationships in yeast tubulins. Mol. Biol. Cell 11 1887–1903. [DOI] [PMC free article] [PubMed]
- Roos, D. S., R. G. Donald, N. S. Morrissette and A. L. Moulton, 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45 27–63. [DOI] [PubMed] [Google Scholar]
- Sage, C. R., A. S. Davis, C. A. Dougherty, K. Sullivan and K. W. Farrell, 1995. a beta-Tubulin mutation suppresses microtubule dynamics in vitro and slows mitosis in vivo. Cell Motil. Cytoskeleton 30 285–300. [DOI] [PubMed] [Google Scholar]
- Sage, C. R., C. A. Dougherty, A. S. Davis, R. G. Burns, L. Wilson et al., 1995. b Site-directed mutagenesis of putative GTP-binding sites of yeast beta-tubulin: evidence that alpha-, beta-, and gamma-tubulins are atypical GTPases. Biochemistry 34 7409–7419. [DOI] [PubMed] [Google Scholar]
- Savage, C., M. Hamelin, J. G. Culotti, A. Coulson, D. G. Albertson et al., 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3 870–881. [DOI] [PubMed] [Google Scholar]
- Savage, C., Y. Xue, S. Mitani, D. Hall, R. Zakhary et al., 1994. Mutations in the Caenorhabditis elegans beta-tubulin gene mec-7: effects on microtubule assembly and stability and on tubulin autoregulation. J. Cell Sci. 107(Pt 8): 2165–2175. [DOI] [PubMed] [Google Scholar]
- Shoji, T., N. N. Narita, K. Hayashi, J. Asada, T. Hamada et al., 2004. Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol. 136 3933–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkermans, T. J., J. D. Schwartzman, K. Keenan, N. S. Morrissette, L. G. Tilney et al., 1996. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 84 355–370. [DOI] [PubMed] [Google Scholar]
- Thitamadee, S., K. Tuchihara and T. Hashimoto, 2002. Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417 193–196. [DOI] [PubMed] [Google Scholar]
- Traub-Cseko, Y. M., J. M. Ramalho-Ortigao, A. P. Dantas, S. L. de Castro, H. S. Barbosa et al., 2001. Dinitroaniline herbicides against protozoan parasites: the case of Trypanosoma cruzi. Trends Parasitol. 17 136–141. [DOI] [PubMed] [Google Scholar]
- Vogel, G., 2006. Malaria. Chloroquine makes a comeback. Science 314 904. [DOI] [PubMed] [Google Scholar]
- Wang, H. W., and E. Nogales, 2005. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature 435 911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. W., S. Long, K. R. Finley and E. Nogales, 2005. Assembly of GMPCPP-bound tubulin into helical ribbons and tubes and effect of colchicine. Cell Cycle 4 1157–1160. [DOI] [PubMed] [Google Scholar]
- Wang, Y., S. Veeraraghavan and F. Cabral, 2004. Intra-allelic suppression of a mutation that stabilizes microtubules and confers resistance to colcemid. Biochemistry 43 8965–8973. [DOI] [PubMed] [Google Scholar]