Abstract
Developing genetically modified crop plants that are biologically contained could reduce significantly the potential spread of transgenes to conventional and organic crop plants and to wild or weedy relatives. Among several strategies, the hereditary mode of transmission of transgenes, whether dominant, recessive, or maternal, could play a major role in interspecific gene flow. Here we report on the gene flow between foxtail millet (Setaria italica), an autogamous crop, and its weedy relative, S. viridis, growing within or beside fields containing the three kinds of inherited herbicide resistance. Over the 6-year study, in the absence of herbicide selection, the maternal chloroplast-inherited resistance was observed at a 2 × 10−6 frequency in the weed populations. Resistant weed plants were observed 60 times as often, at 1.2 × 10−4 in the case of the nuclear recessive resistance, and 190 times as often, at 3.9 × 10−4 in the case of the dominant resistance. Because the recessive gene was not expressed in the first-generation hybrids, it should be more effective than dominant genes in reducing gene flow under normal agricultural conditions where herbicides are sprayed because interspecific hybrids cannot gain from beneficial genes.
GENETICALLY modified (GM) crops could generate potential benefits in many areas of agricultural performance, including best uses of agrochemicals and simplified farm management, and they may broaden the offer of plant services, including soil detoxification and production of medicinal substances. However, many areas of scientific uncertainty and public concern remain regarding environmental and health hazards. In particular, the question of the (trans)gene flow to wild relatives of GM crops is a hot topic, and the debate is open in Europe about the coexistence of GM and non-GM crops. Spontaneous gene flow from crops in the fields to their wild relatives has been documented for most important crops (Ellstrand 2003). Designing strategies to prevent (trans)genes from moving into genomes of related species therefore should be of the highest priority.
In addition to agronomical management, technologies for the prevention of transgene flow to nontransgenic plants may significantly reduce concern about its impacts on biodiversity and non-GM crops. They include biotechnology-based switch mechanisms, also called genetic use restriction technologies (Hills et al. 2007); transgenic mitigation using a tandem construct where a gene of choice is linked to a gene that is deleterious for a wild recipient plant but neutral for the crop (Al-Ahmad et al. 2005); and transgene incorporation into the plant chloroplast because cultivated species generally inherit plastids from the mother and chloroplast genes are not carried by pollen (Daniell 2002). Although tested in the laboratory, those technologies remain pure theory and there is still little knowledge of their ecological and agronomic effects.
Other genetic strategies, such as multigenic determinism and recessive expression, are advocated. In particular, nuclear recessive genes are not expressed by heterozygous plants, so there is no risk of the presence of the transgene product in the case of a non-GM field pollinated by adjacent GM fields. In addition, they are not expressed in interspecific hybrids between crops and their wild relatives, so that even a beneficial transgene cannot help hybrids to be selected, whatever the habitat. As interspecific hybrids often suffer some lower fitness due to incompatible gene combinations or an unbalanced hybridization process between distantly related species (Ellstrand 2003; Van Tienderen 2004), this can help reduce the spread of the transgenes. However, homozygous recessive plants occur in hybrid progeny, and these plants could be submitted to favorable selection.
In this article, we aimed at comparing over the course of 6 years the efficiency of this strategy with regard to the present situation of a simple nuclear dominant gene and that of the maternal transgene transmission. We investigated three kinds of herbicide resistance with Mendelian recessive, Mendelian dominant, or maternal inherited genes in the foxtail millet, Setaria italica L., as a model crop. Foxtail millet is the domesticated crop of the late Neolithic civilization of North China (Lu et al. 2005), but its area has decreased dramatically in recent years. It is a predominantly autogamous cereal that, however, produces numerous pollen grains (1200/flower) of which at least 1.4% move away from the plant and can fertilize male-sterile target plants up to 40 m away (Gu 1987; Wang et al. 1997, 2001). It can cross with its putative wild ancestor, the green foxtail Setaria viridis L., a weed widespread worldwide that shares the same genome (2n = 18; Benabdelmouna et al. 2001) and also displays predominant autogamy, with 0.8% outcrossing on average under natural conditions and up to 4.1% under favorable conditions (Till-Bottraud et al. 1992). Seeds produced by green foxtail growing between the rows of a foxtail millet field can contain 0.15–0.27% and up to 3% interspecific hybrids (De Wet et al. 1979; Till-Bottraud et al. 1992), which is high enough to make gene flow inevitable.
The herbicide resistances were obtained through interspecific crosses and classical breeding (Darmency and Pernès 1985; Wang et al. 1996; Wang and Darmency 1997). Some herbicide-resistant varieties are now registered in China because hand weeding is no longer possible. Their development requires more knowledge about their behavior in the farmers' fields and the impact of the transfer of herbicide resistance genes into wild populations of green foxtail. The experiments consisted of recording the number of interspecific hybrids produced by green foxtail plants within the foxtail millet fields and the adjacent or external crop-free rings over 6 years in two locations of different agro-ecology in China.
MATERIALS AND METHODS
Plant material:
Resistance to three different herbicides in foxtail millet was obtained by interspecific crosses between the Chinese cultivars of S. italica and herbicide-resistant Canadian and French accessions of S. viridis in the Weed Laboratory in Dijon. Resistance to triazines was shown to be maternally inherited and endowed by the chloroplast psbA gene encoding the D1 protein (Darmency and Pernès 1985; Tian and Darmency 2006). Resistance to dinitroanilines was shown to be due to a nuclear α2-tubulin gene recessive to the wild type (Wang et al. 1996; Délye et al. 2004; Tian et al. 2006). Resistance to cyclohexanediones was due to the nuclear-encoded chloroplast ACCase gene inherited as a dominant trait (Wang and Darmency 1997; Délye et al. 2002). Lines derived from these hybrids were further bred at the Crop Germplasm Institute in Beijing through backcrossing and progeny selection, and homozygous lines were selected for this study: AR638 resistant to atrazine with maternal inheritance, TR2 and TR68 resistant to trifluralin with nuclear recessive inheritance, and SR3522 resistant to sethoxydim with nuclear dominant inheritance (the latter line was certified as an elite cultivar in China in 2004).
Field location:
The herbicide-resistant material was cultivated for 4 successive years, from 1999 to 2002, on the same fields in two locations. The Chicheng location is 150 km north of the Great Wall and belongs to Zhangjiakou city, Hebei province, a typical spring-sowing millet area. The Xinji location is 300 km south of Beijing and belongs to Shijiazhuang city, Hebei province, a typical summer-sowing millet area. In each location, the maternal, recessive, and dominant inherited resistant lines were planted in three different places separated by 1.7–4 km. All the experimental places were >600 m away from other foxtail millet fields to prevent any interference between experiments and other pollen sources.
Experimental design:
Every field had almost the shape of a disk and covered an area of ∼1.1 ha. The central 60-m-diameter disk, i.e., ∼0.28 ha, was planted with one of the resistant lines. The resistant foxtail millet was sown and cultivated according to routine practices in the corresponding region, with a density of 520,000 plant (pl) ha−1 in Chicheng and 750,000 pl ha−1 in Xinji (0.33 m between rows). The remaining area around the central 0.28-ha disk was not ploughed and not sown with the crop. Weed control was carried out against broad-leaved species, and any foxtail millet volunteer identified in that area was destroyed before flowering.
Seed sampling:
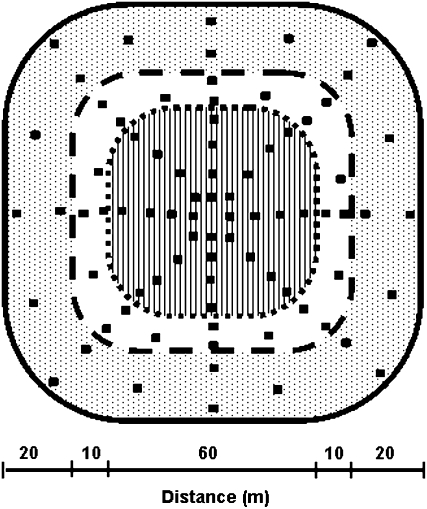
Every field was divided into three areas to sample green foxtail seeds (Figure 1). The central part where the herbicide-resistant crop was grown (0.28 ha) was harvested on 32 plots of 2 × 2 m size, representing 4.5% of the crop area. The adjacent 10-m-large ring (0.22 ha) and the external 20-m-large ring (0.63 ha) were harvested on 24 plots each, representing 4.4 and 1.5% of each area, respectively. The sampling plots were designed following eight directions. The directions were changed every year to avoid sampling in the same place. Seed collection was carried out every year of the experiment, from 1999 to 2002, and during 2 additional years in the absence of the resistant crop, in 2003 and 2004. The plots were signaled by four bamboo sticks connected by a wire netting to prevent any effect of rodents. The total number of green foxtail plants per plot was recorded, and several whole plants were bagged at random after flowering using plastic net bags to avoid seed shedding and bird damage. Once the bags had been collected, the resistant crop area was harvested and the yield was measured. Seeds of green foxtail from the same area were bulked and counted.
Figure 1.—
Field map showing the crop area where the resistant millet lines were grown (hatched central part), the adjacent (open area), and external (dotted area) crop-free rings and the location of the sampling plots of green foxtail plants (distance scale in meters).
Germination and resistance tests:
To overcome the dormancy problem of wild plants and to increase seed germination ratio to at least 60%, the seeds were kept at room conditions for >6 months, and then each of the three following treatments was assayed on a 1000-seed sample of every location, field, and year, and the one resulting in higher germination was used in each case (Wang et al. 2001): (1) seeds were dipped in 3% H2O2 for 8–10 hr and then air dried; or (2) seeds were put in 2.88 × 10−6 mol/liter gibberellic acid solution at 2°–6° for 50 hr and then air dried; or (3) seeds were churned slowly in 98% H4SO4 for 5 min and then immediately and abundantly washed with water, air dried, dipped in water for 8–10 hr, and finally air dried. Around 30% of the collected seeds were conditioned with the appropriate pretreatment and then sown in plastic trays (67 × 46 × 15 cm) on a filter paper supported by 1 cm vermiculite containing a herbicide solution: 0.1 ppm for trifluralin (Treflan EC, Dow Elanco, France) and 62 ppm for sethoxydim (Nabu, Jagri, France). The herbicides have no significant effect on the early phase of germination (Wang et al. 2001). After 3 days at 25°, the germinated seeds had roots >0.5 cm even if they were inhibited by the herbicide. The nongerminated seeds with roots generally 0.2–0.3 cm were discarded while seedlings with 0.5- to 0.8-cm-long roots were counted as susceptible and those with 2- to 3-cm-long roots were counted as resistant. Seeds to test atrazine resistance were sown in trays loaded with mixed vermiculite soil (3:1) and treated with three times the recommended dose at the four-leaf stage, i.e., 3600 g ha−1 active ingredient of atrazine (Youqujin, Xuanhua Agricultural Chemical Factory). All emerged seedlings were counted and the mortality was observed 2 weeks after the treatment. A few of the resistant seedlings were transplanted in pots in the greenhouse and their hybrid status (F1 or F2) was always confirmed by their morphology intermediate between the crop and its wild relative (Darmency and Pernès 1987; Darmency et al. 1987). We assumed that all the hybrid seeds have germinated due to the paternal contribution of the crop that lacks seed dormancy, but dominance of seed dormancy and attributes contributing to dormancy are not yet clearly established (Dekker 2003; Darmency 2005). Therefore, the rate of herbicide-resistant hybrids was the number of resistant seedlings divided by the total seed number (we assumed that nongerminated seeds were dormant), thus running the risk of underestimation of the frequency of hybrid-resistant seeds.
Statistical analyses:
To check that experimental conditions for pollen flow were similar among the two locations, places (for each resistance), areas (crop, adjacent, or distant), and years, the number of plants per plot was analyzed through a fixed-model ANOVA including location, resistance (within location), area, and year as factors. Mean values were given with SEM. Results of herbicide treatments were bulked per area, and regression of the percentage of hybrid in terms of the number of years elapsed from the beginning of the experiment was performed using SYSTAT software version 10 (SPSS, 1999).
RESULTS
General data:
The number of green foxtail plants recorded in the fields over the years was rather similar among locations, fields, crop areas, and crop-free rings: 1.53 ± 0.05 pl m−2 (ANOVA results not shown). Each sampled plant produced on average 1430 ± 50 seeds on the main ear and tillers. This represented a low pollen pressure compared to the 52 (in Chicheng) or 75 (in Xinji) pl m−2 of the foxtail millet in the crop area. The average weight of 1000 seeds was 2.72 ± 0.03 g for the crop vs. 0.80 ± 0.01 g for the wild species. Not a single large seed had ever been observed in the trays, indicating that there was no seed contamination. The average germination rate was 68.9 ± 0.8% with few variations among locations, areas, and years because the best conditioning treatment was chosen in each case. The resistant seedlings always had a seed size and shape similar to that of green foxtail and never had that of the crop, as expected for hybrids with the wild species as mother. The total number of resistant seeds was 501 for >3 million seeds analyzed, with large differences among the three resistance inheritances (Table 1). Resistant seeds were observed within the crop area, but also in the adjacent and external areas. The average percentage of resistant seeds over the 6 years of study decreased as the distance from the pollen source increased (Figure 2).
TABLE 1.
Herbicide-resistant seeds found in the seed samples of green foxtail over 6 years and in the millet lines displaying a nuclear dominant, sethoxydim resistance; a nuclear recessive, trifluralin resistance; and a chloroplast-inherited, atrazine resistance
| Resistant line | No. of tested seeds | Resistant seeds
|
||
|---|---|---|---|---|
| Location | No. | % | ||
| Sethoxydim | Xinji | 537,367 | 210 | 0.0391 |
| Sethoxydim | Chicheng | 463,909 | 168 | 0.0362 |
| Trifluralin | Xinji | 527,974 | 60 | 0.0114 |
| Trifluralin | Chicheng | 475,740 | 61 | 0.0128 |
| Atrazine | Xinji | 557,350 | 2 | 0.0004 |
| Atrazine | Chicheng | 453,285 | 0 | 0 |
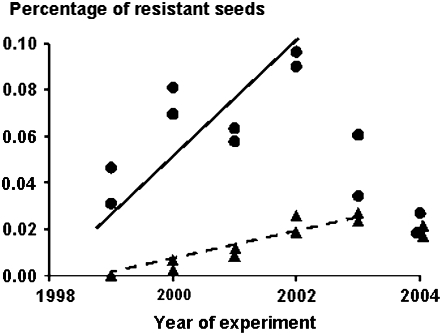
Figure 2.—
Percentage of resistant seeds produced by green foxtail plants growing in two locations within the crop area from 1999 to 2002 and then in 2003 and 2004 in the same area but in the absence of the crop, when the crop displayed a nuclear dominant sethoxydim resistance (circle) or a nuclear recessive trifluralin resistance (triangle).
The nuclear dominant resistant line:
In the first year, the average percentage of resistant seeds produced by the green foxtail plants growing within the crop area of the sethoxydim, nuclear dominant, resistant line was 0.039%. Subsequently, it increased linearly, with the equation y = 0.0248x (y is percentage and x the number of years after 1998; F1,7 = 108.3, P < 0.001, adjusted R2 = 0.94; SE of parameter = 0.0024). The maximum percentage was reached in 2002 in Xinji with 0.097% resistant hybrids. Then, in the absence of the resistant pollen donor in 2003 and 2004, the frequency decreased to an average of 0.023% (Figure 3). The former increase was probably due to the sum of the new hybrids of the year plus the resistant seed production by one or several of the hybrids released the previous year. Only hybrid-resistant progeny accounted for the last 2 years. In the adjacent and external areas, only one or two hybrids were found in the first 3 years. Then a peak was observed in 2002, with up to 0.057% of the collected seeds in Chicheng. The resistant seeds finally decreased to 0.010% in both areas in the absence of a pollen donor in 2004 (Figure 4).
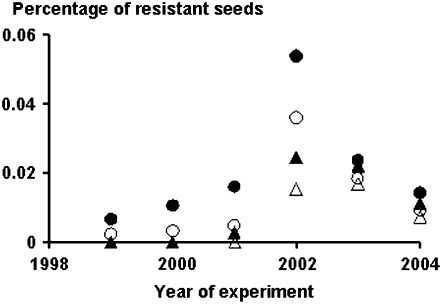
Figure 3.—
Mean values of the percentage of resistant seeds produced by green foxtail plants growing outside the resistant crop area in the 10-m-large crop-free adjacent ring (solid symbols) and in the 20-m-large external ring (open symbols), when the crop displayed a nuclear dominant sethoxydim resistance (circle) or a nuclear recessive trifluralin resistance (triangle).
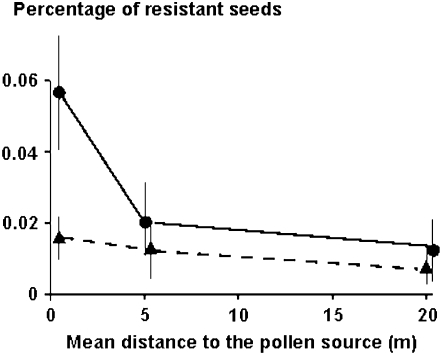
Figure 4.—
Mean percentage of resistant seeds in terms of the mean distance of the target plants from the pollen source for 1999–2004 in the case of the sethoxydim, nuclear dominant resistance (circle) and for 2000–2004 in the case of the trifluralin, nuclear recessive resistance (triangle).
The nuclear recessive resistant line:
In the first year, no resistant seed was detected in any of the three areas, as expected when the resistance gene is recessive and therefore not expressed in hybrids. Resistant seeds appeared during the second year within the crop area, the third year in the adjacent area, and the fourth year in the external area (Figures 3 and 4). In the crop area it increased linearly at a lower rate than for the nuclear dominant inherited resistance, with the equation y = 0.0063x (y is percentage and x the number of years after 1999; F1,9 = 202.2, P < 0.001, adjusted R2 = 0.96; SE of parameter = 0.0004). The maximum percentage was reached in 2003 in Xinji with 0.027% resistant hybrids. Then, in 2004, which was the second year in the absence of the resistant pollen donor, the frequency decreased slightly to an average of 0.019% (Figure 3). A similar trend, but with lower values, was observed in the adjacent and external areas (Figure 4).
The cytoplasmic maternal resistant line:
Of 1 million seeds analyzed, only 2 resistant seeds were found within the crop area. The resistant seed found in 2003 was certainly not a F1 hybrid, but a progeny of some previous hybrid, because there was no more resistant millet grown that year.
DISCUSSION
Sampling strategy:
Since the resistant seeds could be true F1 hybrids in the first year only, and then F1 hybrids or hybrid progeny the following years, casual bagging and the harvest of F1 hybrids originated in the field the previous year could have caused the overestimation of the frequency of the resistance gene. However, previous studies showed that hybrids had 3 times fewer tillers and spikes (Darmency et al. 1987), 4 times less spikelet fertility for the main spike (Darmency and Pernès 1985; Wang et al. 2001), and 12 times less fertility for secondary tillers (Wang et al. 2001) than their green foxtail parents. Similar results were found in Li et al. (1945) and reported in Gu (1987). Although no direct estimate of the relative fitness of hybrids has ever been carried out, it is likely that the contribution of one hybrid to a seed sample would be ∼30 times less than for a green foxtail, that is, a few seeds only, of which 75 or 25% are resistant in the case of a nuclear dominant or a recessive gene, respectively. Therefore, collecting the seed output of a hybrid would not greatly change the frequency of the resistant phenotype in the sample. The scores of one or two homozygous-resistant seeds observed in the samples of 2000 and 2001 for the recessive resistance, although the mother plant was necessarily a hybrid, confirmed this assumption. Similarly, although the entire progeny of a hybrid having inherited a cytoplasm-encoded resistance should be completely resistant (Wang et al. 2004), only one resistant seed was found on two separate occasions, indicating that our sampling strategy was very appropriate for representing the whole foxtail millet population. Finally, we must remark that our experiment left the uncropped areas uncultivated to leave the seeds on the surface and to maximize the chance of growing plants from the last seed rain. Therefore, we overestimated the resistance frequency by preventing resistant seed from entering the soil seed bank and susceptible seeds from coming up from deep soil layers, as in a normal ploughed field.
Pollen dispersal:
Both nuclear dominant and recessive-resistant seeds were found in the adjacent and external areas, indicating pollen movement. The only data that rely effectively on direct hybridization are those of the first year: 0.039, 0.007, and 0.002% at 0, 5, and 20 m, respectively, for the dominant resistance. These values are >10 times lower than those observed in previous works: 1.1, 0.08, and 0.018% at the same distances for an interspecific cross (Wang et al. 2001) and 1, 0.09, and 0.002% for an intraspecific cross (Wang et al. 1997), respectively. Such low values may be explained by a higher pollen competition due to the green foxtail at a density of 1.53 pl m−2 over all the fields, while in the previous experiments there were few target plants placed along radiating lines only, so that the pollen competition was lower (Wang et al. 1997, 2001). When data were averaged over years, from 1999 to 2004 for the dominant resistance and from 2000 to 2004 for the recessive resistance, resistance percentages in the adjacent and external areas were only two to three times lower than within the crop area (Figure 2). Progeny of hybrids and seed movement among areas could explain this low decrease compared to the quick drop of pollen dispersal in 1-year studies, and this feature should be taken into account when modeling the regional dispersal of resistant plants from transgenic fields.
Comparison of the three inheritance systems:
Assuming that the hybridization rate was 0.039% as in 1999—the first year with the dominant resistant line—and that the chloroplast gene transmission was 0.03% as observed over >700,000 hybrids in a previous work (Wang et al. 2004), no more than 0.1 atrazine-resistant F1 hybrid was expected over 1 million seeds. With so few effectively identified hybrids, it is therefore difficult to conclude that there is any difference between the expected and the observed results, a 2 × 10−6 frequency in the weed populations. The strategy of using the chloroplast gene resulted in the occurrence of 190 times fewer resistant plants among the weedy relatives than the use of the dominant resistance. The transmission of plastids is considered as exceptional, but low rates and large numbers of target plants make it unavoidable (Wang et al. 2004; Svab and Maliga 2007). The resulting gene flow is of the same order of magnitude as the spontaneous mutation rates typically cited for plants, although mutations in chloroplast could occur at even lower rates (Jaseniuk et al. 1996). Therefore, the strategy appears to be as safe as possible, apart from the fact that the reciprocal cross (i.e., a foxtail millet plant pollinated by a green foxtail) could also occur (Till-Bottraud et al. 1992) and would inevitably produce a resistant hybrid within a shorter period of time than that necessary for a mutant to occur.
The strategy of using the nuclear recessive gene resulted in three times fewer resistant plants than with the dominant gene. However, the frequency of resistant plants was very similar for both nuclear genes at the end of the experiment, when no more pollen flow from the crop could create new hybrids. The main difference between the two kinds of gene inheritance lies in the lack of expression of the resistance in the first-generation hybrids in the case of the recessive gene. Consequently, under usual agricultural conditions where herbicides are sprayed, the interspecific hybrids will die (which was not the case in our experiment because no herbicide was used). Indeed, very few examples of herbicide resistance occurring in weeds are known to be recessively inherited (Jaseniuk et al. 1996), which suggests that it is a practical barrier to the emergence of this kind of mutation in field conditions. Engineering herbicide-resistant recessive transgenes is perhaps a gene flow mitigation process to be investigated further.
Impact of the pollen dispersal:
None of the three strategies was 100% effective. The three kinds of gene resulted in the presence of resistant plants in the fields and their neighborhoods, even 1 or 2 years after the growing of a resistant crop had stopped and in the absence of herbicide selection pressure. The estimated hybridization rate of 0.039% represents 0.85 hybrid m−2 produced within the crop area and 0.07 hybrid m−2 produced on average in the surrounding areas. This is a huge quantity of new hybrids released within the crop area (2400) and in the neighborhood (600) every year for a field as small as 0.28 ha, which offers a large possibility of gene flow. Compared to other autogamous cereals, gene flow from foxtail millet to its closest wild relative was lower than in the case of wheat to Aegilops sp. (Morrison et al. 2002; Loureiro et al. 2007), but fell in a range similar to that of rice to wild and weedy rice (Geally 2005). However, the proximity of the crop and the wild genomes make the hybrids more productive than in wheat, so that the persistence of crop genes into the wild populations is certainly higher. As indicated above, hybrid fitness is largely reduced compared to that of its wild parent, which is often the case for interspecific hybrids but not always (Arnold and Hodges 1995; Ellstrand and Schierenbeck 2000), although, given the high tiller and seed production of the Setaria group, the release of progeny is warranted. By segregation of traits in the F2 progeny and further generations, typical green foxtail individuals that produce as many seeds as green foxtail and with the same dormancy behavior can be released. The presence of resistant plants over the last 2 years in the absence of the resistant crop confirmed that resistant seeds were continuously produced and had the opportunity of being buried in the soil, making herbicide-resistant genes prone to disperse in space and time into both local and adjacent wild populations and to enter the soil seed bank. While containment strategies recommend rotating herbicides or using herbicide mixtures (Wrubel and Gressel 1994), the good practice recommendations as regards the recessive gene to reduce the risk of dispersal would be a continuous treatment with the same herbicide(s) to allow the destruction of all the hybrids, thus stopping the gene flow process at its very early stage. Obviously, in addition to dispersal of pollen, some other mechanisms could favor gene dispersal, including seed losses during harvest, conditioning, and truck transportation. However, although some foxtail millet plants do appear in waste areas, roadsides, and villages in production areas in China, they are not reported to form feral populations (Darmency 2005), and therefore they do not represent hot spots where interspecific hybridization and gene transfer could occur. Gene flow between foxtail millet and green foxtail certainly occurred from the early domestication of the crop, resulting in the giant green foxtail [S. viridis ssp. pycnocoma (Steud) Tzvelez (Darmency 2005], but the transfer of herbicide-resistant genes could now modify its fitness value and desirability in modern agriculture fields. Associating biological containment tools and field management strategies is a necessary task to control the spread of herbicide-resistant weeds.
Acknowledgments
The authors are especially grateful to Z. Zhao and the millet research group of the Zhangjiakou Institute of Agriculture Sciences for advice and field experiment assistance. This research was supported by the European Commission contract INCO-DC (no. ERB-IC18-CT-98-0391) and by a consecutive project from the Chinese Ministry Of Science and Technology (grant nos. 2004BA525B04 and 2006BAD13B03).
References
- Al-Ahmad, H., S. Galili and J. Gressel, 2005. Poor competitive fitness of transgenically mitigated tobacco in competition with the wild type in a replacement series. Planta 222 372–385. [DOI] [PubMed] [Google Scholar]
- Arnold, M. L., and S. A. Hodges, 1995. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 10 67–71. [DOI] [PubMed] [Google Scholar]
- Benabdelmouna, A., Y. Shi, M. Abirached-Darmency and H. Darmency, 2001. Genomic in situ hybridization (GISH) discriminates between the A and the B genomes in diploid and tetraploid Setaria species. Genome 44 685–690. [PubMed] [Google Scholar]
- Daniell, H., 2002. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 20 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmency, H., 2005. Incestuous relations of foxtail millet (Setaria italica) with its parents and cousins, pp. 81–96 in Crop Ferality and Volunteerism, edited by J. Gressel. CRC Press, Boca Raton, FL.
- Darmency, H., and J. Pernès, 1985. Use of wild Setaria viridis (L.) Beauv. to improve triazine resistance in cultivated S. italica (L.) by hybridization. Weed Res. 25 175–179. [Google Scholar]
- Darmency, H., and J. Pernès, 1987. An inheritance study of domestication in foxtail millet using an interspecific cross. Plant Breed. 99 30–33. [Google Scholar]
- Darmency, H., C. Ouin and J. Pernès, 1987. Breeding foxtail millet (Setaria italica) for quantitative traits after interspecific hybridization and polyploïdization. Genome 29 453–456. [Google Scholar]
- Dekker, J., 2003. The foxtail (Setaria) species-group. Weed Sci. 51 641–656. [Google Scholar]
- Délye, C., T. Wang and H. Darmency, 2002. An isoleucine-leucine substitution in chloroplastic acetyl-CoA carboxylase from green foxtail (Setaria viridis L. Beauv.) is responsible for resistance to the cyclohexanedione herbicide sethoxydim. Planta 214 421–427. [DOI] [PubMed] [Google Scholar]
- Délye, C., Y. Menchari, S. Michel and H. Darmency, 2004. Molecular bases for sensitivity to tubulin-binding herbicides in green foxtail. Plant Physiol. 136 3920–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wet, J. M. J., L. L. Oestry-Stidd and J. I. Cubero, 1979. Origins and evolution of foxtail millets (Setaria italica). J. Agric. Trad. Bot. Appl. 26 53–56. [Google Scholar]
- Ellstrand, N. C., 2003. Dangerous Liaisons? When Cultivated Plants Mate With Their Wild Relatives. Johns Hopkins University Press, Baltimore.
- Ellstrand, N. C., and K. Schierenbeck, 2000. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 97 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geally, D. R., 2005. Gene movement between rice (Oryza sativa) and weedy rice (Oryza sativa): a US temperate rice perspective, pp. 323–354 in Crop Ferality and Volunteerism, edited by J. Gressel. CRC Press, Boca Raton, FL.
- Gu, S. L., 1987. Foxtail Millet Cultivation in China. China Agriculture Press, Beijing.
- Hills, M. J., L. Hall, P. G. Arnison and A. G. Good, 2007. Genetic use restriction technologies (GURTs): strategies to impede transgene movement. Trends Plant Sci. 12 177–183. [DOI] [PubMed] [Google Scholar]
- Jaseniuk, M., A. L. Brûlé-Babel and I. N. Morrison, 1996. The evolution and genetics of herbicide resistance in weeds. Weed Sci. 44 176–193. [Google Scholar]
- Li, H. W., C. H. Li and W. K. Pao, 1945. Cytological and genetical studies of the interspecific cross of the cultivated foxtail millet, Setaria italica (L.) Beauv., and the green foxtail millet, S. viridis L. J. Am. Soc. Agro. 37 32–53. [Google Scholar]
- Loureiro, I., M. C. Escorial, J. M. Garcia-Baudin and M. C. Chueca, 2007. Hybridization between wheat (Triticum aestivum) and the wild species Aegilops geniculata and A. biuncialis under experimental field conditions. Agric. Ecosyst. Environ. 120 384–390. [Google Scholar]
- Lu, H., X. Yang, M. Ye, K. B. Liu, Z. Xia et al., 2005. Millet noodles in late Neolithic China. Nature 437 967–968. [DOI] [PubMed] [Google Scholar]
- Morrison, L. A., O. Riera-Lizarazu, L. Crémieux and C. A. Mallory-Smith, 2002. Jointed goatgrass (Aegilops cylindrica host) × wheat (Triticum aestivum L.) hybrids: hybridization dynamics in Oregon wheat fields. Crop Sci. 42 1863–1872. [Google Scholar]
- Svab, Z., and P. Maliga, 2007. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc. Natl. Acad. Sci. USA 104 7003–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, X., and H. Darmency, 2006. Rapid bidirectional allele-specific PCR identification for triazine resistance in higher plants. Pest Manag. Sci. 62 531–536. [DOI] [PubMed] [Google Scholar]
- Tian, X., C. Délye and H. Darmency, 2006. Molecular evidence of biased inheritance of trifluralin herbicide resistance in foxtail millet. Plant Breed. 125 254–258. [Google Scholar]
- Till-Bottraud, I., X. Reboud, P. Brabant, M. Lefranc, B. Rherissi et al., 1992. Outcrossing and hybridization in wild and cultivated foxtail millets: consequences for the release of transgenic crops. Theor. Appl. Genet. 83 940–946. [DOI] [PubMed] [Google Scholar]
- Van Tienderen, P. H., 2004. Hybridization in nature: lessons for the introgression of transgenes into wild relatives, pp. 7–25 in Introgression From Genetically Modified Plants Into Wild Relatives, edited by H. C. M. den Nijs, D. Bartsch and J. Sweet. CABI Publishing, Wallingford, UK.
- Wang, T., and H. Darmency, 1997. Inheritance of sethoxydim resistance in foxtail millet, Setaria italica (L.) Beauv. Euphytica 94 69–73. [Google Scholar]
- Wang, T., A. Fleury, J. Ma and H. Darmency, 1996. Genetic control of dinitroaniline resistance in foxtail millet (Setaria italica). J. Hered. 87 423–426. [Google Scholar]
- Wang, T., H. B. Chen, X. Reboud and H. Darmency, 1997. Pollen-mediated gene flow in an autogamous crop: foxtail millet (Setaria italica). Plant Breed. 116 579–583. [Google Scholar]
- Wang, T., Z. Zhao, H. Yan, Y. Li, X. Zhu et al., 2001. Gene flow from cultivated herbicide resistant foxtail millet to its wild relatives. Acta Agro. Sin. 27 681–687. [Google Scholar]
- Wang, T., Y. Li, Y. Shi, X. Reboud, H. Darmency et al., 2004. Low frequency transmission of a plastid-encoded trait in Setaria italica. Theor. Appl. Genet. 108 315–320. [DOI] [PubMed] [Google Scholar]
- Wrubel, R. P., and J. Gressel, 1994. Are herbicide mixtures useful for delaying the rapid evolution of resistance? A case study. Weed Tech. 8 635–648. [Google Scholar]