Abstract
The centrosomal kinase Aurora A (AurA) is required for cell cycle progression, centrosome maturation and spindle assembly. However, the way it participates in spindle assembly is still quite unclear. Using the Xenopus egg extract system, we have dissected the role of AurA in the different microtubule (MT) assembly pathways involved in spindle formation. We developed a new tool based on the activation of AurA by TPX2 to clearly define the requirements for localization and activation of the kinase during spindle assembly. We show that localized AurA kinase activity is required to target factors involved in MT nucleation and stabilization to the centrosome, therefore promoting the formation of a MT aster. In addition, AurA strongly enhances MT nucleation mediated by the Ran pathway through cytoplasmic phosphorylation. Altogether, our data show that AurA exerts an effect as a key regulator of MT assembly during M phase and therefore of bipolar spindle formation.
Keywords: Aurora A, centrosome, microtubule, RanGTP, spindle assembly
Introduction
Cell division entails a dramatic reorganization of most cellular components and the assembly of the bipolar spindle, a microtubule (MT)-based apparatus that segregates the chromosomes. The orderly progression of events required for the error-free execution of this process, relies in large part, on several interconnected networks of reversible phosphorylation–dephosphorylation reactions involving several kinase and phosphatase families. The Aurora kinase family includes three members in metazoans that are frequently overexpressed in human cancers and have multiple functions during cell division tightly related to their specific localizations. Aurora A (AurA) is a centrosomal kinase that participates in cell cycle progression and spindle assembly with its best characterized role being in centrosome maturation in late G2 and prophase, when the pericentriolar material expands by recruiting additional components, such as the γ-tubulin ring complex (Barr and Gergely, 2007). Consistent with its role in M phase, AurA protein levels and activity peak in early mitosis and drop during anaphase B, when it is targeted for degradation through the cdh1-activated APC pathway. Although AurA kinase can self-activate by autophosphorylation, several mitotic proteins have been reported to function as activators (Barr and Gergely, 2007). One of them is the nuclear protein TPX2, a direct downstream effector of the RanGTP gradient that triggers an acentrosomal MT assembly pathway around the chromosomes during spindle formation (Gruss and Vernos, 2004). Structural studies have shown that the interaction between the N-terminal 43 amino acids of human TPX2 with the catalytic domain of AurA is sufficient to lock the kinase in an active conformation (Bayliss et al, 2003). Interestingly TPX2–AurA interaction is promoted by RanGTP, suggesting that the RanGTP signalling pathway centred around the chromatin may trigger an AurA-dependent phosphorylation network (Tsai et al, 2003). Although relatively few AurA substrates have been identified so far, many of them are directly involved in spindle assembly. Studies performed in a variety of model systems have clearly established that AurA regulates both the centrosomal localization and function of TACC family members that together with chTOG/XMAP215 family members promote MT assembly from the centrosome (Gergely, 2002; Barr and Gergely, 2007).
Although there is already a wealth of information concerning AurA function, a clear picture of its precise role during M phase is still lacking. Here, we aimed at getting a comprehensive understanding on the role of AurA in spindle assembly. We took advantage of the unique possibilities of the Xenopus egg extract system to examine separately the different MT assembly pathways that participate in spindle formation. Using a peptide corresponding to N terminus of TPX2 to specifically activate AurA in egg extract in combination with depletion and add-back experiments, we show that AurA is a key regulator of MT assembly during M phase. Our data show that AurA functions through two different mechanisms to ensure spindle formation and function.
Results
AurA localization requires its kinase activity
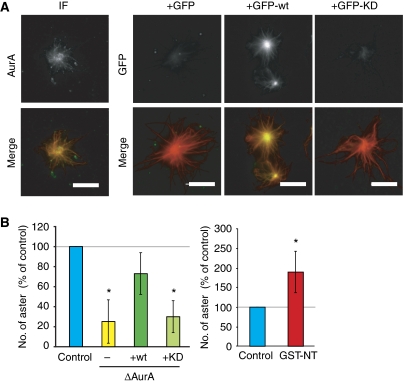
Although the localization of endogenous AurA in the Xenopus egg extract system has been previously reported, it relied on the addition of antibodies to the extract before fixation (Tsai and Zheng, 2005). Using our anti-AurA antibody (Peset et al, 2005), we have been able to visualize AurA by immunofluorescence on spindles assembled in cycled egg extract. As shown in Figure 1A, AurA localizes to the spindle poles and along spindle MTs. A similar localization was observed for recombinant GFP-AurA (GFP-wt) when added to the egg extract at endogenous concentrations (100–200 nM) (Figure 1A). To test whether AurA localization requires its kinase activity, we introduced a single amino-acid substitution (D281A) that completely abolishes kinase activity in vitro (Haydon et al, 2003). The localization of the AurA kinase-dead (KD) protein, GFP-KD, was overall strongly reduced. GFP-KD did not associate with spindle MTs but did localize to the spindle poles albeit to a lesser extent than GFP-wt (Figure 1A). Similar results were obtained with another point mutation (K169R) that has also been shown to strongly reduce AurA kinase activity (data not shown). We conclude that AurA kinase activity is required for the localization of AurA to the spindle.
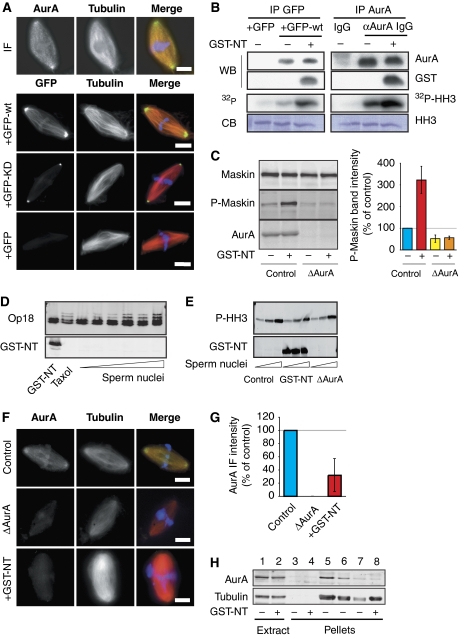
Figure 1.
AurA localization and activation in Xenopus egg extract. (A) Localization of AurA on spindles assembled in cycled egg extract. The endogenous kinase was detected by immunofluorescence using the polyclonal affinity-purified anti-AurA antibodies (upper panels). The localizations of GFP, GFP-AurA (GFP-wt) and GFP-AurA (D281A) (GFP-KD) are shown in the lower panels. In the merged images, AurA and GFP are in green, MTs are in red and DNA is in blue. Bars, 10 μm. (B) GST-NT activates recombinant and endogenous AurA after IP from the egg extract. Left panels: GFP or GFP-wt was incubated in CSF extract as indicated and immunoprecipitated with anti-GFP antibodies (IP GFP). The IP proteins were analysed by western blot (WB) using anti-AurA (AurA) and anti-GST (GST) antibodies. In parallel, the kinase activity of the IP proteins was tested on HH3 in vitro. The autoradiography (32P-HH3) of the Coomassie-stained gel (CB) is shown. Right panels: endogenous AurA was immunoprecipitated with anti-AurA antibodies from egg extract as indicated. The IP proteins were analysed as described above. (C) GST-NT increases Maskin phosphorylation by AurA on Ser626. Western blot of control and AurA depleted (ΔAurA) M-phase cycled extract as indicated, and probed with an anti-Maskin (Maskin), anti-phospho-Maskin (P-Maskin) and anti-AurA (AurA) antibodies. The graph on the right shows the quantification of the phospho-Maskin band intensities normalized with the band of total Maskin and shown as percentages of control. The data are the mean of three independent experiments. Error bars stand for s.d. (D) GST-NT does not promote Op18 hyperphosphorylation. Western blot analysis with an anti-Op18 antibody (Op18) and an anti-GST (GST-NT) of CSF extract incubated with GST-NT, 20 μM taxol or increasing concentrations of sperm nuclei (1, 2, 5, 10 and 20 nuclei per nanolitre). (E) GST-NT addition does not increase HH3 phosphorylation. Western blot analysis with anti-phospho-HH3 (P-HH3) and anti-GST (GST-NT) antibodies of CSF extract: mock (control) containing GST-NT (GST-NT) or AurA depleted (ΔAurA) and incubated with increasing concentrations of sperm nuclei (1 and 10 nuclei per nanolitre). (F) GST-NT displaces AurA from the spindle. Representative images of the localization of AurA detected by immunofluorescence with the anti-AurA antibodies. Three conditions are shown: spindles assembled in control extract, extract containing GST-NT and in AurA-depleted extract. In the merged images, AurA is in green, MTs are in red and DNA is in blue. Bars, 10 μm. (G) Total AurA fluorescence intensities associated with the spindles formed in (F). The background fluorescence intensity detected in ΔAurA was substracted from the values obtained in the other conditions. The fluorescence intensity of the control was set at 100%. The graph shows the mean of two independent experiments. Error bars stand for s.d. (H) Western blot analysis of mitotic structures assembled around sperm nuclei in cycled extracts, in the absence or presence of GST-NT. The initial extracts are shown. The blots were probed with the anti-AurA (AurA) and an anti-α-tubulin (tubulin) antibodies. The pellets from extracts containing nocodazole were used in parallel. 1 and 2: 1 μl of initial extracts; 3 and 4: pellets from 40 μl of extract containing nocodazole; 5–7: pellets from 40, 20 and 10 μl of extract, respectively; 8: pellet from 40 μl of extract containing GST-NT.
The N terminus of TPX2 activates AurA in Xenopus egg extract in a RanGTP-independent manner and displaces it from the centrosome
The N-terminal 39 amino acids of Xenopus TPX2 tagged with GST (GST-NT) has been shown to interact with AurA in egg extract in a RanGTP-independent manner (Bayliss et al, 2003). We reasoned that this peptide could be a useful tool to get additional insight into AurA function during M phase. We first checked whether it could trigger AurA activation when added to an M-phase egg extract. As a first approach, recombinant GFP-wt or GFP alone as a control were added to CSF-arrested egg extracts in the presence or absence of GST-NT (10–20 μM) and pulled down with anti-GFP antibodies. The kinase activity of the pulled-down complexes was then monitored in vitro after incubation with histone H3 (HH3) in the presence of 32P. Autoradiography analysis showed that GFP-wt in complex with GST-NT had a higher kinase activity than GFP-wt alone. Similar results were obtained by immunoprecipitating endogenous AurA from extract with or without GST-NT (Figure 1B). These results suggested that GST-NT activates the kinase in the M-phase egg extract in a RanGTP-independent manner. However, it was important to examine whether this activation resulted in the phosphorylation of AurA substrates in the egg extract. One of them is the Xenopus TACC3 orthologue, Maskin (Kinoshita et al, 2005; Peset et al, 2005). To monitor its phosphorylation by AurA, we generated a phospho-specific antibody directed against the conserved Ser626 shown previously to be phosphorylated by AurA (Supplementary Figure 1). Cycled egg extract with or without sperm nuclei (control or AurA-depleted extracts both in the presence or absence of GST-NT) was analysed by western blotting with the anti-phospho-Maskin antibody. The proportion of phosphorylated Maskin increased by a factor of three in extracts containing GST-NT (Figure 1C). This demonstrates that Maskin becomes phosphorylated on Ser626 when AurA is activated by GST-NT.
To validate the use of GST-NT as a specific activator of AurA in egg extract, we tested whether the activated kinase could also phosphorylate spuriously non-natural substrates. We therefore examined whether two known substrates of the closely related kinase Aurora B (AurB), Op18 and HH3, were phosphorylated in extracts containing GST-NT. Western blot analysis showed that GST-NT did not promote the appearance of the low mobility bands of phosphorylated Op18 seen upon addition of taxol or sperm nuclei (Kelly et al, 2007) (Figure 1D). We did not detect either any effect of GST-NT on the level of HH3 phosphorylation as detected on western blot with an anti-phospho-HH3-specific antibody (Figure 1E). We conclude that GST-NT activates AurA efficiently in the egg extract leading to the phosphorylation of at least some of its endogenous substrates without lowering its specificity.
As TPX2 has been shown to be required for targeting AurA to the spindle, we next examined whether GST-NT might interfere with AurA localization, spindles were assembled in cycled egg extract in the presence or absence of GST-NT and processed for immunofluorescence with the anti-AurA antibody (Figure 1F). Quantification of the anti-AurA immunofluorescence signal on spindles assembled in different conditions showed that, on average, the amount of AurA associated with spindles assembled in egg extract containing GST-NT was reduced by 70% compared with controls (Figure 1F and G).
To gain further support, we pelleted the spindles formed under different conditions to quantify the amount of co-pelleting AurA by western blot analysis (Figure 1H). We found that in control extract a small amount of AurA co-pelleted specifically with the spindles as shown by the lack of AurA in pellets from extracts containing the MT-depolymerizing drug, nocodazole. The amount of AurA co-pelleting with spindles assembled in extracts containing GST-NT was almost negligible (average from three independent experiments, 1.42±1.33% of the control) (Figure 1H). Another interesting observation was that GST-NT protects AurA from degradation through the cdh1–APC-dependent pathway in egg extract released from the metaphase arrest by the addition of calcium (Supplementary Figure 2). This suggests that the interaction between GST-NT and AurA is quite stable in different cell cycle states.
Therefore, not only GST-NT activates AurA efficiently in extracts but it also displaces the kinase from the spindle.
AurA activity and localization are required for centrosome activity in M phase
Different experimental approaches in different systems support the idea that AurA has a function in M-phase entry, centrosome maturation and bipolar spindle assembly (Barr and Gergely, 2007). However, we still lack a unifying picture for its function and a clear understanding of its precise role during M phase. We took advantage of the Xenopus egg extract system to examine separately the putative role of AurA in the two MT assembly pathways that participate in spindle assembly: namely the centrosomal pathway and the RanGTP-dependent pathway, in the absence of any potential role in M-phase entry.
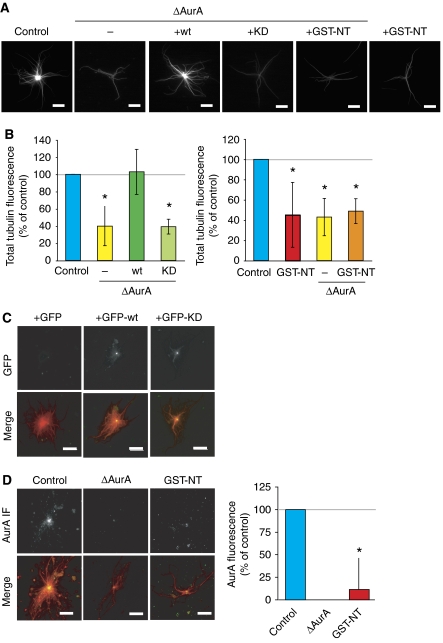
We first studied the role of AurA in centrosome activity in M phase by incubating purified centrosomes in control or AurA-depleted CSF egg extracts (Figure 2A). Centrosomes formed MT asters in both conditions but those incubated in AurA-depleted extract formed less dense asters than controls. To quantify this effect, we measured the size and the total tubulin fluorescence of the centrosomal asters. Although the asters had a similar average size in all conditions (data not shown), those assembled in AurA-depleted extracts showed a clear reduction in total tubulin fluorescence intensity, suggesting that the centrosomes had a reduced capacity for generating an MT aster (Figure 2B). These data indicated that AurA is required for efficient centrosomal aster formation during M phase. To determine whether AurA kinase activity was required, we performed rescue experiments adding recombinant wt or KD AurA proteins (either His or GFP tagged) to the depleted extract (Figure 2A–C; Supplementary Figure 5). Although both GFP-wt and GFP-KD localized precisely at the centre of the centrosomal asters (Figure 2C), only the recombinant wt protein rescued the depletion phenotype (Figure 2B). We conclude that AurA kinase activity is essential for the formation of a robust MT aster by the centrosome in M-phase egg extract.
Figure 2.
Role of AurA on centrosomal aster formation in egg extract. (A) Representative images of asters formed by purified centrosomes in mock-depleted (control) extract, AurA-depleted extract (ΔAurA), AurA-depleted extract containing recombinant AurA (+wt), kinase-dead AurA (+KD) or GST-NT (+GST-NT) and mock-depleted extract containing GST-NT (+GST-NT). Bars, 10 μm. (B) Total tubulin fluorescence associated with asters assembled as in (A). The fluorescence intensity of control asters was set at 100%. The mean results from three independent experiments are shown. Error bars stand for s.d. *P<0.05 compared with control. (C) AurA localizes to the centre of centrosomal asters independently of its activity. Asters were formed in the presence of GFP, GFP-wt or GFP-KD at 150 nM final concentration. In the merge GFP is in green, MTs are in red. Bars, 20 μm. (D) GST-NT displaces AurA from the centrosomal asters. Representative pictures of AurA localization in MT asters formed by centrosomes in mock (control), AurA-depleted extracts (ΔAurA), or in extracts containing GST-NT (GST-NT). AurA is detected by immunofluorescence with the anti-AurA antibodies. The merge shows MTs in red and AurA in green. Bar, 20 μm. The graph shows the quantification of AurA fluorescence intensity at the centre of asters formed in different conditions. The background fluorescence measured in ΔAurA extracts was substracted from the values obtained in the other conditions and the AurA fluorescence intensity of the control was set to 100%. The average values from three independent experiments are shown. Error bars stand for s.d. *P<0.05 compared with control.
We then examined the MT aster formation capacity of centrosomes in extracts containing GST-NT. Unexpectedly, centrosomal asters formed in this condition were quite similar to those assembled in AurA-depleted extract, having a reduced total tubulin fluorescence intensity but similar average size as controls (Figure 2A and B; data not shown). We then examined AurA localization by immunofluorescence on centrosomal asters formed in different conditions. As shown in Figure 2D, AurA was strongly enriched at the centre of asters assembled in control extract. By contrast, very little AurA was present at the centre of asters formed in extracts containing GST-NT (Figure 2D). Quantification of the AurA fluorescence showed that it was indeed reduced by more than 80% of the control levels. Therefore, GST-NT also interferes with AurA localization to the centrosome.
We conclude that the role of AurA in centrosome function relies both on its kinase activity and its localization to the centrosome, suggesting that local phosphorylation is involved in centrosomal aster formation.
Localized active AurA increases MT nucleation at the centrosome
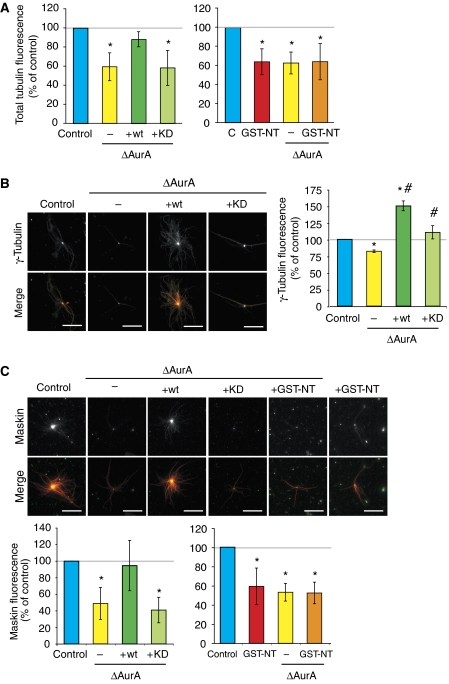
The steady-state size and density of MT asters formed around purified centrosomes in egg extract are determined both by the rate of MT nucleation and the parameters of MT dynamic instability (Carazo-Salas et al, 2001). To examine the role of AurA on the intrinsic nucleation capacity of centrosomes, independently of any effect on MT dynamics, we used the assay described by Carazo-Salas et al (2001). Sperm nuclei from Xenopus laevis are associated with an immature centrosome unable to nucleate MTs in pure tubulin. Upon incubation in M-phase egg extract, the immature centrosomes recruit cytoplasmic components and become fully competent. These reconstituted centrosomes can be re-isolated by centrifugation and their intrinsic nucleation capacity can be quantified in pure tubulin. Sperm-associated immature centrosomes were reconstituted by incubation in control, AurA-depleted extracts or extract containing GST-NT. Quantification of the MT asters formed in pure tubulin showed that centrosomes reconstituted in AurA-depleted extracts nucleated, on average, less MTs than those reconstituted in control extract (Figure 3A). This effect was fully rescued by the addition of GFP-wt but not with GFP-KD to the depleted extract. Interestingly, centrosomes reconstituted in extracts containing GST-NT had a similar activity to those reconstituted in depleted extracts. These results indicated strongly that active AurA increases the intrinsic nucleation activity of the centrosome. They also showed that phosphorylation of AurA substrates in the cytoplasm is not sufficient but that the active kinase has to be at the centrosome.
Figure 3.
AurA depletion decreases the intrinsic nucleation capacity of centrosomes. (A) Quantification of the MT nucleation capacity of sperm centrosomes reconstituted in egg extract. Mock-depleted extract (control), AurA-depleted extract (ΔAurA) AurA-depleted extract with wt AurA (+wt) or with kinase-dead AurA (+KD), mock-depleted extract with GST-NT (+GST-NT). The total tubulin fluorescence was measured for more than 50 structures for each condition and the control was set to 100%. The graphs show the mean of three independent experiments. Error bars stand for s.d. *P<0.05 compared with control. (B) Quantification of γ-tubulin recruitment to centrosomes incubated in extracts. Left, immunofluorescence with an anti-γ-tubulin antibody. Mock-depleted (Control) extract, AurA-depleted extract (ΔAurA), AurA-depleted extract containing recombinant wt AurA (+wt) or kinase-dead AurA (+KD). Right, quantification of the fluorescence signal for γ-tubulin measured in a constant area at the centre of asters. The fluorescence intensity in the control was set at 100%. The graph shows the mean of two independent experiments. Error bars stand for s.d. *P<0.05 compared with control. #P<0.05 compared with ΔAurA. In the merge, γ-tubulin is in green, MTs are in red. Bars, 20 μm. (C) Quantification of Maskin recruitment to centrosomes incubated in extracts as in (B). Upper panels: immunofluorescence with an anti-Maskin antibody. Lower panels, quantification of the fluorescence signal for Maskin measured in a constant area at the centre of the asters. The fluorescence intensity in the control was set at 100%. Graph shows the mean of three independent experiments. Error bars stand for s.d. *P<0.05 compared with control. In the merge, Maskin is in green, MTs are in red. Bars, 20 μm.
AurA centrosomal localization is essential for the recruitment of factors involved in MT nucleation and stabilization
As γ-tubulin is one of the best characterized component of the MT nucleation machinery and AurA has been shown to be required for its recruitment to the centrosome during G2 in other systems (reviewed in Barr and Gergely, 2007), we examined whether its recruitment to the centrosome was altered by depleting or activating AurA in the egg extract. Immunofluorescence analysis of centrosomal asters formed in egg extract showed that depletion of AurA resulted in a reduction of γ-tubulin at the centrosome to 74.3±6.56% (average of four independent experiments) of control levels. The addition of recombinant AurA (wt) to the depleted extract resulted in the efficient accumulation of γ-tubulin at the centrosome (Figure 3B). Recombinant KD also increased the recruitment of γ-tubulin, although less efficiently than wt. These results may suggest that other factors involved in MT nucleation may be altered in this case, as although the levels of γ-tubulin at the centrosome are close to normal, these centrosomes have an impaired capacity to form an MT aster similar to those incubated in AurA-depleted extract (Figures 2A and 3A). In any case, these data show that AurA kinase activity efficiently promotes the recruitment of γ-tubulin to the centrosome. When quantifying the level of γ-tubulin at the centrosome in extracts containing GST-NT, we obtained variable results, although the average of four independent experiments indicate that there was a reduction of 81.9±23.63% from control levels. As this difference was not statistically significant, we cannot make any strong conclusion in this case. Overall, these results indicate that the decrease in MT nucleation activity of centrosomes lacking AurA is, at least in part, due to the inefficient recruitment of γ-tubulin.
MT stabilization is also involved in centrosomal aster formation in Xenopus egg extract through the activity of the TACC3 orthologue Maskin (Kinoshita et al, 2005; Peset et al, 2005). Phosphorylation of TACC3 proteins by AurA has been shown to be required for their recruitment to the centrosomes (Kinoshita et al, 2005). We therefore quantified the amount of Maskin present at the centre of the centrosomal asters under our different experimental conditions. As expected, AurA depletion resulted in a marked reduction of Maskin at the centrosome (Figure 3C) and this effect was fully rescued by the addition of the recombinant wt but not the KD AurA protein to the depleted extract. Surprisingly, we found that GST-NT addition to the egg extract also resulted in a reduction of Maskin accumulation at centrosomes. As we have shown that in these extracts Maskin is efficiently phosphorylated on Ser626 (Figure 1C), this result indicated that Maskin phosphorylation by AurA is not sufficient for its efficient recruitment to the centrosome.
Altogether, these results suggest strongly that the localization of active AurA to the centrosomes promotes the recruitment of various factors involved in MT nucleation and MT elongation or stabilization (similar to Maskin) and therefore for the capacity of the centrosome to generate an MT aster in M phase.
The RanGTP-dependent increase in centrosome nucleation activity requires AurA
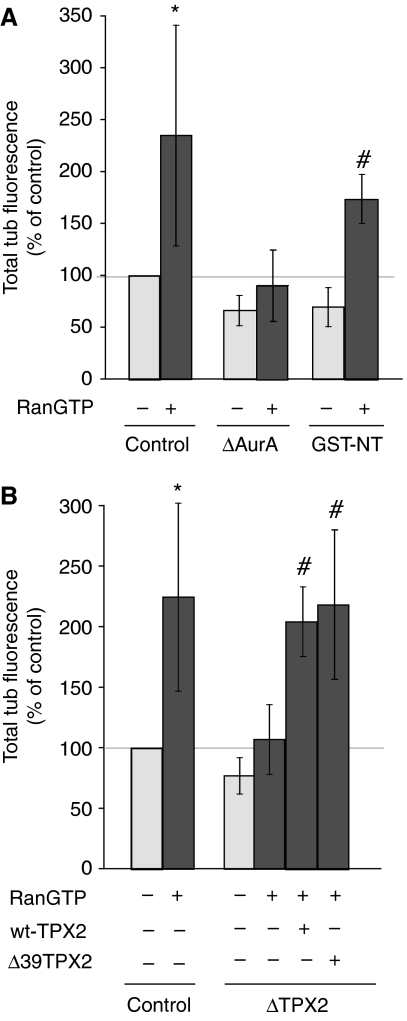
RanGTP has been shown to increase the MT nucleation activity of centrosomes in M-phase extract (Carazo-Salas et al, 2001). We examined whether AurA may participate in this pathway. In agreement with previous reports (Carazo-Salas et al, 2001), centrosomes reconstituted in CSF extract containing RanGTP nucleated almost 2.3 × more (average from eight independent experiments) MTs in pure tubulin than controls. Strikingly, RanGTP did not promote an increase in nucleation activity for centrosomes reconstituted in AurA-depleted extracts (Figure 4A), suggesting that AurA is essential in this pathway.
Figure 4.
The increased nucleation capacity of centrosomes promoted by RanGTP requires AurA. (A) MT nucleation capacity of sperm centrosomes reconstituted in mock-depleted extract (control), in AurA-depleted extract (ΔAurA) or in the presence of GST-NT, with or without RanGTP at 15 μM. The total tubulin fluorescence of more than 50 structures for each condition was measured. The fluorescence intensity of the control (without RanGTP) was set at 100%. The graph shows the mean of three independent experiments. Error bars stand for s.d. *P<0.05 compared with control. #P<0.05 compared with ΔAurA. (B) MT nucleation capacity of sperm centrosomes reconstituted in mock-depleted extract (control), TPX2-depleted extract (ΔTPX2), or TPX2-depleted extract supplemented with recombinant wt TPX2 (TPX2-wt) or a TPX2 fragment lacking the N-terminal 39 amino acids (Δ39TPX2) in the presence or absence of RanGTP. The total tubulin fluorescence of more than 50 structures for each condition was measured. The fluorescence intensity of the control (without RanGTP) was set at 100%. The graph shows the mean of three independent experiments. Error bars stand for s.d. *P<0.05 compared with control. #P<0.05 compared with ΔAurA.
By contrast, although AurA is displaced from the centrosome when GST-NT is present, RanGTP did stimulate the MT nucleation capacity of centrosomes reconstituted in extracts containing GST-NT as efficiently as in controls (by 2.5 × , average of three independent experiments) (Figure 4A). These results therefore indicated that AurA participates in the RanGTP-dependent stimulation of centrosome nucleation activity. However, in this pathway, AurA centrosomal localization is not essential. This suggests that this pathway involves the centrosomal recruitment of RanGTP-regulated factors or complexes, which is promoted by AurA-dependent cytoplasmic phosphorylation.
TPX2 has a role in centrosome nucleation activity
As TPX2 is a direct target of RanGTP (Gruss and Vernos, 2004), we wondered whether it could mediate the role of AurA in the RanGTP-dependent increase in centrosome nucleation activity. We therefore examined the nucleation activity of centrosomes reconstituted in TPX2 depleted egg extracts. Interestingly, although the nucleation activity of these centrosomes was only slightly lower than controls, RanGTP did not stimulate their activity (Figure 4B). We checked that reconstituting the centrosomes in TPX2-depleted extract supplemented with recombinant TPX2 restored the ability of RanGTP to increase their nucleation activity (Figure 4B). To determine whether TPX2 and AurA function through the same pathway, we then performed reconstitution experiments in TPX2-depleted extract supplemented with a truncated form of TPX2 lacking the first 39 amino acids (GST–Δ39TPX2) and therefore unable to bind to AurA (Brunet et al, 2004). As shown in Figure 4B, GST–Δ39TPX2 restored the RanGTP-dependent increase in MT nucleation activity of centrosomes. This indicated strongly that TPX2 and AurA function through different pathways in this process.
AurA kinase activity stimulates the RanGTP acentrosomal pathway
Immunofluorescence studies with our anti-AurA antibody showed that AurA concentrated at the centre of Ran asters (Figure 5A). GFP-wt showed a similar localization, whereas GFP-KD did not localize to the asters. This suggests that the localization of AurA on Ran asters requires its kinase activity.
Figure 5.
Active AurA is required for RanGTP-dependent aster formation. (A) Localization of AurA on MT asters assembled upon RanGTP addition to M-phase egg extract. Endogenous AurA was detected by immunofluorescence with the anti-AurA antibody (left panels). GFP, GFP-wt or GFP-KD added to egg extracts is visualized through the GFP fluorescence. In the merge, AurA and GFP are in green and MTs are in red. Bar, 10 μm. (B) Graphs showing the number of RanGTP-dependent asters formed in mock-depleted (control) extract, AurA-depleted extract (ΔAurA), AurA-depleted extract containing recombinant wt AurA (ΔAurA+wt) or kinase-dead AurA (ΔAurA+KD) and in control extract containing GST-NT. The number of asters in the control was set at 100%. The graphs show the mean of three independent experiments. Error bars stand for s.d. *P<0.05 compared with control. See also Supplementary Figure 4.
We then examined whether AurA is required for the Ran aster formation by counting the number of asters formed 20 min after the addition of RanGTP to control or AurA-depleted extracts. We found that the number of asters formed in AurA-depleted extract was significantly lower than in the control (Figure 5B). The efficiency of aster formation was rescued by the addition of GFP-wt to the depleted extract but not by the addition of GFP-KD (Figure 5B). AurA kinase activity is therefore required for efficient RanGTP-dependent MT assembly in egg extract. Interestingly, we found that activation of AurA by GST-NT stimulated the RanGTP-dependent pathway substantially. Indeed, extract containing GST-NT and RanGTP formed around twice the number of asters formed in control conditions (Figure 5B). These results strongly suggested that AurA-dependent phosphorylation strongly enhances the RanGTP-dependent pathway.
We then examined whether Maskin has a role in this pathway. RanGTP-dependent MT asters were formed in Maskin-depleted extract, although the kinetics was slower than in control conditions (Figure 6A–C). We conclude that Maskin is not essential for the RanGTP-dependent MT assembly pathway, although it may facilitate it. Interestingly, both recombinant Maskin and Maskin-3A (that cannot be phosphorylated by AurA) (Peset et al, 2005) were able to rescue the kinetics of RanGTP aster formation when added at endogenous concentrations (Supplementary Figure 5). This strongly suggests that Maskin is not the substrate of AurA that promotes the RanGTP pathway.
Figure 6.
The absence of Maskin delays, but does not impair, RanGTP-dependent MT aster formation in egg extract. (A) Representative images of MT asters formed after the addition of 15 μM RanGTP to mock (control), Maskin-depleted extract (ΔMaskin) and Maskin-depleted extract containing recombinant wt Maskin (ΔMaskin+wt) or Maskin-3A (ΔMaskin+3A). A portion of 1 μl aliquot from the samples was squashed at different times of incubation as indicated. Bars, 20 μm. (B, C) Quantification of the number of MT asters assembled as in (A). The mean results from three independent experiments are shown. The bundling of asters formed in the presence of Maskin-3A after 20 min impaired the accurate quantification of asters.
AurA is required for spindle formation
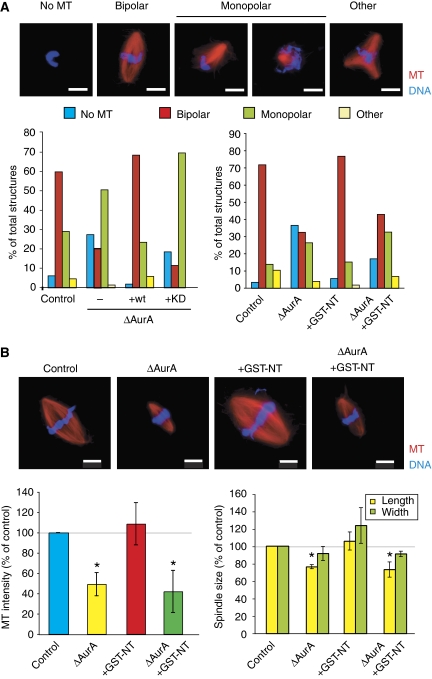
To study the role of AurA in bipolar spindle formation, we used cycled egg extract that more closely mimics physiological conditions because spindles assemble in the presence of duplicated centrosomes and sister kinetochore pairs (Desai et al, 1999). Control extracts contained a majority of bipolar spindles and a minor proportion of monopolar spindles (Figure 7A). The percentage of bipolar spindles formed in AurA-depleted extracts was strongly reduced and concomitantly, monopolar spindles and nuclei with no associated MT became more frequent (Figure 7A). The addition of GFP-wt to the depleted extract rescued this phenotype, whereas the GFP-KD did not (Figure 7A). We also noticed that the bipolar spindles formed in AurA-depleted extract were 20% smaller than controls (Figure 7B) and contained, on average, 50% less MTs than control spindles.
Figure 7.
Role of AurA in spindle assembly in cycled egg extract. (A) Representative mitotic structures formed in cycled egg extracts containing sperm nuclei. Percentages of the different types of structures formed in mock-depleted extract (control), AurA-depleted extract (ΔAurA), AurA-depleted extract containing recombinant wt Aurora (ΔAurA+wt) or kinase-dead protein (ΔAurA+KD) (left graph) and in control or AurA-depleted extract (ΔAurA) containing GST-NT (right graph). The graphs show one representative experiment of three. Bars, 10 μm. (B) Representative images of bipolar spindles formed in the different experimental conditions as in (A). Graphs show the quantification of the total MT fluorescence intensity (left) and the length and width (right) of the bipolar spindles averaged from three independent experiments. Control values were set at 100%. Error bars stand for s.d. *P<0.05 compared with control. DNA is in blue, MTs are in red. Bars, 10 μm.
Extracts containing GST-NT supported bipolar spindle formation as efficiently as controls (Figure 7A), suggesting that AurA localization is not essential. These spindles were slightly wider and had a higher MT content than controls (Figure 7B). These effects were totally dependent on AurA because GST-NT had no effect on spindle formation in AurA-depleted extracts. These results fitted well with our previous data showing that activation of AurA by GST-NT stimulates the RanGTP-dependent MT assembly pathway (Figure 5B).
Altogether, these results indicated that AurA kinase is essential for spindle formation in egg extract and supported the idea that the main role of AurA is to stimulate MT assembly in M phase, in particular the RanGTP-dependent pathway for which it does not require to be localized.
Discussion
A new tool for the functional characterization of AurA
The first 39 amino acids of TPX2 are sufficient for binding to the catalytic domain of AurA and activate the kinase in a RanGTP-independent manner. Although this interaction takes place within a domain highly conserved between the closely related AurA and AurB kinases, the interaction is highly specific as a single amino-acid change in AurA (G198N) was shown to be sufficient to prevent its interaction with TPX2 (Bayliss et al, 2004).
Using phospho-Maskin as a marker, we have shown here that the activation of AurA by the addition of GST-NT to the egg extract results in the phosphorylation of AurA endogenous substrates. Although we cannot rule out completely that the activated AurA-GST-NT complex phosphorylates other proteins in particular AurB substrates, our data indicate that this is unlikely. First, GST-NT does not promote the hyperphosphorylation of the AurB substrates Op18 and HH3 (Figure 1D and E) (Gadea and Ruderman, 2006; Kelly et al, 2007). Second, we consistently found that the phenotypes observed in the presence of GST-NT are fully dependent on the presence of AurA in the extract, ruling out additional unspecific effects. Therefore, GST-NT is a useful tool to study the role of AurA during M phase.
AurA kinase activity is required for its localization during spindle assembly
Using standard immunofluorescence methods in egg extract, we show here that AurA localizes to the centrosome and along spindle MTs. Its enrichment at the spindle poles is probably the result of the concentration of MT ends and of its centrosomal localization. Here, we show that AurA kinase activity is required for its correct localization. Two different KD recombinant proteins (D281A and K169R) fail to localize to MTs while retaining a centrosomal localization (Figures 1 and 2; data not shown). A previous report indicated that KD AurA appeared to localize correctly to the spindle in human cells (Girdler et al, 2006), but the KD proteins were expressed at 3–5 times higher levels than the endogenous protein.
The lack of localization of AurA when GST-NT is present is interesting. As the targeting of AurA to spindle MTs relies on TPX2 (Kufer et al, 2002) and we have previously shown that in egg extract the localization of TPX2 to MTs relies on its C-terminal domain (Brunet et al, 2004), one interpretation is that GST-NT competes with endogenous TPX2 for the interaction with AurA, preventing its MT localization. The strong reduction of AurA localization to the centrosome in the presence of GST-NT is, however, more difficult to interpret. Very little is known about the mechanism of AurA targeting to the centrosome except for its dependence on Plk1 (De Luca et al, 2006). GFP-AurA has been shown to move rapidly in and out of the centrosome in an MT-independent manner (Stenoien et al, 2003), indicating a continuous exchange of AurA at the centrosome. If the activity of the kinase has a function in this dynamic behaviour, one possibility is that by keeping the kinase locked in an active conformation with GST-NT, the equilibrium is broken and the kinase becomes mainly cytoplasmic. Another possibility is that GST-NT interferes with the interaction of AurA with another protein required for its targeting to the centrosome. Unravelling the mechanism involved in the displacement of AurA from the centrosome by GST-NT is at present an open question requiring further studies that may improve our understanding of AurA centrosome targeting.
AurA activity is essential for MT assembly in M phase
Previous studies in a variety of systems have shown that AurA is required for centrosome maturation in G2 (Barr and Gergely, 2007). In agreement with these studies, we find that AurA promotes MT assembly by the centrosome in M phase by recruiting factors involved in MT nucleation and stabilization. In addition, we show that the recruitment of these factors is promoted both by AurA kinase activity and its localization to the centrosome, suggesting that it involves the local phosphorylation of substrates or their direct interaction with the kinase or both.
Centrosome activity increases further after nuclear envelope breakdown through the influence of the RanGTP gradient emanating from the condensed chromosomes (Carazo-Salas et al, 2001). In addition, RanGTP triggers an acentrosomal MT assembly pathway that participates actively in spindle formation. Our data show that AurA strongly enhances the RanGTP-dependent pathway both at the centrosome and in the cytoplasm. Several data strongly suggest that this occurs through the direct phosphorylation of RanGTP-regulated factors and/or factors that participate indirectly in this pathway. First, KD AurA cannot restore the levels of RanGTP-dependent acentrosomal aster formation in AurA-depleted extract. Second, AurA activation by GST-NT stimulates RanGTP-dependent MT assembly in the cytoplasm and MT nucleation at the centrosome despite the lack of localization of the kinase. Currently, a few proteins are known to be both direct targets of RanGTP and substrates of AurA kinase. This includes TPX2 (Gruss and Vernos, 2004), HURP (Koffa et al, 2006) and possibly Maskin (Albee et al, 2006) (but see below).
While we were working on the revised version of this paper, Zhang et al (2008) reported that AurA phosphorylates MCAK at two sites, negatively regulating its depolymerase activity and its localization to the centre of Ran asters and to spindle poles. As the activation of AurA by GST-NT is likely to promote the phosphorylation of MCAK, this may be one of the pathways involved in the increased efficiency of the RanGTP pathway upon addition of GST-NT. It is, however, unclear why this does not happen at the centrosome as well.
Role of AurA in the establishment and maintenance of spindle bipolarity
AurA-coated beads incubated in extracts containing RanGTP have been shown to function as an MT-organizing centres and to enhance MT organization into mini-spindles (Tsai and Zheng, 2005). Consistently, we describe here that in cycled egg extract, one major phenotype of AurA depletion is the presence of many nuclei lacking associated MTs, a phenotype that is fully consistent with the strong impairment of the RanGTP pathway also supported by our results in extracts supplemented with RanGTP. However, we found that when MTs are present they can organize into a variable proportion of monopolar or bipolar spindles that are significantly smaller than controls. In the light of our results showing a clear requirement for AurA in all the different pathways for MT assembly, we interpret these phenotypes as a result of the strong reduction of the steady-state number of MTs that can embark into spindle formation. This primary effect may also explain the reduced efficiency of bipolar versus monopolar spindle formation and the variability of reported phenotypes in different systems (De Luca et al, 2006; Hoar et al, 2007). Spindles assembled in extracts containing the AurA activator GST-NT are also bipolar, although the kinase is displaced from the spindle. As the RanGTP pathway is very active in this system, this fits well with our data showing that the activation rather than the localization of AurA promotes this pathway. We propose that a major primary role of AurA is to promote MT assembly during M phase both at the centrosome and through the acentrosomal pathways.
Functional relevance of TPX2–AurA interaction
We have previously shown that TPX2–AurA interaction is not essential for bipolar spindle assembly in cycled egg extract (Brunet et al, 2004), although different results have been published by other groups (Tsai and Zheng, 2005). Here, we show that robust bipolar spindles form even when GST-NT displaces the kinase from centrosomes and MTs. These results are consistent with our previous findings and indicate that at least in the egg extract system, TPX2-dependent localization of AurA to spindle MTs is not essential. However, AurA kinase activity is. Therefore, this suggests that in addition to the TPX2-dependent activation pathway, other proteins or mechanism may be involved. In this context, it is worth mentioning that AurA can be activated by several proteins in addition to TPX2 (reviewed in Barr and Gergely, 2007). We cannot rule out at this point that by activating the kinase with GST-NT we interfere with the interaction of AurA with these proteins and therefore with other pathways of kinase activation.
Interestingly, we show here that TPX2 also participates in the RanGTP stimulation of centrosomal MT nucleation activity independently from its interaction with AurA. At this point, we can only speculate on a possible mechanism. As RanGTP promotes the formation of a complex containing TPX2 that triggers MT nucleation and stabilization (Groen et al, 2004), the recruitment of this cytoplasmic complex to the centrosome would efficiently enhance centrosome nucleation activity. Interestingly in this context, more than 50% of TPX2-silenced human cells show a defective recruitment of γ-tubulin at the centrosome (De Luca et al, 2006). It is also possible that, considering the proposed role of TPX2 in keeping centrosome and spindle pole integrity, TPX2 may have a structural role keeping the nucleating material at the centrosome.
Role of the AurA substrate Maskin
It has previously been shown that Maskin phosphorylation by AurA is essential for its function in M phase (Kinoshita et al, 2005; Peset et al, 2005). In agreement with previous reports, we show now that AurA does phosphorylate Maskin on Ser626 in M-phase egg extract and that its phosphorylation is required for its localization to the centrosome. However, the higher level of Maskin phosphorylation observed in extracts containing GST-NT does not correlate with its enrichment at the centrosome but, in contrast, with a clear reduction in its localization. This suggests that phosphorylation is probably not the main mechanism involved in the recruitment of Maskin to the centrosome.
A previous report proposed that Maskin is a direct target of RanGTP during M phase (Albee et al, 2006) and that it is required for RanGTP MT aster formation. We show here that Maskin has only a minor function in the kinetics of RanGTP aster formation, presumably by helping MT elongation (the role it has at the centrosome) and that this role does not require its phosphorylation by AurA. Therefore, further work will be required to determine whether AurA enhances the RanGTP pathway by direct phosphorylation of RanGTP targets.
Through a comprehensive analysis of the role of AurA during spindle assembly, we show here that this kinase functions through two distinct mechanisms to stimulate MT assembly in M phase. One of them involves local phosphorylation of substrates promoting their recruitment at the centrosome to actively counteract the activity of MT-destabilizing factors localized there, similar to, for example, MCAK. The other mechanism involves the phosphorylation of cytoplasmic factors promoting the self-organization of molecules into organized structures. The next challenge will be to identify the potentially numerous substrates of AurA to get a better understanding of the key functions of by this kinase during M phase.
Materials and methods
AurA constructs
The Xenopus AurA cDNA was obtained from T Lorca and subcloned in pET-28a and pHAT2-EGFP (Peset et al, 2005). The point mutations D281A or K169R were introduced by site-directed mutagenesis (Stratagene).
Protein and antibody preparation
AurA recombinant proteins were expressed in Escherichia coli BL21(DE3)pLysS and purified on TALON metal affinity resin (Clontech), according to the manufacturer's instructions. Buffer was subsequently exchanged to 25 mM K-Hepes pH 7.5, 250 mM KCl, 2 mM MgCl2, 10% glycerol and 1 mM DTT. GST-NT was purified as previously described (Bayliss et al, 2003). RanQ69L-GTP, GFP-TPX2 and GFP-TPX2-Δ39 expression and purification are described in Brunet et al (2004).
Recombinant Maskin proteins and the Xenopus AurA and Maskin polyclonal antibodies are described in Peset et al (2005). The 1C1 monoclonal anti-Xenopus AurA antibody and the anti-Op18A antibody were obtained from C Prigent and R Heald, respectively. The polyclonal anti-phospho-Ser626 Maskin antibody was produced as described in Kinoshita et al (2005) and affinity-purified over the phospho-peptide (Pierce). Anti-phospho-HH3 antibody was purchased from Upstate.
Egg extract preparation, spindle and aster formation
Methods for Xenopus egg extract preparation and immunodepletions and add-back experiments in cycled spindle assembly experiments are described in Peset et al (2005). For western blot of mitotic spindles, cycled extracts containing sperm nuclei were centrifuged through a 40% glycerol cushion for 12 min at 20°C, 12 000 r.p.m. in a TLS-55 rotor. The cushion was washed twice with BRB80–30% glycerol and the pellets were resuspended in SDS loading buffer. Centrosomal asters were assembled as described (Brunet et al, 2004). The quantification of asters formed by RanGTP was carried out by either counting the number of asters per field ( × 63 magnification) or the total number of asters per microlitre of extract. The intrinsic nucleation capacity of centrosomes was determined as described in Carazo-Salas et al (2001) (Supplementary Figure 3).
Immunofluorescence and quantifications
The affinity-purified anti-Maskin, anti-AurA and anti-γ-tubulin (GTU-88; Sigma) antibodies were used for immunofluorescence analysis of asters (Peset et al, 2005). In Figures 2A and 3C, MTs were visualized by immunofluorescence with the anti-α-tubulin antibody (DM1A; Sigma). All pictures were taken with a Leica DMI6000B microscope equipped with a Leica DFC 350FX camera, using the HCX PL APO CS × 63 objective and equally processed with Adobe Photoshop. Quantifications of MT average length and MT intensity were performed as described in Peset et al (2005). More than 50 structures were analysed for each experimental condition. The measured fluorescence intensities were normalized as percentage of control values to calculate the average of several experiments. In Figure 5B, the control values for each experiment were set to 100%. The raw data are shown in Supplementary Figure 4.
Immunoprecipitation and kinase assay
For immunoprecipitation experiments, protein A-conjugated Dynabeads 280 were coated with the corresponding antibodies. To analyse endogenous AurA activity, anti-AurA-coated beads were incubated in 50 μl of extract containing GST or GST-NT 20 μM for 1 h on ice. Beads were retrieved on a magnet and washed twice with 500 μl CSF-XB and split into two. One half was washed twice with 500 μl PBS-T and processed for SDS–PAGE and western blot analysis. The other half was immediately resuspended in 10 μl kinase buffer (20 mM Hepes, pH 7.5, 200 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 1 mM DTT, 0.05% Triton X-100, 50 μM ATP). After the addition of 32P-ATP (Amersham), samples were incubated for 10 min at 30°C. Reactions were stopped with loading buffer and run on SDS–PAGE before autoradiography. The same procedure was followed for measuring recombinant AurA activity.
Supplementary Material
Supplementary Figures 1–5
Acknowledgments
We are grateful to J Pines for the cdh1 clone, T Lorca for Xenopus AurA cDNA clone, C Prigent for the 1C1 anti-Xenopus AurA antibody, R Heald for the anti-Op18 antibody and T Surrey for the anti-Eg5 antibody. We thank L Bejarano, R Pinyol and P Aloy for comments on the paper and L Bejarano and J Seiler for excellent technical assistance. This study was supported in part by the European Union Research Training Network MRTN-CT-2004-512348 and a grant from the Spanish Ministry of Education and Science BFU2006-04694.
References
- Albee AJ, Tao W, Wiese C (2006) Phosphorylation of maskin by Aurora-A is regulated by RanGTP and importin beta. J Biol Chem 281: 38293–38301 [DOI] [PubMed] [Google Scholar]
- Barr AR, Gergely F (2007) Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120: 2987–2996 [DOI] [PubMed] [Google Scholar]
- Bayliss R, Sardon T, Ebert J, Lindner D, Vernos I, Conti E (2004) Determinants for Aurora-A activation and Aurora-B discrimination by TPX2. Cell Cycle 3: 404–407 [PubMed] [Google Scholar]
- Bayliss R, Sardon T, Vernos I, Conti E (2003) Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell 12: 851–862 [DOI] [PubMed] [Google Scholar]
- Brunet S, Sardon T, Zimmerman T, Wittmann T, Pepperkok R, Karsenti E, Vernos I (2004) Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol Biol Cell 15: 5318–5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Gruss OJ, Mattaj IW, Karsenti E (2001) Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat Cell Biol 3: 228–234 [DOI] [PubMed] [Google Scholar]
- De Luca M, Lavia P, Guarguaglini G (2006) A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle 5: 296–303 [DOI] [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE (1999) The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol 61: 385–412 [DOI] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV (2006) Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci USA 103: 4493–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely F (2002) Centrosomal TACCtics. Bioessays 24: 915–925 [DOI] [PubMed] [Google Scholar]
- Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS (2006) Validating Aurora B as an anti-cancer drug target. J Cell Sci 119: 3664–3675 [DOI] [PubMed] [Google Scholar]
- Groen AC, Cameron LA, Coughlin M, Miyamoto DT, Mitchison TJ, Ohi R (2004) XRHAMM functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr Biol 14: 1801–1811 [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Vernos I (2004) The mechanism of spindle assembly: functions of Ran and its target TPX2. J Cell Biol 166: 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon CE, Eyers PA, Aveline-Wolf LD, Resing KA, Maller JL, Ahn NG (2003) Identification of novel phosphorylation sites on Xenopus laevis Aurora A and analysis of phosphopeptide enrichment by immobilized metal-affinity chromatography. Mol Cell Proteomics 2: 1055–1067 [DOI] [PubMed] [Google Scholar]
- Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N, Ecsedy JA (2007) MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol 27: 4513–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H (2007) Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell 12: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Noetzel TL, Pelletier L, Mechtler K, Drechsel DN, Schwager A, Lee M, Raff JW, Hyman AA (2005) Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J Cell Biol 170: 1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW (2006) HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol 16: 743–754 [DOI] [PubMed] [Google Scholar]
- Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA (2002) Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol 158: 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peset I, Seiler J, Sardon T, Bejarano LA, Rybina S, Vernos I (2005) Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J Cell Biol 170: 1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenoien DL, Sen S, Mancini MA, Brinkley BR (2003) Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil Cytoskeleton 55: 134–146 [DOI] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y (2003) A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol 5: 242–248 [DOI] [PubMed] [Google Scholar]
- Tsai MY, Zheng Y (2005) Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol 15: 2156–2163 [DOI] [PubMed] [Google Scholar]
- Zhang X, Ems-McClung SC, Walczak CE (2008) Aurora A phosphorylates MCAK to control Ran-dependent spindle bipolarity. Mol Biol Cell 19: 2752–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–5