Abstract
Background
Glyoxalases (Glo1 and Glo2) are involved in the glycolytic pathway by detoxifying the reactive methylglyoxal (MGO) into D-lactate in a two-step reaction using glutathione (GSH) as cofactor. Inhibitors of glyoxalases are considered as anti-inflammatory and anti-carcinogenic agents. The recent finding that various polyphenols modulate Glo1 activity has prompted us to assess curcumin's potency as an Glo1 inhibitor.
Methodology/Principal Findings
Cultures of whole blood cells and tumor cell lines (PC-3, JIM-1, MDA-MD 231 and 1321N1) were set up to investigate the effect of selected polyphenols, including curcumin, on the LPS-induced cytokine production (cytometric bead-based array), cell proliferation (WST-1 assay), cytosolic Glo1 and Glo2 enzymatic activity, apoptosis/necrosis (annexin V-FITC/propidium iodide staining; flow cytometric analysis) as well as GSH and ATP content. Results of enzyme kinetics revealed that curcumin, compared to the polyphenols quercetin, myricetin, kaempferol, luteolin and rutin, elicited a stronger competitive inhibitory effect on Glo1 (Ki = 5.1±1.4 µM). Applying a whole blood assay, IC50 values of pro-inflammatory cytokine release (TNF-α, IL-6, IL-8, IL-1β) were found to be positively correlated with the Ki-values of the aforementioned polyphenols. Moreover, whereas curcumin was found to hamper the growth of breast cancer (JIMT-1, MDA-MB-231), prostate cancer PC-3 and brain astrocytoma 1321N1 cells, no effect on growth or vitality of human primary hepatocytes was elucidated. Curcumin decreased D-lactate release by tumor cells, another clue for inhibition of intracellular Glo1.
Conclusions/Significance
The results described herein provide new insights into curcumin's biological activities as they indicate that inhibition of Glo1 by curcumin may result in non-tolerable levels of MGO and GSH, which, in turn, modulate various metabolic cellular pathways including depletion of cellular ATP and GSH content. This may account for curcumin's potency as an anti-inflammatory and anti-tumor agent. The findings support the use of curcumin as a potential therapeutic agent.
Introduction
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a polyphenol derived from the plant Curcuma longa. The medical use of this plant has its roots in the Indian Ayurveda medicine for over 6000 years. Extensive research over the last decades has indicated that curcumin exhibits anti-inflammatory, anti-oxidant, anti-viral and anti-infectious activities [1], [2]. Furthermore, in various animal models, curcumin was found to suppress symptoms associated with type II diabetes [3] rheumatoid arthritis [4], Alzheimer disease [5], promoted wound healing [6] and was protective in an animal model of sepsis [7]. These effects seem to be linked to the anti-inflammatory effect of curcumin that, in turn, may be attributed to its ability to inhibit cyclooxygenase-2, lipoxygenase and inducible nitric oxide synthase (iNOS) [8]. Recently, the involvement of the peroxisome proliferator-activated receptor-gamma has been shown [9].
Curcumin such as other polyphenols is as strong anti-oxidant [10]. It significantly decreases lipid peroxidation, regulates anti-oxidant enzymes and scavenges hyperglycemia-induced reactive oxygen species (ROS) [11]. Oxidative stress and inflammation are closely associated with tumor growth [12]. Therefore, it is not surprising that curcumin possesses anticancer effects by blocking different stages of the tumor development [13]. The low incidence of colon cancer among Indians has been attributed to the use of curcumin in cooking in addition to other dietary compounds [14]. However, a possible dominance of certain genetic prerequisites in these populations can not be excluded.
Anti-proliferative effects of curcumin are suggested to be mediated by induction of apoptosis in a mitochondria-dependent manner via caspase-3 activation [15]. In addition, curcumin could affect cellular proliferation by modulating of various cell signaling pathways including NF-κB, growth factor receptor kinases and mitogen-activated protein kinases [16]. Although, the multiple biological functions of curcumin have been described, the prime driving mechanism underlying its action remains unknown [17].
In the present study we provide evidence that the diverse actions of curcumin may be mediated by inhibiting cellular glyoxalases. Glyoxalases are involved in the detoxification of the reactive MGO formed para-metabolically and para-enzymatically from triosephosphates when glucose is degraded. Glo1 catalyzes the conversion of cytotoxic MGO to nontoxic hemithioacetal using GSH as cofactor. This enzyme is ubiquitously expressed in all mammalian cells and is suggested to be involved in cellular aging and cell death [18].
Inhibitors of Glo1 have long been sought as possible anticancer agents. High affinity inhibitors were prepared as GSH analogues such as S-p-bromobenzylglutathione and were shown to display anti-proliferative action [19]. However, the ubiquity of GSH and its involvement in vital cellular reactions may limit the use of native GSH analogues as inhibitors. We found that curcumin acts as a strong inhibitor of Glo1, causes depletion of cellular ATP and GSH and thus has a potential impact at cellular metabolism predominantly in cells whose energy gain relies on the glycolytic pathway. Inhibition of Glo1 seems to be an important route by which this dietary agent may exert its diverse biological effects.
Results
Curcumin inhibits glyoxalase 1 with high affinity
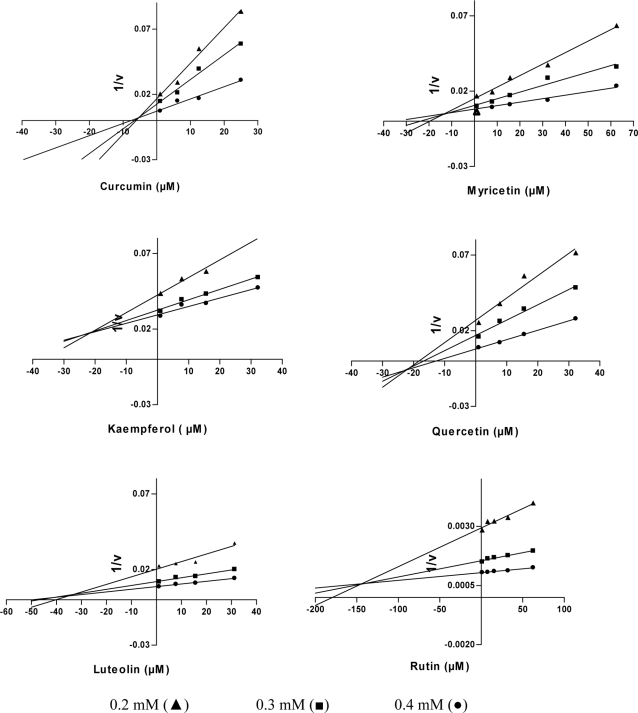
Recently, some polyphenols such as quercetin, luteolin, myricetin, and kaempferol have been shown to inhibit Glo1 [20]–[22]. To see whether curcumin inhibits the enzyme similar to these dietary compounds, we analyzed the enzyme activity in the presence of increasing amounts of inhibitors at different concentrations of the substrates MGO and GSH. The Dixon plot analysis revealed that enzyme inhibition by curcumin was competitive with Ki = 5.1±1.4 µM (Fig. 1). Compared to curcumin, inhibition by other polyphenols turned out to be significantly lower and yielded Ki-values of 23±4.5 µM, 13±2.9 µM, 21±3.8 µM, 35±6.3 µM, 140±15 µM for quercetin, myricetin, kaempferol, luteolin and rutin, respectively.
Figure 1. Inhibition of glyoxalase 1 activity by polyphenolic compounds.
Purified Glo1 (70 mU) was incubated with increasing concentrations of different polyphenols at three substrate concentrations; and enzyme activity was recorded (n = 3). The inhibitor concentration was plotted vs. the reciprocal of enzyme activity (1/v) in Dixon plots.
Curcumin exerts an anti-inflammatory effect on LPS-stimulated blood cells
Polyphenols are known for their anti-inflammatory effects on immune cells. To compare the effect of curcumin with other polyphenols displaying flavonoid structure, we applied whole blood assays whereby monocytes were stimulated with LPS at 37°C/5% CO2 and incubated for 6 h (Fig. 2). LPS-stimulated blood cells release pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β and IL-8. Upon addition of curcumin and other selected flavonoids at increasing concentrations to the cultured cells, we observed a strong suppression of the pro-inflammatory cytokine release as exemplified by IL-1β (Fig. 2). The results of the current study rank curcumin at the top of the investigated polyphenols with respect to inhibition of pro-inflammatory cytokine production. IC50 values of 7.9 µM, 20 µM, 34.6 µM and 68 µM have been estimated for curcumin, quercetin, kaempferol, and luteolin, respectively. None of these compounds was found to induce IL-1β release in the absence of LPS. Moreover, at concentrations above 200 µM, these compounds induced red blood cell lysis. A strong positive correlation was found between the anti-inflammatory activity (IC50) and the in vitro Ki-values of curcumin, quercetin, kaempferol and luteolin (Spearman's R = 0.90) indicating that Glo1 inhibition may be a possible mechanism to explain the anti-inflammatory effects of these polyphenols.
Figure 2. Effect of polyphenols on IL-1β release from LPS-stimulated blood cells.
Heparinized whole blood was stimulated by LPS in the absence or presence of polyphenols at increasing concentrations and incubated for 6 h at 37°C with 5% CO2. (A) Released IL-1β as measured in cell supernatants. Samples without additives but LPS were set at 100%. (B) The calculated LD50 values of the respective polyphenols. Data represent mean±S.D. of independent experiments (n = 6).
Curcumin inhibits growth of cancer cells via targeting glyoxalase 1
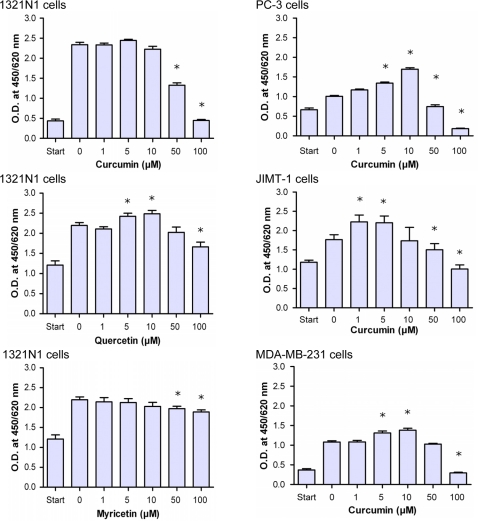
It is known that inhibitors of Glo1, structurally related to GSH, have anti-proliferative properties [19]. To study the action of curcumin on cell growth, we incubated different tumor cells with increasing concentrations of curcumin for 24 h and measured changes in cell proliferation applying WST-1 assay (Fig. 3). Curcumin effectively inhibited the growth of different cancer cell lines derived from prostate cancer (PC-3), breast cancer (MDA-MB-231, JIMT-1), and brain astrocytoma (1321N1). Curcumin-treated cells manifested a dose-dependent reduction in cell proliferation (Fig. 3A). Obviously, the cellular activity of curcumin is biphasic. At low concentrations, it is stimulatory rather than inhibitory, especially in the range between 1 µM and 10 µM. This effect was observed predominantly in prostate and breast cancer cells and was absent in astrocytoma cells. However, strong anti-proliferative effects were observed at concentrations above 50 µM for all cancer cells tested. Not only did curcumin inhibit cell growth as seen for 1321N1, MDA-MB-231 and JIMT-1 cells, but it also exerted even a toxic effect at 100 µM on PC-3 cells. In this case, the normal cellular morphology got lost indicating necrotic cell death. Comparable to curcumin, both of quercetin and myricetin, which also inhibited Glo1 activity, were less anti-proliferative to 1321N1 cells. This indicates that the growth suppressing effect of the studied polyphenols may be related to the Ki-values for Glo1 inhibition as shown in figure 1.
Figure 3. Growth inhibition of different tumor cell lines by polyphenols.
Tumor cells (5000 cells/well) were seeded (start) and cultured at 37°C/5% CO2 in the presence of increasing concentrations of curcumin or the flavonoids quercetin or myricetin. Following 24-h incubation, cell proliferation was evaluated using the WST-1 assay.
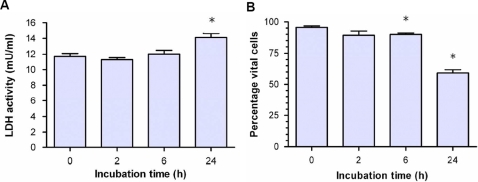
Following 6-h incubation of 1321N1 cells with curcumin (50 µM), cell shrinking and chromatin condensation accompanied by a massive loss of cytoplasm was observed (data not shown). This may be indicative to metabolically depressed tumor cells. Surprisingly, cell membranes integrity at this stage was apparently not altered as indicated by L-LDH release that was not significantly increased (Fig. 4A), as well as by the percentage of vital cells measured by trypan blue exclusion that was only slightly diminished (89.5%±2.5% vs. 95.6%±2.5%) (Fig. 4B). However, longer incubation (24-h) increased the LDH release and significantly reduced the number of vital cells to 60%±6.1% relative to the control.
Figure 4. Release of L-lactate dehydrogenase (L-LDH) and vitality of 1321N1 cells upon incubation with 50 µM curcumin.
Tumor cells were cultured and treated as mentioned in Fig. 3. (A) LDH activity was measured in cell supernatants. (B) Cell vitality as assayed by the trypan blue exclusion test. Data represent the mean±S.D. of independent experiments (n = 6).
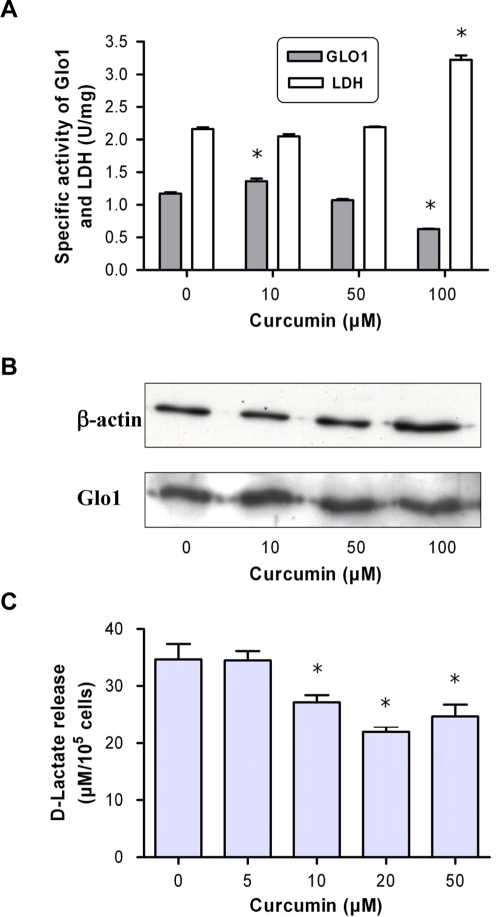
To proof that inhibition of the Glo1activity accounts for cellular effects, we exposed JIMT-1 breast carcinoma cells to curcumin for 24 h. After cell harvesting and solubilisation, we found that specific Glo1 activity in treated cells was lower than in non-treated cells and decreased dose-dependently (Fig. 5A). As a control, we measured the LDH activity, another cytosolic enzyme, which we found to be increased at higher curcumin concentrations. This indicates the very selective effect of curcumin at the para-glycolytic enzyme Glo1. Again, a small augmentation of Glo1 activity was observed at 10 µM curcumin which may reflect the findings shown in figure 3. Estimating the protein level, Western blot analysis revealed no alteration of Glo1 content (Fig. 5B). This substantiates the assumption that curcumin acts as an enzyme inhibitor rather than through modulating the expression or degradation of Glo1. We found similar effects with other tumor cells. To corroborate the results, we measured the concentration of D-lactate in the medium of cultured 1321N1 cells incubated with increasing concentrations of curcumin for 24 h. D-lactate is produced in the MGO pathway as the end-product of the Glo2 enzymatic reaction. Inhibition of glyoxalases is known to result in a decrease in D-lactate release. Our results demonstrated that D-lactate decreased from 34.6±6 µM in the control cells to 22±1.9 µM at 20 µM curcumin.(Fig. 5C). This indicates that cytosolic Glo1 is inhibited by curcumin and thereby may result in accumulation of MGO.
Figure 5. Cytosolic activity and protein content of glyoxalase 1 upon treatment of JIMT-1 cells with curcumin.
(A) JIMT-1 cells were treated with curcumin at 37°C and 5% CO2 for 24 h. Cells were harvested and the specific activity of Glo1 and LDH was determined in the cytosolic extract. (B) Semi-quantitative analysis of protein content as performed by Western blot using specific antibodies against β-actin and Glo1. The blot was developed by chemoluminescense detection. (C) D-lactate release as determined in supernatants of astrocytoma 1321N1 cells following 24-h incubation with curcumin. Data represent the mean±S.D. of independent experiments (n = 6).
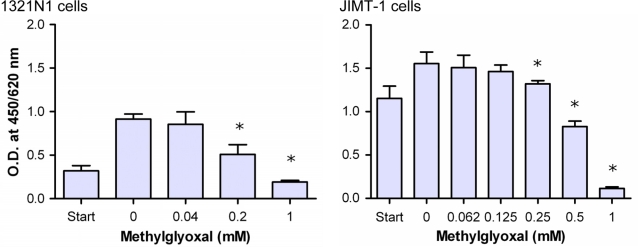
Because MGO rapidly forms hemithioacetal with GSH, elevated MGO will, in turn, deplete cellular GSH levels especially at low expression level of Glo2. Therefore, we investigated whether MGO may, at least partially, account for the anti-proliferative effect of curcumin by treating 1321N1 and JIMT-1cells with increasing concentrations of the reactive aldehyde (Fig. 6). The results disclose the anti-proliferative properties of MGO. Because MGO exerts its effect predominantly intracellular, we expected that the concentration of MGO that reaches the cell is much lower than what was added to the medium. This means that MGO rapidly reacts with amino and sulfhydryl groups of proteins abundantly present in the culture medium (10% fetal calf serum). In this regard, it has been reported that the intracellular concentrations of MGO in normal growing cells can be elevated up to 300 µM [23].
Figure 6. Effect of methylglyoxal on proliferation of astrocytoma and breast cancer cells.
Astrocytoma 1321N1 (5000 cells/well) and breast cancer cells JIMT-1 were seeded (start) and cultured in the absence or presence of increasing concentrations of MGO for 24 h. Cell vitality was recorded by WST-1 assay. Data represent the mean±S.D. of independent experiments (n = 12).
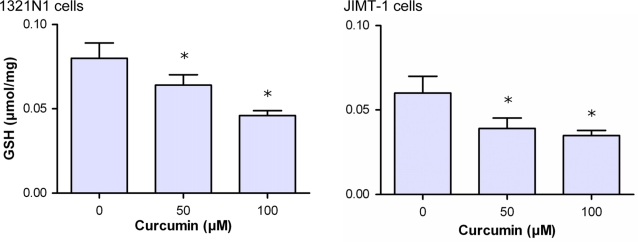
As shown in figure 7, curcumin-treated 1321N1 and JIMT-1 cells revealed decreased GSH levels. GSH was diminished by approximately 20% in cells treated with 50 µM curcumin, a concentration which yields approximately 30% inhibition of cell proliferation as presented in figure 3. This may be linked to the increasingly formed hemithioacetal that might trap GSH. This trapping can be magnified in case of a low Glo2 activity, an enzyme that hydrolyzes the S-lactoylglutathione, the end product of Glo1, to release D-lactate and to regenerate GSH. Therefore, we measured the Glo2 activity in cytosolic cell extracts (Table 1). The results demonstrated between 10- and 70-fold decrease in specific activity of Glo2 compared to Glo1 in different tumor cell lines. Thus, the low expression of Glo2 is suggested to impair the regeneration of GSH. Obviously, this could add additional effects to the GSH depletion.
Figure 7. Effect of curcumin on glutathione (GSH) level of 1321 N1 and JIMT-1 cells.
Astrocytoma 1321N1 (A) and JIMT-1 (B) cells were cultured for 24 h at 37°C and 5% CO2 in the absence or presence of curcumin. Cells were harvested and GSH concentration was determined in the cytosolic extract. Data represent the mean±S.D. of independent experiments (n = 6).
Table 1. Specific activity of Glo11, Glo2 and GSH2 content in various tumor cells.
| Cell type | Glo1 (U/mg protein) | Glo2 (U/mg protein) | GSH (µmol/mg protein) |
| Brain astrocytoma 1321N1 | 0.9±0.08 | 0.02±0.004 | 0.08±0.005 |
| Breast cancer MDA-MD-231 | 0.4±0.04 | 0.03±0.008 | 0.03±0.003 |
| Prostate cancer PC-3 | 1.4±0.09 | 0.02±0.009 | 0.05±0.009 |
| Breast cancer JIMT-1 | 1.17±0.02 | 0.02±0.01 | 0.06±0.01 |
Cytosolic extracts were prepared from cultured tumor cells and specific activity of enzymes and GSH was determined as described in Materials and Methods. The values represent means±S.D. determined in triplicates (n = 6).
glyoxalase 1 & glyoxalase 2.
glutathione.
Curcumin leads to metabolic depletion of ATP
It has been shown that MGO affects energy metabolism [24]. If curcumin-induced Glo1 inhibition leads to accumulation of MGO, both of curcumin and MGO are expected to have similar effects at metabolic activity of tumor cells. Therefore, we analyzed the ATP-content of 1321N1 and JIMT-1 cells treated with various concentrations of curcumin and MGO. As shown in figure 8, curcumin decreased cellular ATP in a concentration-dependent manner and MGO disclosed comparable effects.
Figure 8. Effect of curcumin and methylglyoxal (MGO) at the ATP level in tumor cells.
Astrocytoma 1321N1 cells (A) and JIMT-1cells (B) were seeded (5000 cells/well) and cultured in the presence of curcumin (0–100 µM) or MGO (0–1000 µM) for 6 h. Cellular ATP content was determined using the CellTiter-Glo® Luminescent Cell Viability Assay. Data represent the mean±S.D. of independent experiments (n = 12).
Curcumin is cytotoxic to cancer cells but not to primary human hepatocytes
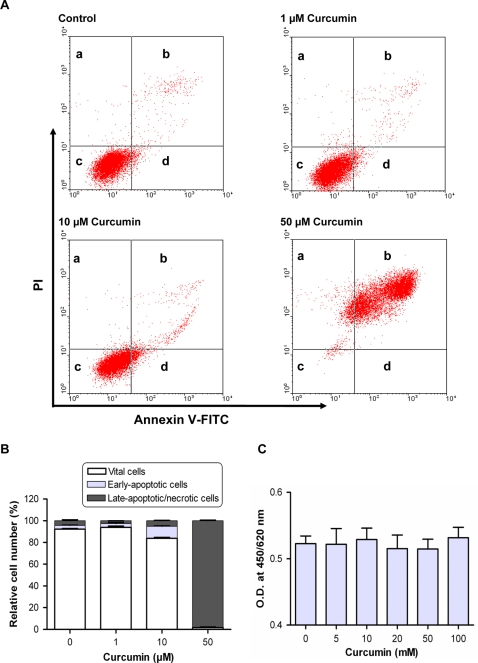
Increased MGO [25], depletion of GSH [26] and ATP [27] are known to initiate apoptotic cell death. We questioned whether curcumin inhibits tumor cell growth by induction of apoptosis. Therefore, 1321N1 cells were treated with increasing amounts of curcumin and analyzed for annexin V-5-fluorescein isothiocyanate (FITC)/propidium iodide (PI) binding (Fig. 9A and 9B).
Figure 9. Effect of curcumin on apoptosis/necrosis of tumor and normal cells.
Apoptosis/necrosis was evaluated using annexin V-5-fluorescein isothiocyanate (FITC)/propidium iodine (PI) staining followed by flow cytometric analysis. (A) Representative plots of showing annexin V-FITC/PI staining of Astrocytoma 1321N1 cells cultured in the presence of curcumin. The proportion of dead cells (a: annexin V-FITC−/PI+), late apoptotic/necrotic cells (b: annexin V-FITC+/PI+), non-apoptotic cells (c: annexin V-FITC−/PI−) and early apoptotic cells (d: annexin V-FITC+/PI−). (B) Results of 3 independent experiments. (C) Vitality response of primary human hepatocytes to 24-h incubation with curcumin as assessed by WST-1 test. Data represent the mean±S.D. of three independent experiments.
The results show that curcumin induced apoptosis in 1321N1 cells. Curcumin at a concentration of 10 µM was found to induce early apoptosis of 11.0% of cells characterized by the intactness of membrane, affinity towards annexin V-FITC and devoid of PI staining. At higher concentration of curcumin (50 µM), the number of late apoptotic/necrotic cells increased up to 98.2%. This accords the results shown in figure 4.
Primary human hepatocytes, on the other hand, were not affected by curcumin as shown by the lack of any change in the cell vitality in the presence of increasing concentrations of curcumin (Fig. 9C).
Discussion
Anti-proliferative and anti-inflammatory activity of curcumin
Curcumin possesses a wide range of pharmacological properties including anti-inflammatory, anti-infectious, and anti-carcinogenic activities [28]. A number of different targets have been proposed to mediate these different effects which include transcription factors, enzymes, cytokines and cell receptors [1], [13]. In the present work we have added a further target of curcumin, the Glo1. Notwithstanding the numerous studies, Glo1 has never been reported or suggested to be a binding target of curcumin.
Inhibitors of glyoxalases have long been sought as possible anti-cancer drugs. In this sense, analogues of GSH such as S-p-bromobenzylglutathione have been prepared and used to inhibit the growth of cancer cells [19]. However, the use of high affinity glutathione-based inhibitors has many drawbacks due to interference with many other GSH-dependent enzymes in vivo.
Recently, natural inhibitors of Glo1 have been selected from flavonoids, polyphenolic compounds found in many plants [22]. This prompted us to test whether the polyphenol curcumin exerts similar properties. We found that curcumin showed even a stronger inhibitory effect on Glo1 than the flavonoids quercetin, myricetin, kaempferol, luteolin and rutin. Its inhibitory activity expressed by the Ki value was in the range of that of S-p-bromobenzylglutathione known to be the strongest inhibitor of Glo1 [22].
Growth inhibition curve of cancer cells treated with curcumin showed a biphasic feature. At low concentrations, curcumin was found to stimulate cell proliferation rather than being inhibitory. The reason for this biphasic action is currently not known, but it may be related to the polyvalent activity of curcumin, so that different cellular pathways are activated in a concentration-dependent manner. Such a distinct dose-related behavior was already observed in case of stimulated mouse macrophages treated with curcumin [29]. At high concentrations, curcumin was found to act probably via inactivation of NF-κB compared to modulation of the heme oxygenase expression at low concentrations.
Similarly, curcumin was found to act as an anti-oxidant at low doses, whereas at higher doses the pro-oxidant properties prevailed, a virtue that may be of high importance in cell apoptosis and treatment of cancer cells [30]. In addition, the down-regulation of pro-inflammatory cytokines is most likely mediated by inactivation of the transcription factor NF-κB signaling system although the precise mechanism is not known [31]. No chemical modification of the NF-κB signaling pathway by curcumin was found and the site of intervention was supposed to precede the phosphorylation steps.
We could imply that curcumin had a strong impact at the highly proliferative and invasive tumor cells such as 1321N1, PC-3 and MDA-MB-231. Currently, there are no drugs for successful treatment of human astrocytoma and glioma tumors. This might be of interest in the light of the finding that curcumin is capable to cross the blood-brain-barrier [5].
This would increase the list of therapeutic application of curcumin comprehensively described earlier [32].
The striking differences found between the IC50 values for cytokine suppression compared to values for the anti-proliferative activity of curcumin let us assume that this polyphenol possesses an anti-inflammatory potency at low concentrations, whereas higher concentrations are required to mediate an anti-proliferative effect. This can be explained by the different expression of Glo1 in mononuclear blood cells compared to tumor cells. Glo1 is highly expressed in cancerous cells to compensate for the permanent formation of MGO due to the high glycolytic rate. The specific activity of Glo1 in mononuclear blood cells was approximately 1/10 compared to tumor cells (unpublished data). Thus, lower concentrations of Glo1 inhibitors are required to mediate cellular effects.
Proposed mechanism of curcumin action
The expression of glyoxalases is normally adjusted to the flux of glucose through the glycolytic pathway. Many cancer cells gain their energy mainly by oxidation of glucose to lactate showing a high aerobic glycolysis [33]. To compensate the low ATP gain via glycolysis, these cells must increase their glycolytic flux several folds. Consequently, this increases the level of MGO to toxic concentrations for the respective cells [24]. Therefore, most cancer cells show increased expression of Glo1 [34].
The key events in cellular metabolism upon glyoxalase inhibition are the elevated levels of MGO and depletion of GSH. Both alterations will have profound effects on regulatory processes and on cellular bioenergetics. There are reports showing that MGO is toxic to cells by depleting ATP, modulation of mitochondrial membrane potential, induction of apoptosis and ROS-production [24], [35]. MGO is capable to modify the function of very distinct proteins, e.g. activation of transcription factors in yeast [36], distinct covalent modifications of proteins like Hsp27 [37] or modulation of enzyme activity of GAPDH [38]. Results of a recent paper supported our hypothesis in the broadest sense that MGO, by reacting with Cys 38 of NF-κB p65, inhibits its binding to DNA [39]. These data coincide with the observed effects of curcumin at the mitochondrial membrane permeability and inhibition of ATP synthesis [40], as well as suppressed activation of NF-κB. However, the specific target within this signaling pathway has not been identified yet [41]. NF-κB activation enables anti-apoptotic genes to promote carcinogenesis and induces inflammation [42]. Therefore, we suggest that the anti-proliferative and ant-inflammatory effect of curcumin might be at least partially mediated by inhibition of Glo1.
As far as GSH is concerned, increased levels of MGO may cause cellular depletion of GSH due to hemithioacetal formation. Low levels of GSH have been found to decrease resistance of cancer cells to chemotherapeutic drugs [43], boost cell apoptosis [44] and mediate ROS-induced mitochondrial damage [45]. Without knowing the precise site of action, curcumin treatment was associated with decreased intracellular GSH which may account for its pro-apoptotic effects [46] but is inconsistent with the observed anti-oxidant properties of curcumin [47]. Recently, it has been convincingly shown that a number of flavonoids can induce GSH depletion in human tumor cells. However, the mechanism how polyphenols can drive GSH depletion has not been published yet [48]. The observed decrease of intracellular GSH and ATP content of tumor cells may cooperate in mediating toxic effects on cells at higher concentrations of curcumin. As far as ATP depletion is concerned, it can currently not be dissipated whether this may be caused by direct inhibition of glycolysis or by toxic effects of MGO or curcumin on mitochondria.
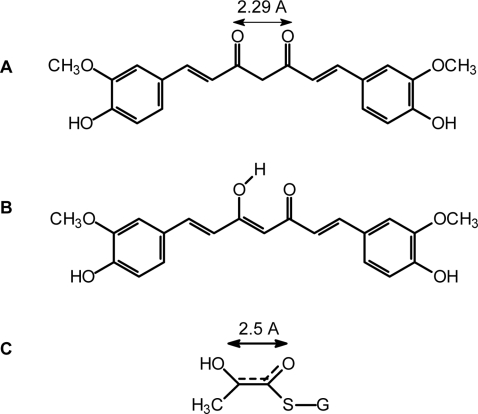
Inhibition of Glo1 by compounds unrelated to GSH has been described recently for flavonoids and indomethacin [22], [49]. Selected from flavonoids, Takasawa et al. [22] proposed that compounds possessing a plane configuration of a ketone and a hydroxy group arranged in a distance of 2.8 A may mimic the enediolate intermediate that yields along the reaction pathway of Glo1 (Fig. 10). As curcumin is endowed with similar structural elements (keto-enol forms), it is conceivable that this pharmacophore directly interacts with the active site of Glo1. Curcumin may exist at equilibrium between the diketo and keto-enol forms; the latter is strongly favored by intramolecular H-bonding. In this line, it has been suggested that these structural elements appear to be important for its anti-tumor activity [50]. Recently, we could evidenced that compounds harboring “dicarbonyl” groups such as ethyl pyruvate possess an anti-inflammatory potency and suppress the expression of immune receptors on human macrophages via targeting human Glo1 [51].
Figure 10. Keto-Enol Tautomeric forms of curcumin and transition-state of glutathione (GSH) and methylglyoxal (MGO).
(A) (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione. (B) (1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-triene-3-one. (C) Transition-state compound of reaction between GSH and MGO.
It has been reported that curcumin can directly react with SH groups of glutathione and cysteine residues in proteins [52]. However, it is not conceivable that this may affect the cellular GSH content because the intracellular concentration of curcumin is expected to be too low compared to that of GSH.
Among the different known targets of curcumin, only Glo1 bridges energy metabolism, especially glycolysis, to inhibition of cell growth. That may be the reason why Glo1 is critical for cell survival [53] and that Glo1 inhibitors may constitute potent chemotherapeutic agents [34]. In addition, it may also explain why curcumin is also active against inflammation, bacteria and protozoa [11], [54], [55]. Inflammation process is always accompanied by activation of glycolytic pathway in specific cells such as monocytes/macrophages or lymphocytes. Furthermore, bacteria as well as protozoa such as malaria parasites and trypanosome are mainly glucose-consuming organisms. As these organisms do not possess NF-κB pathway, one of the predicted targets of curcumin, it is likely that curcumin may act through glyoxalase inhibition, in addition to other proposed mechanisms [56].
In conclusion, the present study may designate the natural product curcumin as a strong competitive inhibitor of Glo1. This will increase our understanding of the anti-inflammatory and anti-proliferative effects of curcumin and may provide new insights into how curcumin exerts its anti-carcinogenic effect.
Materials and Methods
Materials
Curcumin, myricetin, luteolin, kaempferol and quercetin were obtained from Roth (Karlsruhe, Germany). 5,5′-Dithio-bis(2-nitrobenzoic acid) (DTNB), methylglyoxal, lipopolysaccharide (E. coli Serotype O111:B4), S-lactoylglutathione, propidium iodide, trypan blue, L-lactate, D-lactate, D-lactate dehydrogenase (D-LDH) (Lactobacillus leichmannii) and L-lactate dehydrogenase (L-LDH) (bovine heart), EDTA, glycerol, Triton X-100, 1,4-dithiothreitol (DTT), phenylmethanesulfonyl fluoride (PMSF), William Medium E and protease inhibitor cocktail were purchased from Sigma-Aldrich (Taufkirchen, Germany). RPMI 1640 medium was purchased from Biochrom (Berlin, Germany). Cell proliferation reagent WST-1 was obtained from Roche (Mannheim, Germany). BD™ CBA Human Inflammation Kit was purchased from BD Biosciences (Heidelberg, Germany). Annexin V-FITC was obtained from Immunotools (Marseille, France). Dulbecco's modified Eagle Medium (DMEM), RPMI Medium and fetal calf serum (FCS) were obtained from Invitrogen (Karlsruhe, Germany). Anti-human Glo1 monoclonal antibodies were purchased from BioMac (Leipzig, Germany). Tumor cell lines used are prostate cancer PC-3 (CRL-1435; ATCC), brain astrocytoma 1321N1 cells (86030102; ECACC) and human breast JIMT-1 (ACC 589; DSMZ) and MDA-MB-231 (HTB-26; ATCC) cancer cells.
Application of curcumin
A stock solution of curcumin (50 mM) was prepared in DMSO. Just before adding to cultured cells, curcumin was diluted in culture medium as required keeping the final DMSO concentration at 1%.
Whole blood assay
Whole blood assay was performed as previously described [57]. Accordingly, LPS (10 ng/ml), test substances and 200 µl heparinized human blood of healthy donors, obtained from the blood bank of the University of Leipzig, were co-incubated with serum-free medium (RPMI-SF, 1 ml final volume) in 24-well culture plates for 6 h at 37°C with 5% CO2. Samples were removed, centrifuged at 2000×g for 10 min and supernatants were stored at −20°C until assaying for inflammatory cytokines.
Cytokine analysis using cytometric bead array
Assessment of cytokine levels in supernatants of human blood cell cultures was accomplished using Cytometric Bead Array (CBA™, BD Biosciences, San Jose, U.S.A). The procedure was carried out according to the manufacturer's instruction. Fluorescence measurement and analysis were performed using a FACSCalibur and the CellQuest software (BD Biosciences).
Mammalian cell culture
Tumor cells were cultured in RPMI medium containing 10% fetal calf serum (RPMI-FCS) (PC-3, JIMT-1, MDA-MD 231) or in DMEM-FCS (astrocytoma 1321N1 cells) containing penicillin/streptomycin (100 U penicillin/ml; 100 µg streptomycin/ml) at 37°C/5% CO2. Cytosolic protein extracts of tumor cells were prepared using extraction buffer composed of 25 mM Tris, 2 mM EDTA, 10% Glycerol, 1% Triton ×100, 2 mM DTT, 1mM PMSF, pH. 7.0 containing 0.3% protease inhibitor cocktail. Protein content was determined according to Bradford [58]. Specific activity of enzymes analyzed in cell extracts was expressed as U/mg protein.
Primary human hepatocytes were isolated and cultivated in serum-free William Medium E [59]. Tissue samples from human liver resection were obtained from patients undergoing partial hepatectomy for metastatic liver tumor of colorectal cancer. Experimental procedures were performed according to the guidelines of the charitable state controlled foundation (Human Tissue and Cell Research), with the informed patient's consent [60] approved by the local ethical committee.
Purification of glyoxalases
Glo1 was purified from human erythrocytes as described earlier [61]. Briefly, erythrocytes were treated with butanol/chloroform to denature hemoglobin followed by acetone and ammonium sulphate fractionation. Repeated affinity chromatography on S-hexylglutathione-Agarose followed by gel permeation chromatography yielded the enzyme; purity>90%.
Glyoxalase activity measurement
The determination of Glo1 (E.C.4.4.1.5) activity has been described previously [61]. To evaluate the effect of curcumin and other polyphenols on Glo1 enzymatic activity, 2 mM GSH and 2 mM MGO were pre-incubated for 4 min, and then mixed with increasing concentrations of test substances together with 70 mU of the enzyme. The inhibition constant, Ki value of the inhibitors was evaluated by Dixon plot showing the reciprocal of enzyme velocity against the inhibitor concentration at variable concentrations of the substrate. Human Glo2 (E.C.3.1.2.6) was measured using the substrate S-lactoylglutathione as described earlier [62].
LDH activity measurement
LDH activity was spectrophotometrically assayed in the cytosolic extract or in the of cell cultures by adding aliquots to 1 ml reaction buffer (50 mM Na2HPO4, pH 7.0, 0.15 mM NADH, and 0.3 mM pyruvate). Enzyme activity was recorded at 340 nm. It was ascertained that the enzyme activity was not affected by curcumin.
Measurement of L-lactate and D-lactate
The determination of D-lactate and L-lactate in supernatants of astrocytoma 1321N1 cells was performed by an enzymatic method based on the oxidation of L-lactate and D-lactate to pyruvate by NAD in the presence of L-LDH or D-LDH[63].
Apoptosis/necrosis measurement
Astrocytoma 1321N1 cells were treated with increasing amounts of curcumin for 24 h, washed with PBS and after trypsination, apoptosis/necrosis was evaluated using annexin V-FITC/propidium iodine (PI) staining as previously described [64]. Samples were analyzed by flow cytometry using a FACSCalibur and the CellQuest software (BD Biosciences, San Jose, USA). Laser excitation wavelength was set at 488 nm. The green signal from annexin V-FITC was measured at 525 nm and the red signal from PI was measured at 620 nm. Annexin V−/PI− cells are viable; annexin V+/PI− cells are in early apoptosis, whereas annexin V+/PI+ cells are necrotic or in late apoptosis are
Glutathione determination
GSH was determined using DTNB method [65]. Briefly, cytosolic extract of tumor cells were precipitated by 5% sulfosalicylic acid neutralization, aliquots were mixed with DTNB and absorbance was read at 412 nm against a standard solution of GSH.
Cell proliferation/vitality assays
Cell proliferation/vitality of tumor cells (96-well plates; 5000 cells/well) and primary human hepatocytes (24-well plates; 0.25×106 cells/well) was assayed using the WST-1 assay according to manufacturer's instructions. Trypan blue exclusion was performed by staining cells with 0.4% aqueous trypan blue solution according to the manufacturer's instructions.
ATP measurement
Cell ATP content was determined by means of the CellTiter-Glo® Luminescent Cell Viability Assay according to the manufacturer's instructions (Promega, Madison, USA). Briefly, tumor cells were cultured in 96-well plates (5000 cells/well) in the absence or presence of curcumin or MGO, respectively. After 6-h incubation, cells were mixed with test reagents and luminescence was read according to the manufacturer's instructions. A standard curve was prepared from ATP (1 µM to 10 nM) in medium.
Western blot analysis
An amount of 20 µg to 40 µg of cytosolic extract was loaded to SDS-pore gradient gels (4 to 20%) and run under reducing conditions. Proteins were blotted to cellulose nitrate membranes (Whatman Schleicher & Schuell, Dassel, Germany) and Glo1 was detected by anti-Glo1 monoclonal antibodies (1 µg/ml) (BioMac, Leipzig, Germany) in combination with goat anti-mouse Ig-HRP (1∶1000) (Dako, Hamburg, Germany). For comparison, β-actin was analyzed using rabbit anti-β-actin Ig (1∶2000) (Acris, Hiddenhausen, Germany) in conjunction with HRP-labeled goat anti-rabbit Ig (Dianova, Hamburg, Germany). Band visualization was performed by chemiluminescence detection (Boehringer, Mannheim, Germany).
Statistical analysis
Kinetic data (Fig. 1) were analyzed with the GraphPad Prism 3.03 (GraphPad Software Inc. San Dieago, CA) using the Marquardt-Levenberg method with simple weighting. A goodness-of-fit criterion (Akaike's information criterion, AIC) was used for falsification of incompatible inhibition models. It turned out that the considered inhibitors act mainly in a competitive mode. The quality of the fit was characterized by 95% confidence intervals of the estimated parameters and the total residual error. Spearman's rank correlation test was applied to evaluate the correlation between the IC50 values of pro-inflammatory cytokine release and the Ki-values of various polyphenols. Data were presented as means±S.D. of at least three independent experiments. The enzyme inhibition by polyphenols (Ki-values) are considered significant at p = 0.1 (Wilcoxon's rank-sum test). In all other tests, significance has been considered at values of p<0.05.
Acknowledgments
We thank Ms. E. Usbeck for her skilful technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported in part by Wilhelm Sander-Stiftung (No. 2006.053.1; GB and IOe), by the BMBF-grant (HepatoSys project 0313081 F; TW and RG) and by Hans Böckler Stiftung (356283; AH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 4.Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 6.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 8.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 9.Alexander JA, Castillo M, Hoffman JC., Jr Magnetic resonance findings in a patient with internuclear ophthalmoplegia. Neuroradiological-clinical correlation. J Clin Neuroophthalmol. 1991;11:58–61. [PubMed] [Google Scholar]
- 10.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann N Y Acad Sci. 2005;1042:372–378. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- 12.Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007;10:724–728. doi: 10.1097/MCO.0b013e3282f0cef2. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol. 1999;18:118–121. [PubMed] [Google Scholar]
- 15.Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J Agric Food Chem. 2001;49:1464–1474. doi: 10.1021/jf001129v. [DOI] [PubMed] [Google Scholar]
- 16.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 17.Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- 18.Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–269. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 19.Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, et al. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol. 1996;51:1365–1372. doi: 10.1016/0006-2952(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 20.Douglas KT, Gohel DI, Nadvi IN, Quilter AJ, Seddon AP. Partial transition-state inhibitors of glyoxalase I from human erythrocytes, yeast and rat liver. Biochim Biophys Acta. 1985;829:109–118. doi: 10.1016/0167-4838(85)90074-3. [DOI] [PubMed] [Google Scholar]
- 21.Douglas KT, Quilter AJ, Shinkai S, Ueda K. Trapping of reactive intermediates in enzymology. Exogenous flavin reduction during catalytic turnover of substrate by glyoxalase I. Biochim Biophys Acta. 1985;829:119–126. doi: 10.1016/0167-4838(85)90075-5. [DOI] [PubMed] [Google Scholar]
- 22.Takasawa R, Takahashi S, Saeki K, Sunaga S, Yoshimori A, et al. Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg Med Chem. 2008 doi: 10.1016/j.bmc.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Chaplen FW, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1998;95:5533–5538. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Arriba SG, Stuchbury G, Yarin J, Burnell J, Loske C, et al. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells–protection by carbonyl scavengers. Neurobiol Aging. 2007;28:1044–1050. doi: 10.1016/j.neurobiolaging.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Shangari N, O'Brien PJ. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol. 2004;68:1433–1442. doi: 10.1016/j.bcp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Naoi M, Maruyama W, Yi H, Yamaoka Y, Shamoto-Nagai M, et al. Neuromelanin selectively induces apoptosis in dopaminergic SH-SY5Y cells by deglutathionylation in mitochondria: involvement of the protein and melanin component. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05329.x. [DOI] [PubMed] [Google Scholar]
- 27.Obajimi O, Melera PW. The depletion of cellular ATP by AG2034 mediates cell death or cytostasis in a hypoxanthine-dependent manner in human prostate cancer cells. Cancer Chemother Pharmacol. 2008;62:215–226. doi: 10.1007/s00280-007-0593-6. [DOI] [PubMed] [Google Scholar]
- 28.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, et al. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomed Pharmacother. 2008 doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Manson MM. Inhibition of survival signalling by dietary polyphenols and indole-3-carbinol. Eur J Cancer. 2005;41:1842–1853. doi: 10.1016/j.ejca.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 33.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 34.Sakamoto H, Mashima T, Sato S, Hashimoto Y, Yamori T, et al. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res. 2001;7:2513–2518. [PubMed] [Google Scholar]
- 35.Vesce S, Jekabsons MB, Johnson-Cadwell LI, Nicholls DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J Biol Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]
- 36.Zuin A, Vivancos AP, Sanso M, Takatsume Y, Ayte J, et al. The glycolytic metabolite methylglyoxal activates Pap1 and Sty1 stress responses in Schizosaccharomyces pombe. J Biol Chem. 2005;280:36708–36713. doi: 10.1074/jbc.M508400200. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto H, Mashima T, Yamamoto K, Tsuruo T. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem. 2002;277:45770–45775. doi: 10.1074/jbc.M207485200. [DOI] [PubMed] [Google Scholar]
- 38.Lee HJ, Howell SK, Sanford RJ, Beisswenger PJ. Methylglyoxal can modify GAPDH activity and structure. Ann N Y Acad Sci. 2005;1043:135–145. doi: 10.1196/annals.1333.017. [DOI] [PubMed] [Google Scholar]
- 39.Laga M, Cottyn A, Van Herreweghe F, Vanden Berghe W, Haegeman G, et al. Methylglyoxal suppresses TNF-alpha-induced NF-kappaB activation by inhibiting NF-kappaB DNA-binding. Biochem Pharmacol. 2007;74:579–589. doi: 10.1016/j.bcp.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Morin D, Barthelemy S, Zini R, Labidalle S, Tillement JP. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495:131–136. doi: 10.1016/s0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 41.Gautam SC, Gao X, Dulchavsky S. Immunomodulation by curcumin. Adv Exp Med Biol. 2007;595:321–341. doi: 10.1007/978-0-387-46401-5_14. [DOI] [PubMed] [Google Scholar]
- 42.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 43.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Carystinos GD, Batist G. Potential for selective modulation of glutathione in cancer chemotherapy. Chem Biol Interact. 1998;111–112:263–275. doi: 10.1016/s0009-2797(97)00166-x. [DOI] [PubMed] [Google Scholar]
- 45.Kachadourian R, Leitner HM, Day BJ. Selected flavonoids potentiate the toxicity of cisplatin in human lung adenocarcinoma cells: a role for glutathione depletion. Int J Oncol. 2007;31:161–168. [PMC free article] [PubMed] [Google Scholar]
- 46.Scott DW, Loo G. Curcumin-induced GADD153 upregulation: modulation by glutathione. J Cell Biochem. 2007;101:307–320. doi: 10.1002/jcb.21179. [DOI] [PubMed] [Google Scholar]
- 47.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med. 2007;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kachadourian R, Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med. 2006;41:65–76. doi: 10.1016/j.freeradbiomed.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato S, Kwon Y, Kamisuki S, Srivastava N, Mao Q, et al. Polyproline-rod approach to isolating protein targets of bioactive small molecules: isolation of a new target of indomethacin. J Am Chem Soc. 2007;129:873–880. doi: 10.1021/ja0655643. [DOI] [PubMed] [Google Scholar]
- 50.Miguel L-L. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Molecular Nutrition & Food Research. 2008;52:S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 51.Hollenbach M, Hintersdorf A, Huse K, Sack U, Bigl M, et al. Ethyl pyruvate and ethyl lactate down-regulate the production of pro-inflammatory cytokines and modulate expression of immune receptors. Biochem Pharmacol. 2008;76:631–644. doi: 10.1016/j.bcp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, et al. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 53.Miller AG, Smith DG, Bhat M, Nagaraj RH. Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem. 2006;281:11864–11871. doi: 10.1074/jbc.M513813200. [DOI] [PubMed] [Google Scholar]
- 54.Nandakumar DN, Nagaraj VA, Vathsala PG, Rangarajan P, Padmanaban G. Curcumin-artemisinin combination therapy for malaria. Antimicrob Agents Chemother. 2006;50:1859–1860. doi: 10.1128/AAC.50.5.1859-1860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang G, Yang S, Jiang L, Zhao Y, Shao L, et al. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull (Tokyo) 2008;56:162–167. doi: 10.1248/cpb.56.162. [DOI] [PubMed] [Google Scholar]
- 56.Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J. 2008;410:147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- 57.Wilson BM, Severn A, Rapson NT, Chana J, Hopkins P. A convenient human whole blood culture system for studying the regulation of tumour necrosis factor release by bacterial lipopolysaccharide. J Immunol Methods. 1991;139:233–240. doi: 10.1016/0022-1759(91)90193-j. [DOI] [PubMed] [Google Scholar]
- 58.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 59.Weiss TS, Pahernik S, Scheruebl I, Jauch KW, Thasler WE. Cellular damage to human hepatocytes through repeated application of 5-aminolevulinic acid. J Hepatol. 2003;38:476–482. doi: 10.1016/s0168-8278(02)00454-3. [DOI] [PubMed] [Google Scholar]
- 60.Thasler WE, Weiss TS, Schillhorn K, Stoll PT, Irrgang B, et al. Charitable State-Controlled Foundation Human Tissue and Cell Research: Ethic and Legal Aspects in the Supply of Surgically Removed Human Tissue For Research in the Academic and Commercial Sector in Germany. Cell Tissue Bank. 2003;4:49–56. doi: 10.1023/A:1026392429112. [DOI] [PubMed] [Google Scholar]
- 61.Mannervik B, Aronsson AC, Tibbelin G. Glyoxalase I from human erythrocytes. Methods Enzymol. 1982;90 Pt E:535–541. doi: 10.1016/s0076-6879(82)90181-1. [DOI] [PubMed] [Google Scholar]
- 62.McLellan AC, Thornalley PJ. Glyoxalase activity in human red blood cells fractioned by age. Mech Ageing Dev. 1989;48:63–71. doi: 10.1016/0047-6374(89)90026-2. [DOI] [PubMed] [Google Scholar]
- 63.Brandt RB, Siegel SA, Waters MG, Bloch MH. Spectrophotometric assay for D-(-)-lactate in plasma. Anal Biochem. 1980;102:39–46. doi: 10.1016/0003-2697(80)90314-0. [DOI] [PubMed] [Google Scholar]
- 64.Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol. 2008;14:2174–2178. doi: 10.3748/wjg.14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]