Abstract
The navigational system of the mammalian cortex comprises a number of interacting brain regions. Grid cells in the medial entorhinal cortex and place cells in the hippocampus are thought to participate in the formation of a dynamic representation of the animal's current location, and these cells are presumably critical for storing the representation in memory. To traverse the environment, animals must be able to translate coordinate information from spatial maps in the entorhinal cortex and hippocampus into body-centered representations that can be used to direct locomotion. How this is done remains an enigma. We propose that the posterior parietal cortex is critical for this transformation.
Keywords: entorhinal cortex, grid cell, place cell, Path integration, spatial memory
Animals have a number of strategies for optimizing movement toward goal locations. The repertoire of navigation strategies ranges from simple approach and avoidance, such as following odor trails and chemical gradients or moving toward prominent visual beacons, to the use of complex representations, such as geometric maps based on perceived and remembered spatial relationships between distributed landmarks, and path integration based on the continuous flow of motion-generated speed and direction signals (1–3). In most species, the mechanisms are complementary and used in combination. Landmarks and geometrical relationships enable the animal to store maps and routes for individual environments, but on their own these cues provide limited information about the direction and distance that the animal has moved from a given reference position. Conversely, path integration can be used to build a metric representation of the animal's position in the environment, but without regular calibration against perceived or recalled landmarks and geometric boundaries, errors will accumulate and the representation will drift. The composite nature of navigation suggests that multiple brain regions and mechanisms may be involved.
Place Cells in the Hippocampus
Our understanding of the neural representation of navigational space began with the discovery of “place cells” by O'Keefe and Dostrovsky in 1971 (4). By using microelectrodes chronically implanted in freely behaving rats, these authors found that the firing of putative pyramidal cells in the dorsal hippocampus exhibited a striking behavioral modulation, where cells fired only when animals occupied particular locations, or “place fields,” in the recording environment (Fig. 1A). Outside of these fields, the cells were mostly silent. Subsequent work showed that neighboring hippocampal cells had nontopographically organized place fields such that the entire surface of the environment could be represented by a group of neurons in a local circuit (5). Cells with firing fields in two environments fired at unrelated locations in those environments (6). Following these observations, it was proposed that the concerted activity of place cells provides the physiological substrate for a “cognitive map,” where cell populations throughout the hippocampus maintain a coherent, up-to-date representation of allocentric space and the animal's location in that space at any point in time (1).
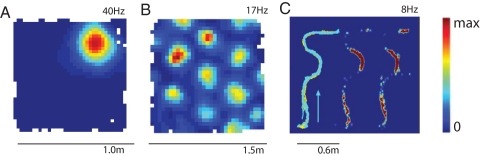
Fig. 1.
Spatial firing properties of neurons in hippocampus (A; place cell), medial entorhinal cortex (MEC) (B; grid cell), and posterior parietal cortex (PPC) (C). In A and B, the rat runs freely in an open-field environment; in C, the rat traverses a complex maze with multiple segments and turns (Left, path of the animal; Center, rate map with lights on; Right, rate map in darkness). Firing rates are color-coded, with red showing maximal rate and blue minimum (scale bar to the right). Note that the parietal neuron fires at specific epochs along the trajectory and that firing is independent of visual inputs. [C is adapted with permission from ref. 66 (Copyright 2006, Neuron).]
Grid Cells and the Spatial Map in the Medial Entorhinal Cortex
Despite major advances in understanding hippocampal spatial computation, the neural mechanisms for computing a dynamic representation of the animal's own location have remained elusive. The large number of nonoverlapping spatial representations stored in the hippocampus (7, 8) pointed to an extrahippocampal location for general navigational computations (5, 9, 10). This possibility was supported by the persistence of place fields in CA1 in rats where intrahippocampal connections were disrupted but direct projections from the entorhinal cortex were spared (11, 12). For a long time, however, the possibility of an extrahippocampal origin was neglected because neurons in the entorhinal cortex, from which the hippocampus receives most of its cortical inputs, showed only weak spatial modulation (13, 14). A closer look at the firing properties of entorhinal neurons finally revealed that neurons in more dorsomedial parts of the structure, far more dorsal than where activity was recorded in the earlier studies, exhibit fine spatial tuning similar to that observed in CA1 (15). The multiple firing fields of cells in this region of entorhinal cortex formed a strikingly regular grid-like pattern composed of equilateral triangles tessellating the entire environment covered by the animal (16; Fig. 1B). Based on the firing patterns of small numbers of grid cells, the position of a moving animal could be reconstructed on a second-by-second basis (15), suggesting that self-position was represented already in inputs to the hippocampus.
Grid cells in the entorhinal cortex are organized in a map-like manner according to the basic parameters of the grid (15, 16). All grid fields display the same iterative triangular geometry, but they may differ in spacing (distance between fields), orientation (the angle to which the maps are tilted), and phase (xy offset of the fields relative to an external reference). Grids of neighboring cells have similar orientation and spacing, but randomly shifted vertices, such that the fields of adjacent cells do not overlap more than expected by chance. Collectively, grid cells with different spatial phase, orientation, and spacing provide unambiguous information about the animal's current position (17). Grid cells are the predominant cell type of the medial entorhinal cortex (MEC), but in layers III–VI of this area they intermingle with head direction-responsive cells and cells with conjunctive grid and head direction properties (18).
Path Integration and Entorhinal Grid Cells
The expression of a strongly periodic spatial firing pattern in the presence of constantly changing running speed and running direction suggests that the grid must rely on path integration computations (16, 19, 20), where changes in velocity and direction are integrated over time to allow a constant representation of space (3, 19, 21). The relative invariance of the grid representation is consistent with this idea (22). Unlike place cells in the hippocampus, grid cells are activated in a stereotypic manner across environments, irrespective of the particular landmarks of the environment. Two cells in different parts of MEC whose grid fields are shifted and rotated 30° relative to each other in one environment will show the same relative shift and rotation in a different environment. The proposed dependence on path integration is further supported by the fact that grid-like spacing is expressed immediately as an animal starts to explore an environment and that, like place fields, the grids persist after removal of external sensory cues (16).
The continued firing of place cells and grid cells in the absence of environmental cues does not mean they are not normally anchored to extrinsic inputs. Spatial representations in both the hippocampus and MEC rotate in accordance with the displacement of distal visual cues (6, 7, 16), firing fields in both regions can be transformed by extending or contracting the geometric boundaries of the recording enclosure (23, 24), and self-motion-driven firing patterns in the hippocampus can be overridden by input from external landmarks (22, 25). These observations demonstrate that, although the discharge patterns of place cells and grid cells may be generated primarily by self-motion, the locations of firing must be influenced to a great extent by external sensory cues.
If grid cells and place cells are necessary for path integration-based spatial representation, animals with lesions in MEC and hippocampus should display navigational impairments. Several decades of research have indeed shown that lesions of the hippocampus disrupt the ability to navigate efficiently to the goal location in various kinds of mazes (see ref. 26) for a review). In the majority of these studies, the impairments in navigation can unfortunately not be distinguished from effects on memory. However, in one informative series of studies, path integration was assessed more directly by analyzing the trajectories of animals that returned to a starting refuge after searching for food on a slowly rotating arena (27, 28; Fig. 2A). In this task, the way back could only be found by integrating self-movement on the outward journey. Whereas control rats returned to the location that the refuge had held at the beginning of the trial, rats with lesions in the hippocampus had long return paths with no apparent preference for the original start location. A similar impairment was observed after lesions of the entorhinal cortex (29; Fig. 2B). The conclusions from these studies are somewhat contradicted by a study in which rats with partial hippocampal lesions did return to the start position (30). The authors suggested that the hippocampal part of the circuit is not necessary for path integration. Some details of the experimental procedure might weaken this conclusion, however. In experiments conducted in near total darkness by using a circular, open field, the control and hippocampal lesion rats were able to accurately return to the start location only in a condition in which a food cup was dragged by hand across the surface of the open field from the start location to the center of the open field. This was done to lead the rat away from the start location to the center. Although the experimenters varied the route by which the cup moved from the start location to the center from trial to trial, this procedure may have provided useful cues on the table surface. Rats are uncommonly good at tracking even subtle odors across the surface of an open field (31). With the uncertainty about the availability of odor trails in mind, we suggest that the study is not decisive in negating the hypothesis that the hippocampus is essential to complex path integration in the rat (but see note added in proof).
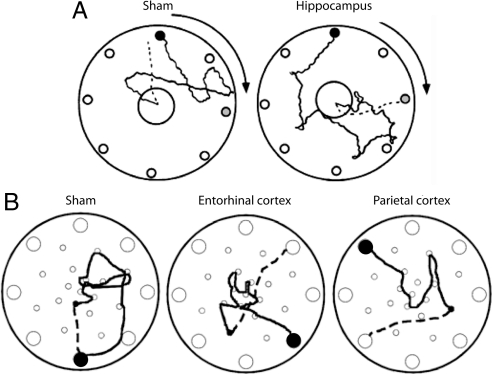
Fig. 2.
Disrupted path integration in rats with lesions of hippocampus (A), entorhinal cortex, or PPC (B). The rats were trained to leave a starting refuge, enter a large circular open field, search for a large food pellet, and bring the food pellet back to the refuge. The start box was placed beneath one of eight holes along the periphery of the circle (small filled circle). In A, the arena was slowly rotated, at subvestibular speed, as the rat searched for food. Gray circles indicate position of the start box after rotation. In B, there was no rotation. Outward paths are shown as solid lines in both A and B; return paths are shown as dashed lines (only from the food location to the first choice). Control animals generally chose the shortest path back to the point where they started, whereas rats with lesions in hippocampus, entorhinal cortex, or PPC generally chose the wrong hole, indicating that path integration was disrupted. Note that, although the lesioned rat in A ran directly to the novel target location, the dispersion of first choices was large in the hippocampal group as a whole. [Adapted with permission from ref. 27 (Copyright 2004, Hippocampus) and ref. 29 (Copyright 2004, Experimental Brain Research).]
The overall evidence thus implies an essential role for entorhinal and probably hippocampal neurons in path integration-based spatial representation and navigation in rats. However, the entorhinal-hippocampal circuit alone cannot be sufficient for implementing the full array of computations necessary for spatial behavior. In addition to possessing a universal map of space, animals must also be able to convert the spatial information into goal-oriented motor output. Previous models have suggested that the parietal cortex mediates the translation between external and first-person-based spatial representations, a process that may be instrumental in route planning (32). We propose here that this translation involves a transformation of output from the MEC circuit into body-based coordinates in parietal cortex, and that this transformation, aided by strong connections from parietal cortex to motor and premotor cortex (33, 34), is necessary for guiding locomotor output during goal-oriented navigation.
Spatial Behavior and the PPC in Primates
Parietal cortex is typically divided into two regions, an anterior region with primarily somatosensory functions and a more complex multisensory posterior region, the posterior parietal cortex (PPC) (Box 1 and Fig. 3). A large body of work, involving rats, monkeys, and humans, has consistently supported the conclusion that PPC is essential for key aspects of spatial representation (35). Through much of this work the emphasis has been on sensory or perceptual functions of the PPC. For example, in the case of visual information, a distinction has been made between dorsal and ventral visual streams (36). The ventral stream, which provides the major source of visual input to the temporal lobe, has been suggested to be critical in representing features of objects enabling their identification or recognition. The dorsal stream, which provides the PPC with its visual input, has been described as being critical for spatial perception, enabling the representation of the locations of objects in space (36). This distinction fits well with symptoms of visual agnosia, the inability to recognize objects, often displayed by patients with temporal neocortical injury (37) and with the various forms of spatial disorientation shown by patients with PPC damage (35, 38).
Box 1: What Is (Posterior) Parietal Cortex?
The parietal cortex is that part of the cortex that is covered by the parietal bone. Defined in this way, the parietal cortex includes at least two functionally different regions, the somatosensory cortex and an area that is considered part of the multimodal association cortex (99, 100). The latter region is referred to as PPC. The definition of this area is commonly based on a number of criteria, such as topological relationships among cortical areas, as well as cytoarchitecture, myeloarchitecture, and expression patterns of different neuroactive substances and neurotransmitters. Combined with connectional criteria focusing on thalamocortical and/or corticocortical connectivity and functional (electrophysiological and behavioral) comparisons, it is apparent that a PPC can be defined in most if not all species. The PPC is located in between somatosensory cortex anteriorly and visual cortex posteriorly (61, 101). Ventrally, PPC is bordered by areas that belong to the temporal association cortex, whereas the medial border abuts parts of the cingular domain.
Studies in various species report that the PPC is characterized by a connection with the higher-order associative posterior thalamus, but that it lacks either one of the unimodal visual or somatosensory thalamic inputs. In non-human primates, PPC is characterized by thalamic input from the posterior domain of the thalamus, in particular, the pulvinar. It lacks input from the ventral thalamic tier, which sends projections to the somatosensory part of parietal cortex, and from the lateral geniculate nucleus, which almost exclusively targets unimodal visual domains (102, 103). In other mammals, including the rat, thalamic inputs to PPC arise predominantly from the lateral posterior nucleus and adjacent nuclei, including laterodorsal and posterior nuclei. These nuclei are considered to be comparable to the primate pulvinar and posterior thalamic domain. Similar to the primate, thalamic projections from the ventral nuclear complex and lateral geniculate in the rat do not seem to innervate the PPC (76, 104–107). This combination of thalamic inputs thus provides useful borders between PPC, somatosensory cortex, and visual cortex, respectively. These borders coincide with both cyto- and myeloarchitectonic criteria (76, 100, 108), as well as with changes in a number of other histochemical markers (108).
The PPC receives input from cortical areas representing all main sensory modalities and communicates back to these domains as well (33, 77, 78, 88, 109). Whether the somatosensory input includes vestibular and proprioceptive information has not been unequivocally established, but data in the monkey and the rat seem to support this idea (107, 110–112). The PPC is also strongly and reciprocally connected to almost all of the other multimodal association cortices, such as (pre)frontal, temporal, and limbic association cortex (see main text for details). It further is connected with motor and premotor areas of the cortex (33, 34, 76, 96). A final feature which helps to differentiate the PPC from the adjacent somatosensory cortex is that only the former exhibits strong callosal connections (96, 113).
Notwithstanding the overall agreement that PPC is present in the rat, the definition of this area still generates some controversy about how to specifically delineate it and whether it can be subdivided in the same way as in the macaque monkey, where four areas—7a, b, m, and ip—are distinguished (99, 108, 113). It is likely that a more systematic study of the connectivity pattern, including the connections with the thalamus, will eventually enable us to clearly define the PPC of the rat.
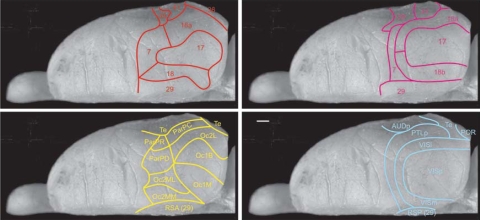
Fig. 3.
Dorsal view of the right hemisphere of the rat brain showing the different delineations of the parietal cortex of the rat as reported by Krieg (100), in red; Palomero-Gallagher and Zilles (108), in yellow; Miller and Vogt (109), in magenta; and Burwell and Amaral (67), in blue. Whether or not to include parts of Oc2 in the PPC (i.e., the lateromedial extent of the PPC, or area 7) is still controversial. The figures are based on the original diagrams but have been redrawn onto a standard representation. (Scale bar, 1 mm.)
More recently however, the emphasis in work on the PPC in primates has shifted away from the idea that it constructs an all-purpose framework for locating objects in space toward the notion that there are multiple spatial representations, each specialized to support a different action, such as eye movements, head movements, or grasping (39, 40). On this view, much of the information processing in the PPC is directed toward putting visual and other sensory information into register with the different coordinate systems of the eye, head, and body axis to support accurate eye, head, limb, and whole body movements to targets. This shift in emphasis from a general coding of spatial location to a more specialized role in the coregistration of sensory and motor systems for planning specific actions was motivated in large part by data from electrophysiological recordings from PPC neurons in behaving monkeys (41–43) and from finer-grained analyses of deficits in cortically damaged humans (44). These studies indicate that world-referenced and body-referenced signals come together in PPC neurons that respond specifically to conjunctions of the two types of input. Visual representations of objects and scenes in PPC thus contain spatial information that can be used in accurately guiding specific actions, such as targeting and grasping. Such a multiplicative mechanism is exactly what is needed to translate the allocentric entorhinal representation into goal-directed movements.
Much of the single unit recording experimentation with monkeys has measured activity during two types of movements, saccadic eye movements and reaching (41–43, 45–47). PPC neurons respond to target locations within retinotopic coordinates, and their firing rates for a specific target location are modulated by eye, head, and limb position. Frequently these neurons will fire transiently in response to a target onset and to a specific movement of the eyes or limb to the target (48, 49; Fig. 4). Thus, the response characteristics are determined by a complex coregistration of target location, movement type, and position of body parts. Andersen and coworkers (50, 51) have analyzed the activity of PPC neurons during delay periods between a cue signaling target location and the launching of a target-directed eye or limb movement. Cells fire according to the properties of the next movement to be made in this task. That is, if the cell prefers to fire when a limb movement is made to a specific location, then it will increase its activity during the delay interval between the delivery of a cue signaling that the specific limb movement should be made and the opportunity to move the limb. If a second cue is delivered during the delay interval signaling that an eye movement and not a limb movement should be made then the cell stops firing, even when the target location for the eye movement is the same as for the initial limb movement (48, 49; Fig. 4). These data, along with others, have led to the idea that cells in the PPC form a distributed representation of movement plans or intentions to make movements to specific goal locations (39).
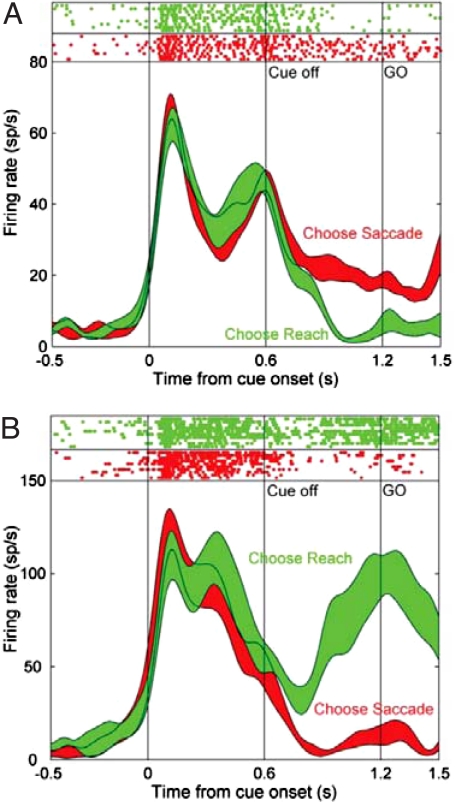
Fig. 4.
Predictive relationship between firing in monkey PPC and the next movement to be made in a choice task. The monkey was trained to indicate the location of a target on a screen by using either an eye movement (saccade, when the cue was red) or an arm movement (reach, when the cue was green). Firing rates are shown for two cells as a function of time, including onset and offset of the cue and initiation of the response (Go). (Top) Raster plots; (Bottom) smoothed peristimulus time histograms. The cell in A is from the lateral intraparietal area; the cell in B is from the parietal reach region. When the cue was on, firing was similar in the two regions. When the cue was turned off, different patterns emerged. Saccades were preceded by preferential firing in the lateral intraparietal area; reaches were preceded by stronger firing in the parietal reach region. Thus, firing during the delay period depends on the motor output required to solve the task, suggesting that the PPC represents intended movements to specific locations. [Reproduced with permission from ref. 49 (Copyright 2007, Neuron).]
Several unit recording experiments have extended the analysis of movement planning by monkeys into the domain of computer-based virtual navigation, where animals can plan and follow long paths despite the physical constraints of the experimental setting. In one such study, 77% of neurons recorded from a medial region of PPC responded to conjunctions of specific movements and specific locations during virtual navigation (52). In another study, approximately one quarter of the PPC cells were spatially tuned to path direction in a computerized maze task (53). This spatial tuning did not depend on saccade directions or location of visual receptive fields, but likely reflected the operation of a spatial cognitive process related to solving the maze that was not driven by concurrent movement or concurrent changes in visual input. In humans, activation of the PPC has been observed during spatial navigation along remembered routes. When participants were asked to virtually navigate or imagine navigating along a route in a very familiar real-world environment, activity increased in the PPC, as well as the hippocampus, parahippocampal regions, and retrosplenial cortex (54, 55), suggesting that the parietal cortex may possibly be involved not only in targeting of microscopic movements but also in planning of large-scale spatial trajectories.
Spatial Navigation and the Posterior Parietal Cortex in Rats
Much of our knowledge regarding the functions performed by the PPC, such as sensorimotor transformations and fine-tuned motor planning, is derived from recordings carried out in head-restrained monkeys and from work in humans. While the functions described are likely indispensible to efficient spatial navigation, movement restrictions inherent to the head-restrained system prevent an analysis of the specific contributions made by the PPC during free locomotion. Because of this limitation, most functional investigations of the PPC in navigational tasks have used freely behaving rats, with the most common approach being to characterize behavioral performance after lesioning the PPC. Although an exact anatomical definition for the PPC in rodents remains a subject of debate (Box 1, Fig. 3), most lesion studies have focused on an area extending ≈2–6 mm posterior to bregma and 2–5 mm lateral to the midline, which includes most of the posterior neocortical area described as PPC.
A growing body of such studies has pointed to the PPC as a critical component of the rat's navigational system. As in the primate literature, deficits after lesions in PPC have traditionally been interpreted as perceptual impairments, reflecting a proposed role for this brain area in forming a spatial image based on visual and somatosensory inputs. Some of the earliest evidence in support of this view comes from studies reporting that, in a water-maze task in which distal landmarks served as visual cues, PPC damage consistently increased error in initial heading as well as swim latencies to reach the platform (56–58). The errors in initial heading persisted when the animals were given a proximal but discontiguous visual landmark in the form of a cue card hung behind the hidden platform on the wall of the water tank (57). In this version of the task, the PPC-lesioned animals adopted a looping strategy along the wall of the water-maze tank until they happened to find the platform, indicating that they had learned the objective of the task despite a presumptive failure to use strong spatial information provided by nearby visual beacons. It was also found that rats with lesions of the PPC were particularly impaired at detecting changes in the spatial arrangement of familiar objects in an open field (59). The spatial deficit is not confined to the visual domain, because rats with lesions of the PPC are also strongly impaired in path integration-based navigation under conditions where visual inputs are irrelevant or unavailable. For example, when such animals leave a refuge to search for a randomly located food reward on a circular arena surrounded by a curtain, they are not able to return directly to the refuge based on their outbound movements (29; Fig. 2B). Rats with PPC lesions also fail to use self-motion information to locate the escape platform in a water maze when tested in complete darkness (60). Given that visual discrimination is apparently unaffected (56), a conventional interpretation of these studies might hold that the behavioral deficits stem from an inability to effectively construct a spatial map of the environment based on visual and vestibular-kinesthetic sensory inputs (61, 62).
However, the full spectrum of behavioral dysfunctions observed after PPC damage cannot be attributed solely to deficits in visuospatial perception. It is possible to argue that PPC is instead necessary for the ability to transform the spatial information provided by sensory cues into body-based coordinates required to implement the sequence of actions to take the rat to the goal location, in the same way that this area translates spatial information into targeted eye and limb movements in head-restrained primates. Most behavioral tasks used to assess navigational abilities in rodents require the animals to continuously coregister self-motion information (derived from somatosensory, vestibular, and proprioceptive inputs) with extrinsic information derived from the environment to generate goal-oriented movements. Navigating to a platform in a cued water-maze task, for example, requires animals to continuously update their motor output and reorient their body axis relative to the available cues to maintain a goal-oriented heading and reach the correct location. Impairments in transformation of self-location information to specific locomotor actions may therefore account for a wide spectrum of behavioral deficits in rats with lesions in PPC. Whether they do remains open; at present, the evidence is ambiguous.
Spatial Representation in the Rat Posterior Parietal Cortex
Single-unit recordings in freely behaving rats have verified a role for the PPC in representing position and motion relative to the outside world. The majority of the data in these studies was recorded within the area targeted by the lesion experiments (also see Box 1, Fig. 3). Initially, the firing properties of PPC cells were characterized in rats as they ran a radial-arm maze (63, 64). Consistent with more recent virtual-navigation data obtained from primates (52), it was found that PPC cells represented conjunctions of movement and location. For example, a PPC cell would fire during forward motion, but only when the animal was running outward from the center of the maze, whereas another cell would fire exclusively during left turns executed at the end of a maze arm (63). Subsequent work described head-direction-selective cells in the PPC whose firing rates showed additional modulation by different types of movement, such as right turns, left turns, or forward motion (65). PPC neurons in the rat thus express a multiplicative mechanism very similar to the gain fields of the monkey PPC (43). As in nearly all studies with posterior parietal lesions, the early recording studies were generally interpreted as showing integration of visual and self-motion information at a relatively early stage of perceptual processing, before the information reached the memory systems in the hippocampus or the output systems in motor-planning regions of the cortex. However, more recent work has suggested an alternative interpretation. PPC cells have been shown to encode sequences of behaviors, or navigational epochs, as rats run along complex routes in irregular labyrinth-style mazes (66; Fig. 1C). These cells faithfully tracked the rats' progress through maze sequences irrespective of spatial position or direction of motion, both in darkness and light. The firing fields scaled with the size of the navigational segment, much like place cells in the hippocampus (23) and grid cells in the MEC (24), but the scaling occurred even under conditions where the size of hippocampal place fields remained constant. This dissociation suggests that PPC neurons do not express perceived space. Instead, the observations point to a possible role for PPC neurons in planning and execution of navigational behaviors.
Output Pathways from Medial Entorhinal to Posterior Parietal Cortex
We have argued that the mammalian navigational system includes both the entorhinal-hippocampal circuit and the PPC and we hypothesized that path integrator outputs from MEC and hippocampus are used by neurons in PPC and downstream motor and premotor cortices to plan the sequence of movements needed to bring the animal to the goal location. If this is true, there are several pathways that could pass outputs from hippocampus and MEC to PPC.
There are no direct connections to PPC from the dentate gyrus, hippocampus, or subiculum, but the dorsal part of the lateral band of the MEC, adjacent to the postrhinal cortex, has a direct PPC projection (Fig. 5; ref. 67, figure 4 in ref. 68). This connection is relatively weak, and it is uncertain whether it is able to convey location information to possible action-preparing systems in PPC. In principle, position can be reconstructed from the spike patterns of a very low number of grid cells (15, 17), but it has not been determined whether the medial entorhinal outputs target those PPC neurons that are involved in action preparation.
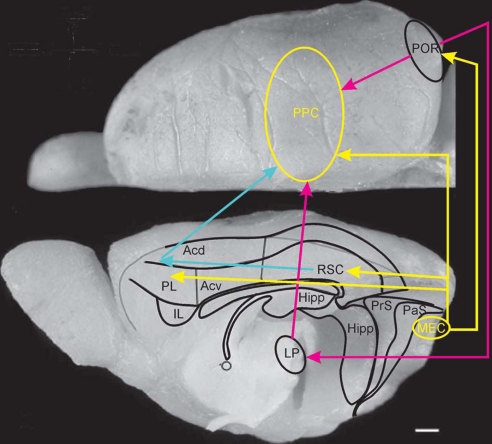
Fig. 5.
Dorsal and midsagittal view of the rat brain indicating the proposed routes by which information from the medial entorhinal grid cell area may reach PPC. The most likely route by way of the connections mediated by the postrhinal cortex is indicated (yellow and purple) as well as the multisynaptic routes using pathways through the lateral posterior complex of the thalamus (purple), and the retrosplenial cortex (RSC) and dorsal portion of medial prefrontal cortex (PL and ACd) (yellow and blue). (Scale bar, 1 mm.)
A more substantial component of the medial entorhinal output may reach the PPC indirectly through the postrhinal, retrosplenial, and prefrontal cortices (Fig. 5). The shortest and densest projection is through the postrhinal cortex (67, 68). The contribution of this pathway is uncertain because animals can still learn a simple water-maze task after relatively large lesions of the postrhinal cortex (69, 70); however, no studies have tested the involvement of postrhinal cortex in path integration tasks where position can only be estimated from the animal's own motion (as in ref. 27). The spatial modulation of postrhinal neurons is weak and unstable (15, 71), but this also does not rule out a function for the postrhinal cortex in relaying output from MEC to PPC, as the signal may be transformed to a nonallocentric code already in postrhinal cortex.
An alternative set of pathways passes through the medial prefrontal cortex. There are significant direct connections to locations in this area from the entorhinal and postrhinal cortices, posterior CA1, and adjacent subiculum. Projections target the orbitofrontal cortex, the prelimbic/infralimbic domain, and the dorsally adjacent part of the anterior cingulate cortex (68, 72–75). These regions have strong connections with the PPC in both rats and primates (33, 57, 76–78). Since the initial report showing impaired initial acquisition of place navigation in a water- maze after aspiration lesions of the medial prefrontal cortex (79), the results of a large number of experiments have supported the conclusion that hippocampal-prefrontal connections are important for certain spatial behaviors. However, with preoperative training or extended postoperative training, rats with medial prefrontal cortex damage are able to navigate directly to a hidden goal (80). Rats with such lesions are also not impaired in using self-motion information to return to a home base in total darkness (81). These findings suggest that, in its simplest form, navigation in a direct path from start to goal does not require hippocampal-frontal connections.
A final intermediate may be the retrosplenial cortex, which receives significant excitatory input from MEC, subiculum, and presubiculum (82–84) as well as inhibitory input from CA1 (85; see also ref. 86). In non-human primates, retrosplenial and adjacent posterior cingulate cortex project further to PPC (77, 87), although it is uncertain whether such direct retrosplenial-parietal connections exist in the rat (82, 83, 88), but see refs. 76 and 89). In any case, signals from retrosplenial cortex are likely to reach PPC indirectly via strong connections to the medial frontal cortex, in particular, the anterior dorsal cingulate cortex (90), which projects in turn to the PPC (57, 76). The potential significance of these pathways is underscored by the observation that, unlike postrhinal and prefrontal lesions, damage to the retrosplenial cortex causes strong and lasting disruption of spatial navigation, both in the water maze (81, 91–93) and in a path-integration task testing the ability to return directly to a home base in darkness (28). The path integration impairment is as severe as after hippocampal damage (27).
Thus, a number of hippocampal-entorhinal output pathways could be involved in transferring information about the animal's current location to the PPC and further on to the motor-planning system (94, 95), using connections from PPC to premotor and motor domains (33, 34, 76, 96). The relative contributions of the various pathways remain uncertain. We are just beginning to scratch the surface of this multitude of navigational control pathways.
Conclusion
The data collected from the PPC of rats during navigation are generally supportive of the idea that the PPC could be central to selection of trajectories in a manner that is similar to the planning of eye or limb movements by primates. Specifically, the experiments with rats have demonstrated that firing of posterior parietal cells is often determined by conjunctions of body position or orientation, positions in a path, and concurrent movement type (i.e., turns or forward locomotion), as would be expected for a system that combines world-centered and body-centered reference frames. Both entorhinal and parietal cells encode location as animals traverse segments of repetitive mazes (14, 97, 98), suggesting that the two cortical areas may work in concert during the performance of navigational tasks. The spatial firing correlates of parietal neurons might thus be interpreted as representing information that could be useful in constructing spatial maps in entorhinal and hippocampal regions. However, in light of the foregoing functional considerations, there is clearly another interpretation. We hypothesize that the essential contribution of the PPC is the translation of the animal's position from a world-centered coordinate system generated by place cells and grid cells into the body-based coordinates of locomotor actions. This translation is necessary for planning the next movement in a navigational sequence.
Note added in proof.
A recent study of amnesic patients suggests that simple path integration operations can be performed in the absence of hippocampus and entorhinal cortex in humans (114). These findings do not rule out a primary role for hippocampus and entorhinal cortex in path integration but suggest that, when the path integration problem is simple, processing in other areas in the human brain can support similar behavior, using different neural computations. The lack of impairment in the human patients contrasts with the severe deficits in path integration observed after specific lesions of the hippocampus and entorhinal cortex in rats (Fig. 2; 27–29).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 2.Biegler R. Possible uses of path integration in animal navigation. Anim Learn Behav. 2000;28:257–277. [Google Scholar]
- 3.Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171.175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 6.O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: Why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 7.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- 9.Sharp PE. Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus. 1999;9:432–443. doi: 10.1002/(SICI)1098-1063(1999)9:4<432::AID-HIPO9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Touretzky DS, Redish AD. Theory of rodent navigation based on interacting representations of space. Hippocampus. 1996;6:247–270. doi: 10.1002/(SICI)1098-1063(1996)6:3<247::AID-HIPO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- 12.Brun VH, et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- 13.Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr The positional firing properties of medial entorhinal neurons: Description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 15.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 16.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 17.Solstad T, Moser EI, Einevoll GT. From grid cells to place cells: A mathematical model. Hippocampus. 2006;16:1026–1031. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- 18.Sargolini F, et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 19.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 20.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 21.Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften. 1980;67:566–567. [Google Scholar]
- 22.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 23.O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 24.Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- 25.Gothard KM, Skaggs WE, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadel L. The hippocampus and space revisited. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- 27.Maaswinkel H, Jarrard LE, Whishaw IQ. Hippocampectomized rats are impaired in homing by path integration. Hippocampus. 1999;9:553–561. doi: 10.1002/(SICI)1098-1063(1999)9:5<553::AID-HIPO9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: Evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 29.Parron C, Save E. Evidence for entorhinal and parietal cortices involvement in path integration in the rat. Exp Brain Res. 2004;159:349–359. doi: 10.1007/s00221-004-1960-8. [DOI] [PubMed] [Google Scholar]
- 30.Alyan S, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113:19–31. doi: 10.1037//0735-7044.113.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Wallace DG, Gorny B, Whishaw Rats can track odors, other rats, and themselves: Implications for the study of spatial behavior. Behav Brain Res. 2002;131:185–192. doi: 10.1016/s0166-4328(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 32.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 34.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: The MIT Press; 1982. pp. 549–586. [Google Scholar]
- 37.Farah M. Visual Agnosia: Disorders of Object Recognition and What they Tell Us about Normal Vision. Cambridge, MA: The MIT Press; 1990. [Google Scholar]
- 38.Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. New York: Worth Publishers; 2003. [Google Scholar]
- 39.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 40.Milner AD, Goodale MA. The Visual Brain in Action. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 41.Andersen RA, Essick GK, Siegel RM. Neurons of area-7 activated by both visual-stimulated and occulomotor behavior. Exp Brain Res. 1987;67:316–322. doi: 10.1007/BF00248552. [DOI] [PubMed] [Google Scholar]
- 42.Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- 43.Snyder LH, Grieve KL, Brotchie P, Andersen RA. Separate body- and world-referenced representations of visual space in parietal cortex. Nature. 1998;394:887–891. doi: 10.1038/29777. [DOI] [PubMed] [Google Scholar]
- 44.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 45.Gnadt JW, Andersen RA. Memory related motor planning acitvity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 46.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. 1. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- 47.Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of monkey—Command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- 48.Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- 49.Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron. 2007;56:552–559. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol. 2001;85:539–544. doi: 10.1152/jn.2001.85.2.539. [DOI] [PubMed] [Google Scholar]
- 51.Mazzoni P, Bracewell RM, Barash S, Andersen RA. Motor intention activity in the Macaque's lateral intraparietal area. 1. Dissociation of motor plan from sensory memory. J Neurophysiol. 1996;76:1439–1456. doi: 10.1152/jn.1996.76.3.1439. [DOI] [PubMed] [Google Scholar]
- 52.Sato N, Sakata H, Tanaka YL, Taira M. Navigation-associated medial parietal neurons in monkeys. Proc Natl Acad Sci USA. 2006;103:17001–17006. doi: 10.1073/pnas.0604277103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowe DA, Averbeck BB, Chafee MV, Georgopoulos AP. Dynamics of parietal neural activity during spatial cognitive processing. Neuron. 2005;47:885–891. doi: 10.1016/j.neuron.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- 55.Spiers HJ, Maguire EA. The neuroscience of remote spatial memory: A tale of two cities. Neurosci. 2007;149:7–27. doi: 10.1016/j.neuroscience.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 56.Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- 57.Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behav Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 58.DiMattia BD, Kesner RP. Spatial cognitive maps: Differential role of parietal cortex and hippocampal formation. Behav Neurosci. 1988;102:471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- 59.Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- 60.Commins S, Gemmell C, Anderson M, Gigg J, O'Mara SM. Disorientation combined with bilateral parietal cortex lesions causes path integration deficits in the water maze. Behav Brain Res. 1999;104:197–200. doi: 10.1016/s0166-4328(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 61.Save E, Poucet B. Hippocampal-parietal cortical interactions in spatial cognition. Hippocampus. 2000;10:491–499. doi: 10.1002/1098-1063(2000)10:4<491::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins TA, Amin E, Pearce JM, Brown MW, Aggleton JP. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: A c-fos expression study. Neuroscience. 2004;124:43–52. doi: 10.1016/j.neuroscience.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 63.McNaughton BL, Leonard B, Chen C. Cortical-hippocampal interactions and cognitive mapping: A hypothesis based on reintragration of the parietal and inferotemporal pathways for visual processing. Psychobiology. 1989;17:230–235. [Google Scholar]
- 64.McNaughton BL, et al. Cortical representation of motion during unrestrained spatial navigation in the rat. Cereb Cortex. 1994;4:27–39. doi: 10.1093/cercor/4.1.27. [DOI] [PubMed] [Google Scholar]
- 65.Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- 66.Nitz DA. Tracking route progression in the posterior parietal cortex. Neuron. 2006;49:747–756. doi: 10.1016/j.neuron.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 67.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 68.Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- 69.Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. J Neurosci. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burwell RD, Hafeman DM. Positional firing properties of postrhinal cortex neurons. Neuroscience. 2003;119:577–588. doi: 10.1016/s0306-4522(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 72.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 73.Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: Cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 74.Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: A double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- 75.Delatour B, Witter MP. Projections from the parahippocampal region to the prefrontal cortex in the rat: Evidence of multiple pathways. Eur J Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- 76.Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: Topography of corticocortical and thalamic connections. Exp Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- 77.Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat Rec. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- 78.Neal JW, Pearson RC, Powell TP. The ipsilateral cortico-cortical connections of area 7b, PF, in the parietal and temporal lobes of the monkey. Brain Res. 1990;524:119–132. doi: 10.1016/0006-8993(90)90500-b. [DOI] [PubMed] [Google Scholar]
- 79.Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: Definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland RJ. The navigating hippocampus: An individual medley of movement, space, and memory. In: Buzsáki G, Vanderwolf CH, editors. Electrophysiology of the Archicortex. Budapest: Akadémiai Kiadó; 1985. pp. 255–279. [Google Scholar]
- 81.Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res. 2001;118:67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 82.van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- 83.van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- 84.van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- 85.Miyashita T, Rockland KS. GABAergic projections from the hippocampus to the retrosplenial cortex in the rat. Eur J Neurosci. 2007;26:1193–1204. doi: 10.1111/j.1460-9568.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- 86.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- 88.McDonald AJ, Mascagni F. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: A Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1996;71:37–54. doi: 10.1016/0306-4522(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 89.Burcham KJ, Corwin JV, Stoll ML, Reep RL. Disconnection of medial agranular and posterior parietal cortex produces multimodal neglect in rats. Behav Brain Res. 1997;86:41–47. doi: 10.1016/s0166-4328(96)02241-3. [DOI] [PubMed] [Google Scholar]
- 90.Jones BF, Witter MP. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus. 2007;17:957–976. doi: 10.1002/hipo.20330. [DOI] [PubMed] [Google Scholar]
- 91.Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sutherland RJ, Hoesing JH. Posterior cingulate cortex and spatial memory: A microlimnology analysis. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Treatise. Boston: Birkhauser; 1993. pp. 461–477. [Google Scholar]
- 93.Vann SD, Aggleton JP. Testing the importance of the retrosplenial guidance system: Efects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behav Brain Res. 2004;155:97–108. doi: 10.1016/j.bbr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- 95.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: Anatomical connectivity and functional considerations. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Akers RM, Killackey HP. Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol. 1978;181:513–537. doi: 10.1002/cne.901810305. [DOI] [PubMed] [Google Scholar]
- 97.Derdikman D, Fyhn M, Hafting T, Moser M-B, Moser EI. Breaking up the entorhinal grid in a hairpin maze. Soc Neurosci Abstr. 2006;32 68.10. [Google Scholar]
- 98.Whitlock JR, Pfühl G, Moser M-B, Moser EI. A comparison of representations in entorhinal and parietal cortices. Soc Neurosci Abstr. 2008 in press. [Google Scholar]
- 99.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbauers. Leipzig: Verlag von Johann Ambrosius Barth; 1909. [Google Scholar]
- 100.Krieg WJS. Connections of the cerebral cortex: I. The albino rat. B. Structure of the cortical areas. J Comp Neurol. 1946;84:277–323. doi: 10.1002/cne.900840302. [DOI] [PubMed] [Google Scholar]
- 101.Krubitzer L. The organization of neocortex in mammals: Are species differences really so different? Trends Neurosci. 1995;18:408–417. doi: 10.1016/0166-2236(95)93938-t. [DOI] [PubMed] [Google Scholar]
- 102.Schmahmann JD, Pandya DN. Anatomical investigation of projections from thalamus to posterior parietal cortex in the rhesus monkey: A WGA-HRP and fluorescent tracer study. J Comp Neurol. 1990;295:299–326. doi: 10.1002/cne.902950212. [DOI] [PubMed] [Google Scholar]
- 103.Cappe C, Morel A, Rouiller EM. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience. 2007;146:1371–1387. doi: 10.1016/j.neuroscience.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 104.Bucci DJ, Conley M, Gallagher M. Thalamic and basal forebrain cholinergic connections of the rat posterior parietal cortex. NeuroReport. 1999;10:941–945. doi: 10.1097/00001756-199904060-00009. [DOI] [PubMed] [Google Scholar]
- 105.McDaniel WF, McDaniel SE, Thomas RK. Thalamocortical projections to the temporal and parietal association cortices in the rat. Neurosci Lett. 1978;7:121–125. doi: 10.1016/0304-3940(78)90154-4. [DOI] [PubMed] [Google Scholar]
- 106.Chandler HC, King V, Corwin JV, Reep RL. Thalamocortical connections of rat posterior parietal cortex. Neurosci Lett. 1992;31:237–242. doi: 10.1016/0304-3940(92)90273-a. [DOI] [PubMed] [Google Scholar]
- 107.Giannetti S, Molinari M. Cerebellar input to the posterior parietal cortex in the rat. Brain Res Bull. 2002;58:481–489. doi: 10.1016/s0361-9230(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 108.Palomero-Gallagher N, Zilles K. Isocortex. In: Paxinos G, editor. The Rat Nervous System. 3rd Ed. Amsterdam: Elsevier; 2004. pp. 729–757. [Google Scholar]
- 109.Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 1984;226:184–202. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- 110.Fukushima K. Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res. 1997;117:1–16. doi: 10.1007/pl00005786. [DOI] [PubMed] [Google Scholar]
- 111.Kawano K, Sasaki M, Yamashita M. Vestibular input to visual tracking neurons in the posterior parietal association cortex of the monkey. Neurosci Lett. 1980;17:55–60. doi: 10.1016/0304-3940(80)90061-0. [DOI] [PubMed] [Google Scholar]
- 112.Guldin WO, Akbarian S, Grusser OJ. Cortico-cortical connections and cytoarchitectonics of the primate vestibular cortex: a study in squirrel monkeys (Saimiri sciureus) J Comp Neurol. 1992;326:375–401. doi: 10.1002/cne.903260306. [DOI] [PubMed] [Google Scholar]
- 113.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 114.Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: Path integration does not require hippocampus or entorhinal cortex. Proc Natl Acad Sci USA. 2008;105:12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]