Abstract
Aboriginal burning in Australia has long been assumed to be a “resource management” strategy, but no quantitative tests of this hypothesis have ever been conducted. We combine ethnographic observations of contemporary Aboriginal hunting and burning with satellite image analysis of anthropogenic and natural landscape structure to demonstrate the processes through which Aboriginal burning shapes arid-zone vegetational diversity. Anthropogenic landscapes contain a greater diversity of successional stages than landscapes under a lightning fire regime, and differences are of scale, not of kind. Landscape scale is directly linked to foraging for small, burrowed prey (monitor lizards), which is a specialty of Aboriginal women. The maintenance of small-scale habitat mosaics increases small-animal hunting productivity. These results have implications for understanding the unique biodiversity of the Australian continent, through time and space. In particular, anthropogenic influences on the habitat structure of paleolandscapes are likely to be spatially localized and linked to less mobile, “broad-spectrum” foraging economies.
Keywords: fire ecology, human behavioral ecology, hunter–gatherers, resource management
Anthropogenic fire is increasingly recognized as an important constructive force in shaping plant communities around the world (1–3), and its impact in Australia has been argued to be particularly significant (4–6). Fire can act as an “intermediate disturbance,” enhancing biodiversity by disrupting the reproductive rate of slowly growing species and promoting greater diversity (7–11). Over evolutionary time scales, fire shapes the life-history traits of plant communities; thus a change in fire regime should trigger a cascade of population extinctions of those species with incompatible life histories (12). Such a “trophic cascade” has been hypothesized to have occurred upon the colonization of Australia ca. 45–50 ka (5). Paleoecological records coincident with the putative arrival of modern humans show possible changes in burning regimes and subsequent trophic-level shifts in ecological communities—a fragmentation of woodlands and forests and an expansion of grasses, particularly Triodia (the inedible spinifex grass)—which may have contributed to Australia's late Pleistocene extinctions (6, 13). Furthermore, some propose that with the establishment of anthropogenic fire mosaics throughout the continent, flora and fauna coevolved with human behavior to such an extent that the cessation of burning with European incursion into the continent precipitated a second major trophic collapse, leading to severe declines in small-to-medium-sized mammal populations (7, 14–17).
Models of the prehistoric shift from lightning to anthropogenic fire regimes rely on analogies with modern Australian Aboriginal burning. Previous work has examined the correlation between Aboriginal presence and landscape-level effects, concluding that (i) in both regimes, fire increases nutrient availability and removes “climax” vegetation (more slowly growing hummock grasses and woody shrubs), enhancing the short-term productivity of herbaceous plants and increasing in-patch diversity, which tends to peak 2–4 years after a fire (18–20); (ii) in anthropogenic regimes, fires are of lower intensity and occur more frequently but only in particular types of habitat (8); (iii) fire mosaics of burnt and regenerating vegetation are more fine-grained (17, 18, 21, 22), (iv) which prevents extensive, late-season fires (22), and this in turn (v) prevents the fragmentation and subsequent contraction of Acacia woodlands (23) and other fire-sensitive species (24).
These studies quantify the ecological outcomes of anthropogenic fire but not its formation processes, assuming that mosaic formation is driven by management strategies designed to increase the availability of key resources (25). Without sanctions that exclude free-riders, management produces common-pool resources and thus becomes a collective-action problem (26). This problem is further exacerbated if the future benefits of management are heavily discounted, as they might be if individuals are risk averse for uncertain future rewards. It can be difficult to overcome the dual problems of collective action and future discounting, especially in small-scale, politically decentralized societies (27). However, there are conditions that might make management strategies less costly to maintain: if individuals gain more from contributing to construction than they gain from free-riding, if the costs of construction are low relative to the future benefits gained or if those paying the costs reap more of the future benefit. In order for burning to be maintained as part of any long-term strategy of ecosystem construction or resource management, there must be future benefits to survival and/or reproductive success that accrue to those who pay the cost of creating and maintaining mosaics. Although this “burning as resource management” hypothesis was first proposed by Rhys Jones (4) and elaborated by Gould (28), no research has yet tested this either by quantifying the adaptive benefits or by linking the process of mosaic formation to particular foraging strategies.
Here, we attempt to address this gap in our knowledge of the processes structuring Aboriginal fire mosaics by linking quantitative observations of foraging by contemporary Aboriginal people (Martu) in the Western Desert of Australia with remotely sensed measures of landscape heterogeneity to test three predictions derived from Jones' “fire-stick farming” hypothesis: (i) anthropogenic landscapes (as compared with those shaped through “natural” or lightning fire) are associated with greater habitat heterogeneity at the spatial scale of the human day range; (ii) reductions in scale and increases in diversity in a local landscape are linked to time spent foraging for resources for which burning offers an immediate increase in foraging returns; (iii) increases in landscape diversity as encountered by human foragers increase foraging efficiency. We first describe the ethnoecological context of Martu foraging before turning to tests of the predictions listed above.
The Ethnoecological Context of Aboriginal Fire
Martu are the indigenous owners of estates that comprise ≈150,000 km2 in Australia's Western Desert [supporting information (SI) Fig. S1]. In the 1980s, Martu established three permanent settlements (“outstations”) in this region when they returned to their homelands after a mid-20th century exodus into missions and pastoral stations on the western and northern fringe of the desert (29, 30). Each outstation has a population that fluctuates between 50–200, with people routinely shifting between communities and traveling extensively throughout the Western Desert and Pilbara regions on logistical, social, and ritual business. Most Martu based in these remote communities hunt and gather daily, acquiring 1,700 kcal per capita per foraging day from bush foods (31), purchasing the remainder from the small outstation stores or on visits to towns in the Pilbara. Contemporary daily foraging typically involves travel by vehicle from an outstation to a foraging camp, which establishes a central place for search and pursuit of hunted and collected resources. The party splits into smaller units for hunting on foot, reconvening at the foraging camp for cooking, distribution, and consumption before returning to the outstation in the evening (31).
Martu country is dominated by spinifex (Triodia spp.) sandplains and dune fields, with smaller proportions of low lying rocky ranges, watercourse margins, and mulga (Acacia aneura) woodland. The vast majority of anthropogenic fires are set during the winter (April–October) in spinifex sandplain, and accordingly, Martu focus on vegetative succession here in categorizing the stages of regrowth after a fire. The stages are defined by their subsistence utility. Nyurnma is a freshly burnt area. Waru-waru is an early successional stage characterized by the presence of yukuri, or green shoots of new and diverse growth. Mukura is a midsuccessional stage reached at ≈1–3 years after a rain in the freshly burned ground, characterized by high densities of edible seed grasses, flowering shrubs, acacia seedlings, Solanum fruit, and other edible plants. Mukura gradually fades into the late-successional stage of mangul, or mature spinifex, as the slowly growing spinifex begins to crowd out edible plants, ≈5–7 years after the first rain. As the spinifex ages to kunarka, it begins to die in the center, and large sterile spaces open up between the hummocks. Only mangul and kunarka contain enough fuel to carry a fireline.
Martu take great pride in their land-use practices and routinely refer to the social and ritually constituted imperatives of mosaic burning. Fires are sometimes set to “clean up country,” especially when visiting remote regions, and to attract bustards (Ardeotis australis) who forage for exposed prey in burned areas, but the majority of burning occurs on women's monitor lizard (Varanus gouldii) hunts (31, 32). Burning of this type is not designed to drive prey: Hunters fire a tract of mangul or kunarka, usually between 1 and 10 ha in extent, follow in behind the advancing fire line to search the cleared area for signs of tracks to pursue to fresh burrows, and then use a specialized digging stick to probe for and excavate an occupied den. Fires are carefully orchestrated to take advantage of the wind and are situated in locations where known firebreaks are downwind. Martu burn almost exclusively in the winter: in the summer, goanna and other reptiles are mobile and tracked on the surface, and a fire would drive them into their deep summer dens from which they are costly to remove. Secondly, winter season fires are more controllable, because the wind is consistent and strong—fires can be set so that they burn out relatively quickly against a downwind firebreak. Being able to control a fire is important because the rights to burn country for hunting are held collectively, but individual hunters are responsible for fires that burn areas to which they do not hold such rights. A hunter whose fire shifts with the wind and threatens a sacred site in an area where his/her burning rights are deemed less than legitimate is subject to severe punitive procedures by the collective body of owners, which today involves ritualized physical punishment and monetary compensation to owners. The use of fire thus has social and ritual meaning that accompanies its immediate hunting utility: Burning is a signal of coownership and obligation and an expression of one's commitment to upholding the Yulupirti, the law handed down from the Jukurrpa, or “Dreamtime.” The mosaic that develops as a result of repeated burning is a signal of its use: It represents the number of hunters and the frequency with which they hunt on the land.
Martu often stress the short-term utility derived from wintertime burning: “Today, we often make a nyurnma to hunt in during the winter months, because there is too much old spinifex and not enough ground already burnt. We prefer to burn a small patch of old spinifex, if it is surrounded by younger growth that won't burn, to keep the fire from spreading or burn when the grass is green and doesn't move very far or very fast. You get more goanna that way—Goanna like to hide in the small patches of unburnt spinifex inside a nyurnma.” (Karrimarra, June 2003).
Martu also maintain a great store of observational knowledge concerning the long- term impacts of anthropogenic fire on plant and animal populations: “Burning mangul, and sometimes wintamarra (mulga woodland), has long-term benefits: It helps to grow more food for both animals and people and prevents big bush fires. Kilu (hopping mouse) and many of the larger animals like kipara (bustard), karlaya (emu), marlu (red kangaroo), and kirti-kirti (hill kangaroo) do better if there is regular patch burning because they eat many of the same things as Martu and get plenty of food when plants starts growing after the fire.” (Jutirangu, July 2003).
Results
Do Anthropogenic Landscapes Show Increased Habitat Heterogeneity?
An analysis of 34 circular landscapes (28 km2) classified via Landsat imagery according to Martu successional stage shows that those centered on foraging locations (anthropogenic regime), have a higher Shannon's diversity index (SDI), higher edge density, and smaller and more numerous patches than control landscapes (lightning regime) (Table 1, Fig. 1., and Fig. S1). Circles placed in remote foraging locations (i.e., mixed regime, n = 4, x̄ SDI = 0.78) are more similar to control than anthropogenic landscapes (Fig. S2).
Table 1.
Habitat heterogeneity under anthropogenic fire regime vs. lightning fire regime
| Parameter | Anthropogenic regime x̄ ± SD, n = 15 | Lightning regime x̄ ± SD, n = 15 | Significance (t test, unequal var) P |
|---|---|---|---|
| No. of patches | 64.33 ± 8.95 | 21.73 ± 3.21 | 0.0003 |
| Patch size, ha | 57.98 ± 9.18 | 189.40 ± 33.70 | 0.0017 |
| Edge density, m/ha | 2.1 ± 0.1 | 1.0 ± 0.1 | <0.0001 |
| SDI | 1.15 ± 0.06 | 0.63 ± 0.08 | <0.0001 |
var, variance.
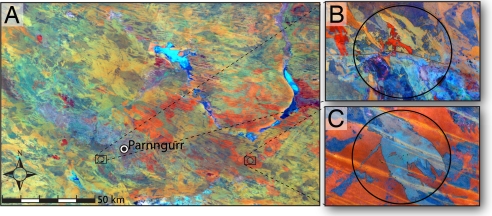
Fig. 1.
Satellite images of habitat heterogeneity in the Martu homelands. (A) Landsat 7 ETM+ image mosaic (bands 7, 4, 2) of the study region surrounding Parnngurr Community taken November and December 2002. Recent fires are shaded red to orange, regrowing habitat appears yellow to green, and mature growth as dark green to blue. (B and C) Images are enlarged to show detail in the habitat mosaic of two 28-km2 landscapes: a landscape under a primarily anthropogenic regime 19 km from community (center 122.437E, 22.910 S) (B); a landscape under a primarily lightning ignition regime 91 km from community (center 123.505 E, 22.915 S) (C).
If anthropogenic influence merely “rescales” habitat structure without loss of variation, then an anthropogenic regime should be more similar to a lightning regime at the regional scale than at the local scale. To test this prediction, we paired 15 anthropogenic landscapes with 15 control landscapes according to their ranked percentage cover of spinifex sandplain habitat, and used the Kullback–Leibler (KL) discrimination function to compare the difference in the distribution of Landsat middle infrared pixel reflectance values, a continuous measure of successional vegetative diversity. High KL entropies would indicate substantial divergence between two probability distributions. We first computed KL entropies from each of the 15 local pairings (0.112–1.607, x̄ = 0.9255, SD = 0.4369; Figs. S3–S17). Next, KL entropy for the regional-scale comparison was calculated by combining all 15 anthropogenic landscapes into an anthropogenic region and all 15 control landscapes into a control region. The KL divergence between the anthropogenic and control regions was 0.1077, which is substantially lower than all KL entropy values for the local comparisons, suggesting that, at the regional scale, the distribution of vegetation diversity differs little between regimes.
To determine the scale of this difference, we used a resampling scheme, calculating the distribution of KL divergence values from 1,000 samples (with replacement) of 45,000 pixels generated from the aggregated reflectance values of the anthropogenic and control regions, respectively, from which KL divergence was calculated. The resulting histogram is presented in Fig. S18. Although the regional-level divergence was well within the range of resampled values (interquartile range: 0.102–0.114), only 1 of the 15 local comparisons fell within this range. This circle happened to be situated near a working windmill ≈140 km along the main road, linking the community with the nearest population center, and exhibited recent signs of anthropogenic hunting fires. Parties traveling to and from the community often stop here for hunting, suggesting that its successional dynamics are governed more by anthropogenic forces than other control regions. The probability that we would observe a difference this great by chance is P < 0.005 (χ2 test for homogeneity, χ2 = 8.067, df = 1).
We also predicted that anthropogenic landscapes (as compared with controls) will have greater variance in the distribution of successional stages at the local scale than at the regional scale, where any differences should diminish. Indeed, 11 of 15 comparisons (Table S1) show higher variance in the anthropogenic samples, providing modest evidence that variance is greater (with >45,000 df, the null hypothesis is almost assured to be false, and the F statistics indeed reflect this). By using a χ2 test for homogeneity, this result is significant at the P < 0.1 level (χ2 = 3.27, df = 1, P = 0.07).
Inspection of the pixel distributions indicates that where control landscapes had greater variance, they were also associated with a bimodal distribution of reflectance values, indicating that the sample contained two distinct subareas with relatively homogenous successional stage. The shape of the pixel densities of anthropogenic landscapes was qualitatively flatter and more uniform in nearly all comparisons (Fig. 2). This suggests that anthropogenic landscapes may be better approximated by a uniform distribution of vegetation types. In 13 of 15 cases, the KL relative entropy for an anthropogenic-uniform comparison is substantially less than the anthropogenic-random comparison (mean difference = 0.5228). A paired t test suggests that this difference is highly significant (t = 4.402, df = 14, P < 0.001). This analysis shows that anthropogenic landscapes are very different from random ones at the local scale, containing a broader and more uniform range of reflectance values. When aggregated to the regional scale, these differences diminish. Rather than causing localized habitat loss, anthropogenic influence protects vegetational diversity at smaller spatial scales, actually preventing the total extinction of late successional habitat.
Fig. 2.
Relative distribution plot comparing reflectance values for an anthropogenic landscape with a control one. The anthropogenic landscape shows a more uniform distribution of reflectance values.
Are Reductions in Scale and Increases in Diversity in a Local Landscape Linked to Time Spent Foraging for Resources for Which Burning Offers an Immediate Increase in Foraging Returns?
We have previously shown that most Martu burning occurs in the context of winter-season hunts for smaller animal prey, particularly monitor lizards, desert pythons (Aspidites ramsayi), blue-tongued skinks (Tilinqua scincoides), and feral cats (32). Such prey are commonly searched for simultaneously and are locally grouped into a single hunting activity (goanna hunting). While goanna hunting, burning late successional habitat (mangul or kunarka) to a nyurnma (n = 152 hunts) results in an increase of >400 kcal in mean harvest size over not burning (from 1,529 to 1,938 kcal, n = 89 hunts; t = −2.27, P = 0.0246). Mean hunting return rates also increase, from 478 to 656 kcal per hour (t = −2.91, P = 0.0040), and mean hunt-failure rate drops from 22% to 4% (t = −3.92, P = 0.0002). As we reported in Bird et al. (32), burning never occurs when collecting plant-based resources and occurs only 12% of the time when hunting larger prey [kangaroo (Macropus robustus) and bustard (Ardeotis australis)]. When it does, there are no significant effects on immediate foraging return rates. This would suggest that only small-game hunting time within a local landscape would have significant effects on measures of landscape heterogeneity and scale.
As predicted, only small-game hunting (as sampled from January to June 2002) correlates with measures of landscape heterogeneity visible on satellite imagery 6 months later (December 2002) within each of the 19 camp-centered circles. Edge density at the landscape scale increases by 1.15 m/ha with each forager-hour allocated to goanna hunting 6 months before in the landscape [ordinary least squares (OLS), r2 = 0.389, F = 8.91, P = 0.0098], but there is no relationship between edge density and large-game (kangaroo and bustard) hunting time (OLS, r2 = 0.078, β = −0.022, F = 1.18, P = 0.2950), or plant collecting time (OLS, r2 = 0.072, β = 0.018, F = 1.25, P = 0.2805). The relationship between goanna hunting time and edge density is a product of the linear relationship between edge density and the number of fires of anthropogenic origin in the circle (OLS, r2 = 0.674, β = 0.083, F = 66.33, P = <0.0001).
The noisy relationship between landscape heterogeneity and foraging intensity is likely due to the fact that our independent variable is a subsample of all foraging time allocated to that landscape. Accordingly, we also use an indirect measure of foraging intensity that correlates with summed foraging time allocation: one-way travel time. According to central place foraging theory, a forager interested in maximizing daily energetic foraging returns should use foraging patches according to their expected profitability relative to the costs of travel to the patch (34). Spatial models of habitat use incorporating central place foraging assumptions have shown that the intensity of utilization of the region surrounding a central place will depend upon conspecific competition, the patchiness of resource distribution, and the variability in patch profitability (35). When resources are evenly distributed and vary little in profitability, patches surrounding a central place should decline in intensity of use as the costs of travel increase. Edge density for all 34 landscape circles shows a highly significant hyperbolic relationship with travel time (OLS, r2 = 0.675 β = 42.87, F = 58.05, P < 0.0001) (Fig. S19). That is, edge density declines approximately linearly until travel time exceeds ≈100 min, the approximate threshold of daily foraging travel time, at which point there is a very weak relationship. The model predicts edge density drops from 3.12 m/ha at 20 min of travel time to 1.41 m/ha at 100 min. At 600 min of travel time, edge density is still 1.05 m/ha. There is also a significant but weaker hyperbolic relationship between travel time and SDI (OLS, r2 = 0.285, β = 15.52, P < 0.0024).
Do Increases in Landscape Diversity as Encountered by Human Foragers Increase Foraging Efficiency?
If mosaic creation provides adaptive benefits over the long term, habitat diversity should be positively correlated with in-patch foraging returns per bout. We constructed a diversity encounter rate (number of different successional ages encountered per hour spent foraging) for 53 follows of goanna hunters during winter 2004 and standardized the associated bout return rates to control for individual variation. As predicted, there is a positive linear relationship between diversity encounter rate and standardized return rate (OLS: r2 = .116, β = 455, P = 0.0215). The relationship is quite noisy, however, and a residual analysis shows that many of the points that are a poor fit to the model are those where foragers burned an area for hunting. Disaggregating the sample by foraging technique (burning vs. no burning), resulted in no effect of diversity on 28 follows when foragers were able to burn while hunting (r2 = 0.002, β = 72, P = 0.8347). There were 25 follows on which no burning occurred. When foragers do not burn in low-diversity habitats, their hunting returns are very low, increasing by 699 kcal/hr with each additional habitat type encountered (r2 = 0.552, β = 699, P = 0.0057) (Fig. S20). This latter model predicts that foragers gain equal returns from burning and not burning when habitat diversity exceeds 1.26 types encountered per hour of foraging and should burn only when diversity is lower than this. Indeed, only 14% of cases involved burning when diversity exceeded 1.3 types per hour. All of the failures to burn in low-diversity habitats occurred because individuals were hunting in regions to which they did not own the rights to burn.
Discussion and Implications
Anthropogenic burning increases biodiversity and prevents habitat loss at the local scale. This pattern is the direct result of human foraging activity, the efficiency of which increases as a result of such treatment. Burning areas of mature spinifex increases foraging return rates in low-diversity landscapes, but the benefits of burning (and thus its frequency) decline as habitat diversity increases. Small-game hunters gain higher foraging returns without burning in anthropogenic landscapes where small-scale successional mosaics are well established and foragers can encounter a high diversity of habitat types. The formation of the small-scale mosaics that maximize habitat diversity (and thus foraging returns) encountered by foot hunters is primarily a function of the cumulative effect of burning while small-game hunting in the winter season. Increased cumulative foraging time spent within a landscape is directly correlated with habitat diversity and the amount of habitat edge, because more time spent hunting results in more anthropogenic fires, and more anthropogenic fires contribute to greater edge density. Thus, as central place foraging models predict, increases in the costs of foraging (travel time to the hunting patch) are correlated with increases in the scale of habitat mosaics as fewer small-game hunters spend less time hunting in regions that are costlier to access. The absolute size of the mosaic generated is a function of a foot-hunter's encounter rate with habitat diversity: at diversity rates >1.3 per hour, burning has no effect on foraging returns, thus reducing incentives for further fragmentation of habitat.
Aboriginal foragers (in this case, mostly women that hunt small game) thus construct their own ecosystem (36). Over time, small-game hunting creates a very different landscape than that under a natural or lightning regime: It rearranges habitat into smaller patches, creating more diversity at spatial scales equivalent to a human foraging range. Importantly, the patchiness that ensues does not eliminate mature habitat, but rather prevents its localized extinction from large-scale fires, such that it persists in all anthropogenic landscapes but is periodically eliminated from natural ones. Such reductions in scale may favor habitat generalists with more intermediate dispersal ranges, as well as promoting recolonization of burnt habitat from unburnt source populations for more specialist species with smaller dispersal ranges. Thus, the immediate increase in foraging efficiency bought by fire treatment feeds back to create more habitat edge that promotes higher population densities of the species Martu hunt: in effect, “farming” small game.
This pattern is specific to contemporary contexts involving a single central settlement, but our focus on the mechanism of mosaic formation allows us to formulate a predictive model of burning and habitat construction that may be informative of both historic and prehistoric foraging contexts. The contemporary pattern links foraging intensity on burrowed prey to the scale of habitat heterogeneity surrounding a residential camp. As hunting time increases, more fires are set, and habitat scale decreases, until it becomes more profitable to cease burning. With decreased residential mobility, localized resource depletions force longer and longer daily path lengths for a central place forager, causing the circle of anthropogenic mosaic to expand. When average returns drop to match those gained by traveling to the next unexploited region, foragers should shift camp and begin the process of mosaic formation anew, hunting and burning as they travel. The creation of the fine-grained mosaic is thus critically linked to intensity of exploitation: With fewer foragers, less-frequent wintertime small-game hunting in a local area, and more frequent camp moves, anthropogenic mosaics may be nearly indistinguishable from natural ones.
Several important paleoecological/archaeological implications follow from this. First, anthropogenic mosaics like those described here are likely to be associated with “broad-spectrum” transitions: increased population density, lower residential mobility, increasing logistical mobility, a focus on small-game subsistence, and a broader range of intensified foraging activities with increasing investment in more-costly resources and elaborate processing technologies. Only after the mid-Holocene, and only after ≈1.5 kya in the arid zone, do archaeological patterns indicate a dramatic increase in human populations and longer-term occupation of sites by larger, less wide-ranging groups with intensified use of a broad spectrum of lower-ranked resources such as grass-seeds (37, 38, 39). We have yet to quantify how plant collecting returns are influenced by mosaic grain, but we suspect that because edible seed grasses (Eragrostis eriopoda, woolybutt, in particular) are limited to early successional sandplain habitats, Acacia spp. to late successional ones, efficient exploitation of seed resources may be critically linked to the scale of habitat mosaics, which are created through small-game hunting. Reducing the scale of habitat mosaics would decrease variance in the availability of small game and seeds within a forager's day range. As such, increasing presence of seed-grinding technologies in the archaeological record should correspond closely with the establishment of anthropogenic fire mosaics. Secondly, our results show clearly that human influence does not extend uniformly across the arid zone; it is scaled to forager day-range and localized around residential camps and along linking pathways. Areas less frequently traversed by small-game hunters, even those just a few dozen kilometers beyond intensively used areas, are always more coarse grained, the fire regime determined primarily by lightning associated with the summer monsoon. Such a pattern is to be expected in the past as well, because of the patchy nature of water and other limiting resources in the arid zone (40). It is thus unlikely that late-Pleistocene/early-Holocene human populations were dense enough or sedentary enough to control the lightning fire regime and maintain anthropogenic landscapes, at least not on a pan-Australian scale. This throws considerable doubt on the hypothesis that immediately after the arrival of humans, continent-wide habitat modification caused a rapid trophic collapse and the extinction of Pleistocene fauna (5, 6, 13). Finally, our data also make it clear that an anthropogenic landscape differs from a natural one in scale, but not in kind, and supports the assertion (23) that anthropogenic fire is protective of diversity. Martu burning does not increase the absolute amount of fire, it rescales its temporal and spatial impact. This adds to evidence showing that it may be difficult to separate the impact of anthropogenic fire from climate-driven changes in fire frequency by using only charcoal or fossil pollen records (15). Any attempts to reconstruct paleoecological communities must rely on “fossils” of successional mosaics, not fossils of fire (e.g., ref. 41). One possible candidate for preserving the scale of habitat mosaics may be stable isotopes as biomarkers of herbivore diet; sedentary generalist species with home ranges similar to the scale of human foraging day range will experience a more consistent presence of a wider array of vegetation types under an anthropogenic regime.
Our test of predictions derived from Jones' fire-stick farming model suggest that daily small-game hunting results in a higher diversity of successional habitats, which, in turn, leads to higher overall foraging efficiency. Our results cast doubt on the hypothesis that human habitat modification initiated late Pleistocene faunal loss but support the notion that the maintenance of biodiversity in the Western Desert relies on women's small-game hunting—in its absence, fine-grained mosaics easily dissolve leading to a decrease in biodiversity at the local scale.
Materials and Methods
Ethnographic data on Martu social organization and (pre)history are available from a variety of sources (e.g., refs. 30, 31, 40, 42, and 43). Details on our ethnographic methods have been described elsewhere relative to Martu mosaic burning, foraging seasonality, gender, and age (31, 32, 44, 45). Data reported here incorporate 1,095 forager hours over 421 bouts on 59 camp-days in 2002 in which we recorded the identity of each participant in the foraging party (the group that leaves camp together), the time they spent traveling to foraging locales, route taken, foraging location, and foraging-bout details of all party participants. We defined a foraging bout as the time each participant in the party spent searching for and handling wild food resources. After each bout, we recorded the number and whole weight of each type of resource that each forager acquired and monitored subsequent food distributions. Whole weight was converted to edible kilocalories by using experimental and published sources (31). On many follows, we also recorded the foraging path using GPS, and the number of different successional stages encountered while foraging. This project was reviewed and approved by the Stanford Institutional Review Board, and informed consent was obtained before commencement of research.
To assess the differences in landscape diversity created through anthropogenic influence, we visually classified successional habitat structure according to the Martu ethnoecological model in 34 circular landscapes (28 km2) on 2002 Landsat imagery. This scale (3-km radii) was chosen because it corresponds with the average one-way foraging distance traversed by individuals hunting on foot. Nineteen circles were centered on foraging camps from which detailed time-allocation data were collected from January to June 2002; 15 of these are frequently visited areas and were considered under an anthropogenic regime, 4 were centered on remote camps that we initiated into the generally unvisited region and were considered under a mixed regime. Fifteen other circles were chosen as controls from a stratified random sample of center coordinates located in regions generally unvisited by Aboriginal foragers and thus under a lightning-ignition regime: Fig. S1 shows the location of all sampled landscapes. Two Landsat 7 ETM+ images (110_76_2002_Nov3, and 109_76_Dec14) were calibrated, orthorectified, mosaicked, and color balanced in ENVI. Bands 7, 4, and 2, which facilitate discrimination of fire scars and the density of regenerating vegetation, were subject to a decorrelation stretch. The images were ground-truthed with 10 10-km transects conducted in June of 2003. All data were imported into ArcGIS. Habitat classification vectors were analyzed with the Patch Analyst 4.0 extension to calculate edge density and SDI. Data were analyzed in JMP 7.0.
To examine differences at the regional scale compared with the local scale, the distribution of pixel digital number values for each circle was collected by converting each circular landscape into region of interest (ROI) in ENVI and calculating statistics for the decorrelated band 7. Band 7 reflectance values provide a continuous measure of the diversity of successional stages within the landscape: immediately after a fire, reflectance in the middle infrared is high and decreases as vegetation cover increases. To assess the differences in the distribution of reflectance values between anthropogenic and control regions, we adopted an information–theoretic approach based on KL discrimination information or relative entropy (33). KL is a measure of the divergence between two probability distributions, ƒ(x) and g(x) or, interchangeably, a measure of the inefficiency of measuring distribution F with distribution G (46). KL relative entropy is defined as:
 |
where ƒ(x) is the target density, and g(x) is the reference distribution. We generated histograms of the reflectance values for all pixels in each anthropogenic and control region and approximated their densities using smoothing splines. We then calculated H(F,G) of the anthropogenic region with respect to its paired control region for all 15 samples. All calculations were done using the reldist library (47) for the R statistical programming language (48).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804757105/DCSupplemental.
References
- 1.Laris P. Burning the seasonal mosaic: Preventative burning strategies in the wooded savanna of Southern Mali. Hum Ecol. 2002;30:155–186. [Google Scholar]
- 2.Sheuyange A, Obaa G, Weladjib RB. Effects of anthropogenic fire history on savanna vegetation in northeastern Namibia. J Environ Manage. 2005;75:189–198. doi: 10.1016/j.jenvman.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Stephens SL, Martin RE, Clinton NE. Prehistoric fire area and emissions from California's forests, woodlands, shrublands, and grasslands. Forest Ecol Manage. 2007;251:205–216. [Google Scholar]
- 4.Jones R. Firestick farming. Aust Nat Hist. 1969;16:224–231. [Google Scholar]
- 5.Flannery T. Pleistocene faunal loss: Implications of the aftershock for Australia's past and future. Archaeol Ocean. 1990;25:25–67. [Google Scholar]
- 6.Miller GH, et al. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science. 2005;309:287–290. doi: 10.1126/science.1111288. [DOI] [PubMed] [Google Scholar]
- 7.Allan G, Southgate R. Fire regimes in the spinifex landscapes of Australia. In: Bradstock RA, Williams JE, Gill A, editors. Flammable Australia. Cambridge, UK: Cambridge Univ Press; 2002. pp. 145–176. [Google Scholar]
- 8.Bowman D, Walsh A, Prior LD. Landscape analysis of Aboriginal fire management in Central Arnhem Land, north Australia. J Biogeogr. 2004;31:207–223. [Google Scholar]
- 9.Latz P. Bushfires and Bushtucker: Aboriginal Plant Use in Central Australia. Northern Territory, Australia: IAD Press, Alice Springs; 1996. [Google Scholar]
- 10.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Syst. 2003;34:487–515. [Google Scholar]
- 11.Smith DA. Garden game: Shifting cultivation, indigenous hunting and wildlife ecology in Western Panama. Hum Ecol. 2005;33:505–537. [Google Scholar]
- 12.Bond WJ, Keeley J. Fire as global “herbivore”: The ecology and evolution of flammable ecosystems. Trends Ecol Evol. 2005;20:387–394. doi: 10.1016/j.tree.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Miller GH, Magee JW, Fogel ML, Gagan MK. Detecting human impacts on the flora, fauna, and summer monsoon of Pleistocene Australia. Clim Past Discuss. 2006;2:535–562. [Google Scholar]
- 14.Bolton B, Latz P. The Western Hare-Wallaby, Lagorchestes hirsutus (Gould) (Macropodidae) in the Tanami desert. Aust Wildlife Res. 1978;5:285–293. [Google Scholar]
- 15.Bowman D. The impact of aboriginal burning on the Australian biota. New Phytol. 1998;140:385–410. doi: 10.1111/j.1469-8137.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 16.Burbidge A, McKenzie NL. Patterns in the decline of Western Australian vertebrate fauna: Causes and conservation implications. Biol Conserv. 1989;50:143–198. [Google Scholar]
- 17.Burrows ND, Burbidge A, Fuller PJ, Behn G. Evidence of altered fire regimes in the Western Desert region of Australia. Conserv Sci West Aust. 2006;5:272–284. [Google Scholar]
- 18.Burrows ND, Christensen PE. A survey of Aboriginal fire patterns in the Western Desert of Australia. In: Nodvin SC, Waldrop TA, editors. Fire and the Environment: Ecological and Cultural Perspectives. Asheville, NC: U.S. Dept of Agric Forest Service, General Technical Report SE-69; 1990. pp. 20–24. [Google Scholar]
- 19.Bradstock RA, Williams JE, Gill AM. Flammable Australia: The Fire Regimes and Biodiversity of a Continent. Cambridge, UK: Cambridge Univ Press; 2002. [Google Scholar]
- 20.Letnic M, Dickman CR, Tischler MK, Tamayo B, Beh C-L. The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. J Arid Env. 2004;59:85–114. [Google Scholar]
- 21.Braithwaite RW. Biodiversity and fire in the savanna landscape. In: Solbrig O, Medina E, Silva JF, editors. Biodiversity and Savanna Ecosystem Processes. Berlin: Springer; 1996. pp. 121–140. [Google Scholar]
- 22.Russell-Smith J, et al. Aboriginal resource utilization and fire management practice in western Arnhem Land, monsoonal northern Australia: Notes for prehistory, lessons for the future. Hum Ecol. 1997;25:159–195. [Google Scholar]
- 23.Bowman D, Boggs GS, Prior LD. Fire maintains an Acacia aneura shrubland—Triodia grassland mosaic in central Australia. J Arid Environ. 2008;72:34–47. [Google Scholar]
- 24.Yibarbuk D, et al. Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: A tradition of ecosystem management. J Biogeogr. 2001;28:325–343. [Google Scholar]
- 25.Murphy B, Bowman D. The interdependence of fire, grass, kangaroos and Australian Aborigines: A case study from central Arnhem Land, northern Australia. J Biogeogr. 2007;34:237–250. [Google Scholar]
- 26.Hawkes K. Why hunter–gatherers work: An ancient version of the problem of public goods. Curr Anthropol. 1993;34:341–361. [Google Scholar]
- 27.Smith EA, Wishnie M. Conservation and subsistence in small-scale societies. Annu Rev Anthropol. 2000;29:493–524. [Google Scholar]
- 28.Gould RA. Uses and effects of fire among the western desert Aborigines of Australia. Mankind. 1971;8:85. [Google Scholar]
- 29.Davenport S, Johnson P, Yuwali . Cleared Out: First Contact in the Western Desert. Canberra, Australia: Aboriginal Studies Press; 2005. [Google Scholar]
- 30.Tonkinson R. The Mardu Aborigines: Living the Dream in Australia's Desert. Rhinehart, and Winston, New York: Holt; 1991. [Google Scholar]
- 31.Bliege Bird R, Bird D. Why women hunt: Risk and contemporary foraging in a Western Desert Aboriginal community. Curr Anthropol. 2008 in press. [PubMed] [Google Scholar]
- 32.Bird D, Bliege Bird R, Parker C. Aboriginal burning regimes and hunting strategies in Australia's Western Desert. Hum Ecol. 2005;33:443–464. [Google Scholar]
- 33.Kullback S, Leibler RA. On information and sufficiency. Ann Math Stat. 1951;22:79–86. [Google Scholar]
- 34.Orians GH, Pearson NE. On the theory of central place foraging. In: Horn DJ, Sairs R, Mitchell RD, editors. Analysis of Ecological Systems. Columbus: Ohio State Univ Press; 1979. pp. 155–177. [Google Scholar]
- 35.Getty T. Analysis of central-place space-use patterns: The elastic disc revisited. Ecology. 1981;62:907–914. [Google Scholar]
- 36.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction: The Neglected Process in Evolution. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 37.O'Connell JF, Allen J. Pre-LGM Sahul (Pleistocene Australia–New Guinea) and the archaeology of early modern humans. In: Mellars P, Boyle K, Bar-Yosef O, Stringer C, editors. Rethinking the Human Revolution. Cambridge, UK: McDonald Institute for Archaeological Research; 2007. pp. 395–410. [Google Scholar]
- 38.Smith MA, Ross J. What happened at 1500–1000 cal. BP in Central Australia? Timing, impact and archaeological signatures. Holocene. 2008;18:379–388. [Google Scholar]
- 39.Smith MA, Williams NA, Turney CSM, Cupper ML. Human environment interactions in Australian drylands: Exploratory time-series analysis of archaeological records. Holocene. 2008;18:389–401. [Google Scholar]
- 40.Veth P. Islands in the Interior: The Dynamics of Prehistoric Adaptations Within the Arid Zone of Australia. Ann Arbor, MI: International Monographs in Prehistory; 1993. (Archaeology Series 3). [Google Scholar]
- 41.Black MP, Mooney SD, Attenbrow V. Implications of a 14,200 year contiguous fire record for understanding human climate relationships at Goochs Swamp, New South Wales, Australia. Holocene. 2008;18:437–447. [Google Scholar]
- 42.Veth P. Martujarra prehistory: Variation in arid zone adaptations. Aust Archaeol. 1987;25:102–111. [Google Scholar]
- 43.Walsh F. An ecological study of traditional aboriginal use of “country”: Martu in the Great and Little Sandy Deserts, Western Australia. Proc Ecol Soc Aust. 1990;16:23–37. [Google Scholar]
- 44.Bliege Bird R, Bird D. Human hunting seasonality. In: Brockman D, van Schaik C, editors. Primate Seasonality. Cambridge, UK: Cambridge Univ Press; 2005. pp. 243–266. [Google Scholar]
- 45.Bird D, Bliege Bird R. Mardu children's hunting strategies in the Western Desert, Australia: Foraging and the evolution of human life histories. In: Hewlett B, Lamb M, editors. Hunter Gatherer Childhoods. New York: Aldine; 2005. pp. 129–146. [Google Scholar]
- 46.Cover TM, Thomas JA. Elements of Information Theory. New York: Wiley; 1991. [Google Scholar]
- 47.Handcock MS, Morris M. Relative Distribution Methods in the Social Sciences. New York: Springer; 1999. [Google Scholar]
- 48.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.