Abstract
A fundamental issue in stem cell biology is whether adult somatic stem cells are capable of accessing alternate tissue sites and continue functioning as stem cells in the new microenvironment. To address this issue relative to neurogenic stem cells in the mouse mammary gland microenvironment, we mixed wild-type mammary epithelial cells (MECs) with bona fide neural stem cells (NSCs) isolated from WAP-Cre/Rosa26R mice and inoculated them into cleared fat pads of immunocompromised females. Hosts were bred 6–8 weeks later and examined postinvolution. This allowed for mammary tissue growth, transient activation of the WAP-Cre gene, recombination, and constitutive expression of LacZ. The NSCs and their progeny contributed to mammary epithelial growth during ductal morphogenesis, and the Rosa26-LacZ reporter gene was activated by WAP-Cre expression during pregnancy. Some NSC-derived LacZ+ cells expressed mammary-specific functions, including milk protein synthesis, whereas others adopted myoepithelial cell fates. Thus, NSCs and their progeny enter mammary epithelium–specific niches and adopt the function of similarly endowed mammary cells. This result supports the conclusion that tissue-specific signals emanating from the stroma and from the differentiated somatic cells of the mouse mammary gland can redirect the NSCs to produce cellular progeny committed to MEC fates.
Keywords: transplantation, niche, plasticity, trans-differentiation, regeneration
Somatic stem cells are defined by how they function physiologically in the presence of heterologous cells, that is, the microenvironment or stem cell niche that balances protecting stem cells from exhaustion and protecting the host from unregulated stem cell growth. The dominance of the niche over the stem cell's autonomous phenotype has been demonstrated in several reports involving cells crossing lineage “boundaries” to regenerate “foreign” tissues. We previously demonstrated that cells isolated from the seminiferous tubules of the mature testis, when mixed together with normal mammary epithelial cells (MECs), would cooperate with these cells and contribute robust numbers of epithelial progeny to normally growing mammary glands in transplanted mammary fat pads (1). In these experiments, the testicular cells included ≈10% germinal stem cells (spermatogonia α and β), ≈20% Sertoli cells, and the remainder primary and secondary spermatocytes and other cells at various stages of spermatogenic cell differentiation. Interstitial and vascular tissue was separated from the seminiferous tubules before dissociation. In these experiments, we were unable to distinguish whether all or just a subpopulation of the isolated seminiferous cells contributed progeny to the mammary epithelial outgrowths.
In the present study, to more directly demonstrate the activity of multipotent cells (i.e., cells with the capacity to differentiate into multiple cell types) in this experimental model, we opted to begin with carefully characterized and stable multipotent stem cell populations that could be maintained indefinitely in vitro and could differentiate into multiple cell types in culture after the addition of serum factors and the removal of culture components responsible for stem cell maintenance. Toward this end, neural stem cells (NSCs) from fetal and adult WAP-Cre/Rosa26R mice were isolated and propagated in serum-free medium. Subsequently, these NSCs were mixed with equal numbers of MECs and inoculated immediately into epithelium-divested mammary fat pads of prepubertal female mice. Both fetal and adult NSCs were shown to interact with MECs on transplantation and to robustly contribute mammary epithelial-specific progeny to normal mammary outgrowths.
Results
Isolation of Multipotent NSCs from WAP-Cre/Rosa26R Mice.
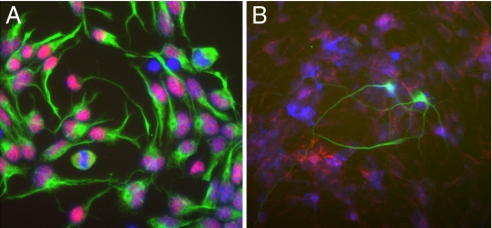
NSCs were isolated from 13.5-day postcoital WAP-Cre/Rosa26R embryos or adult males as reported previously (2, 3). Characterization of the pluripotency of these NSCs in culture demonstrated their multipotentiality. When plated at clonal densities, the cells generated neurons and glia after a 5-day differentiation period initiated by mitogen withdrawal (2). NSCs isolated from WAP-Cre/Rosa26R mice both before and after removal of the mitogen are shown in Fig. 1. Before differentiation, all of the cells were positive for Sox2 and nestin (Fig. 1A). After mitogen removal, the cells differentiated into cells expressing markers specific for neurons, astrocytes, and oligodendrocytes (Fig. 1B).
Fig. 1.
NSCs isolated from WAP-Cre/Rosa26R mice are kept in an undifferentiated state (A) with NSCs expressing nestin (green) and Sox2 (red) or allowed to differentiate (B) as determined by the expression of markers specific for neurons (TUJ1, green), astrocytes (GFAP, pink), and oligodendrocytes (CNPase, red).
Formation of Chimeric Mammary Outgrowths on Transplantation.
Cultured WAP-Cre/Rosa26R NSCs and wild-type FVB/N MECs were combined in a 1:1 ratio (50,000 NSCs and 50,000 MECs) and inoculated directly into epithelium-divested mammary fat pads of 3-week-old Nu/Nu female mice. Mammary duct development proceeded for 6–8 weeks after injection, at which time some hosts were allowed to complete a full-term pregnancy (required for activation and recombination of the reporter transgene). Pups were removed after 1 week, and complete glandular involution was allowed. Subsequently, the regenerated mammary glands were removed and stained as whole mounts for LacZ activity by X-gal. For activation of the reporter, both the reporter gene and the WAP-Cre gene must be in the same cell at the time of Cre–lox recombination. Only the NSCs carried both transgenes, and thus only progeny from NSCs could be LacZ+. After pregnancy-induced Cre recombination, LacZ expression from the Rosa26 promoter was constitutive and served as a lineal marker to identify the location and cell fate of NSC progeny.
The occurrence of blue LacZ+ cells within the regenerated mammary glands indicates the presence of neural-derived progeny within the mammary outgrowth. Cultured cells from freshly isolated embryonic NSCs mixed with MECs produced mammary outgrowths in 75% of the injected fat pads (15/20); 93.3% of these were LacZ+ (14/15). NSCs isolated and cultured from adult brains and mixed with mammary cells produced mammary outgrowths in 70% of the inoculated pads (7/10), all of which (100%) contained LacZ+ epithelium (Table 1). These results suggest that the NSCs were able to interact with MECs in newly formed mammary epithelial growth cones, proliferate along with the mammary cells, and contribute MEC progeny during formation of the regenerated mammary epithelial tree. In most cases, the LacZ+ neural cell progeny were distributed throughout the entire epithelial outgrowth (Fig. 2A), indicating that the interaction between neural and mammary cells occurred early in the formation of the mammary growth cone and continued throughout the formation of the mammary duct system. Secondary transplants from the original chimeras also produced outgrowths, with LacZ+ MECs distributed throughout the regenerated mammary ducts (14/20) (Table 1).
Table 1.
Transplantation results from implantation of dispersed mixtures of central nervous system stem cells and MECs into mammary fat pads
| Cell type | Number of positive takes/number of implants | Number of LacZ+ takes/number of positive takes |
|---|---|---|
| Embryonic central nervous system | 15/20 | 14/15 |
| Adult central nervous system | 7/10 | 7/7 |
| Second transplants/LacZ+ outgrowths | 14/20 | 14/14 |
Cell mixtures were introduced into the epithelium-free mammary fat pads of 3-week-old female mice and allowed to grow for 6–8 weeks. Hosts were maintained as virgins or allowed one cycle of pregnancy, lactation, and involution. Whole mounts were stained by X-gal to reveal β-galactosidase expression. Only outgrowths from impregnated hosts contained WAP-Cre–activated LacZ+ cells.
Fig. 2.
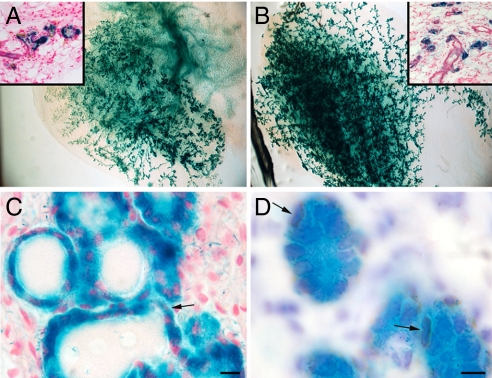
NSC-derived cells are present in chimeric outgrowths. (A) X-gal-stained whole mammary fat pad from a 16-day postlactation host mouse reveals that LacZ+ cells are represented throughout the entire chimeric outgrowth. (B) Regeneration of chimeras in secondary transplants demonstrates that LacZ+ cells are capable of replicating and contributing to all portions of the regenerated mammary outgrowth. Insets are histological sections of whole mounts demonstrating that only epithelial cells are LacZ+ in (A) and (B). (C) Tissue section of the gland in A demonstrating the distribution of LacZ+ epithelial cells. Note that the surrounding stroma remains LacZ−. (D) Tissue section of the gland in B reacted for smooth muscle actin (brown), a myoepithelial marker. Arrows indicate LacZ+ myoepithelial cells. Magnifications: 20 × in A and B, 100 × in the insets. (Scale bars: C, 15 μm; D, 10 μm.)
NSC Progeny Display Mammary Characteristics.
LacZ+ cells (i.e., NSC-derived cells) were positioned together in positively stained groups and also mixed intermittently among nonstaining epithelial cells along mammary ducts and side branches (Fig. 2 A and B). This pattern suggests that the neural cells had proliferated in concert with MECs during ductal morphogenesis in a manner similar to intact WAP-Cre/Rosa26R mammary gland development. NSC-derived cells (LacZ+) were present as luminal epithelial cells (Fig. 2C) lining the duct lumina and also as basally located myoepithelial cells (Fig. 2C, arrow). The latter was confirmed by demonstrating coexpression of β-galactosidase and smooth muscle actin (Figs. 2 D and 3A). From serial sections obtained from five independent chimeric outgrowths, we estimated that ≈5–7% of the mammary cells in chimeras were stained positive for LacZ, indicating their derivation from NSCs. Only WAP-Cre-activated mammary cells that survived involution could be identified as NSC progeny. During pregnancy and lactation, NSC-derived cells differentiated into secretory MECs, as demonstrated by immunologic colocalization of β-galactosidase and a milk protein, β-casein (Fig. 3B) in lactating tissue. Furthermore, progeny of NSC-derived cells surviving in second-generation transplants were able to differentiate into estrogen receptor (ER)-α-expressing luminal mammary cells (Fig. 3C) and progesterone receptor (PR)-expressing MECs (Fig. 3D). These data indicate that NSC progeny are capable of differentiating into mammary cells along different lineages, both luminal epithelial and myoepithelial. The multipotent nature of the LacZ+ NSC-derived mammary cells corresponds to the previously reported multipotent nature of pregnancy-identified MECs (PI-MECs) isolated and characterized from the intact mammary glands of parous WAP-Cre/Rosa26R female mice. In secondary outgrowths, PI-MECs replicated and maintained their multipotent capacity. Similarly, NSC-derived LacZ+ MECs in secondary outgrowths were capable of producing ER+ and PR+ luminal progeny, as well as myoepithelial offspring, in pregnant hosts.
Fig. 3.
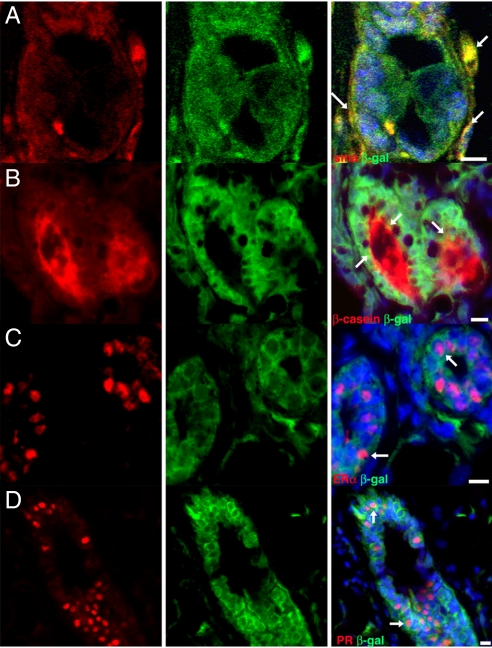
NSC-derived progeny may express myoepithelial or luminal epithelial cell markers. (A) Colocalization of smooth muscle actin (SMA, red) and β-galactosidase (green) in chimeric outgrowth. Arrows indicate myoepithelial cells that express both SMA and β-galactosidase. (B) NSC progeny expressing both casein (red) and β-galactosidase in a mammary chimera in a pregnant host. Arrows indicate cells expressing both casein and β-galactosidase. (C and D) Second-generation transplant sections demonstrating that β-galactosidase-positive (green) NSC-derived progeny express (C) ERα (red) and (D) PR (red). Arrows indicate select cells that co-express β-galactosidase and the nuclear steroid receptor of interest. (Scale bars: 20 μm.)
Cell Cycle Analysis of the Chimeric Outgrowths.
Many reports have confirmed that cell–cell fusion can account for observations of stem cell plasticity in various experimental models. Consequently, we initiated several analyses to test this possibility in our model. A description of the biological and morphogenetic events leading to the development of a regenerated mammary outgrowth from injected cells is in order. The cells were mixed and inoculated immediately into freshly cleared mammary fat pads of 3-week-old females. No adjuvant or growth medium was applied. The cells were scattered along the needle tracks, and over the next several days, they recombined and formed an epithelial complex able to support growth and penetration of the surrounding fat through the formation of terminal end buds (4). Formation of the mammary branching ductal system then proceeded radially from the initiation point until the fat pad was filled or until growth senescence occurred within the advancing buds. This process was ordinarily completed in 6–7 weeks.
During pregnancy, secretory development in the form of acini and lobules occurs at branch points along the ductal system. Further duct development also may occur. Mammary duct and full secretory development in the inoculated fat pad leads to the production of 25–40 × 106 MECs (5). During secretory development, WAP-Cre is abundantly expressed, leading to activation of the Rosa26R gene, but only in cells in which both transgenes are present (on separate chromosomes). Because LacZ+ epithelial cells are commonly seen throughout the mammary duct outgrowth after lactation and involution, it seems justified to conclude that NSCs and their progeny intermingle with MECs that proliferated during duct development and subsequently recombine their reporter transgenes during WAP-Cre expression in pregnancy and lactation. On weaning, some of these cells survive and represent the LacZ+ population in the involuted gland.
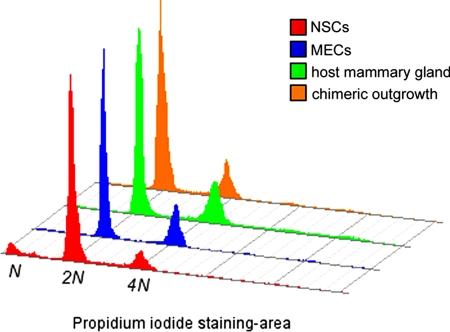
To ascertain whether or not early fusion between NSCs and MECs occurred after mixing and injection into the cleared fat pads, propidium iodide staining and cell cycle analysis were carried out. Separate populations of NSCs and MECs for DNA content along with single cell suspensions prepared from several independent chimeric mammary outgrowths that they generated, were analyzed. For comparison, single cell suspensions also were created from an intact postpubertal mammary gland and analyzed in parallel. Fig. 4 shows a histogram of the relative ploidy in 20,000 individual cells from each of the four test groups. If fusion between an NSC and MEC had occurred, either in the test tube immediately after mixing or at any time after co-injection into the cleared recipient fat pad, then the cell cycle curve from a chimeric outgrowth would reflect this by showing an increased area under the 4N peak (if two 2N cells fused) or an increased number of higher ploidy cells (if a 2N cell fused with a 4N cell or two 4N cells fused). Neither of these effects was observed. The 2N and 4N peaks from the chimeric outgrowths showed similar areas as those from a native mammary gland, strongly suggesting that no detectable fusion had occurred in at least 20,000 cells, which collectively serve as a random representation of what is occurring throughout the chimeric mammary gland. Furthermore, no higher-ploidy cells were observed in the chimeric outgrowth, consistent with normal DNA replication and chromosome segregation during the extensive proliferation of mammary outgrowth.
Fig. 4.
Assessment of DNA content within individual cells by propidium iodide staining followed by flow cytometric cell cycle analysis. A histogram plotting total cellular fluorescence (FL2 area) on the x-axis versus cell number on the y-axis shows the DNA content in each of 20,000 cells from the two starting populations (NSCs and MECs) and single cell suspensions prepared from an intact host mammary gland and an NSC/MEC-injected chimeric outgrowth. The relative G0/G1 (2N) and G2/M (4N) peaks are prominent, with the less frequent S-phase cells accounted for by the valley between the 2N and 4N peaks.
NSC Markers in LacZ+ Cells.
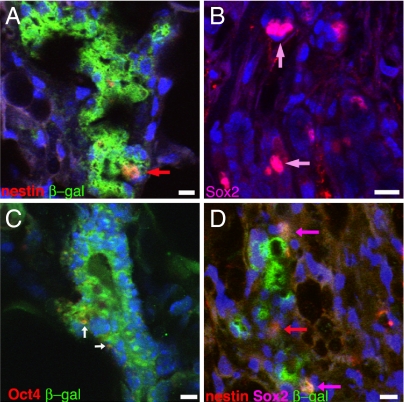
Within the functional chimeric gland, occasional LacZ+ cells that expressed NSC markers were found. Nestin-positive cells were found in luminal or suprabasal positions and expressed nestin and β-galactosidase (Fig. 5A), suggesting that a population of neural-derived cells continued to express NSC markers. Similarly, some LacZ+ cells also stained positively for another NSC marker, Sox2 (Fig. 5B). We found that 6.34% of the cells within the chimeric gland expressed nestin, and 1.06% of the mammary cells expressed Sox2. All of the Sox2+ cells also were positive for nestin. We also found Oct4, a transcription factor associated with stem cell maintenance, in some cells that expressed β-galactosidase (Fig. 5C). LacZ+ cells that expressed nestin or Sox2 also were present in second-generation transplant outgrowths (Fig. 5D), suggesting persistent NSC-specific gene expression after expansion of the LacZ+ population within secondary outgrowths. In wild-type mammary glands, we found nestin expression in 1.08% of the entire cell population but no Sox2 expression by any wild-type cells.
Fig. 5.
LacZ+ cells within chimeras occasionally express NSC markers. Cross-sections of involuted chimeric mammary glands were probed for (A) nestin (red, arrow) and β-galactosidase (green), (B) Sox2 (pink, arrows), or (C) Oct4 (red, arrows) and β-galactosidase (green). (D) Cross-section of second transplantation generation demonstrating the persistence of neural markers nestin (red, arrow) and double-labeled with Sox2 (pink, arrows). All sections were stained with DAPI (blue) for nuclear visualization. (Scale bars: A, 10 μm; B–D, 20 μm.)
NSCs Are Recoverable from Primary Mammary Chimeras.
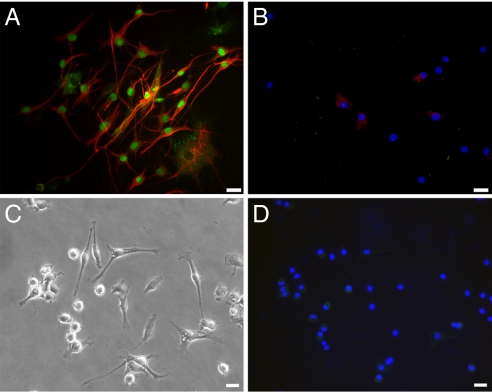
After complete mammary gland formation by the mixture of NSCs and MECs, the chimeric glands from primary outgrowths were excised and dissociated. Subsequently, the recovered cells were cultured under conditions originally used to propagate and maintain the NSCs. Under these conditions, cells with the characteristics of the originally inoculated NSCs were recovered (Fig. 6). The recovered NSCs expressed both nestin and Sox2 (Fig. 6A). Similar attempts to reisolate NSCs from secondary outgrowths proved unsuccessful (Fig. 6B). These results suggest that successful isolation of NSCs from primary implants most likely resulted from the survival of NSCs from the original inoculums, not from the NSC progeny that have adopted MEC fate. Our inability to reisolate NSCs from mammary fat pads containing secondary outgrowths is consistent with this premise. In addition, when freshly isolated MECs were placed in culture originally used to propagate NSCs, some cells were elongated (Fig. 6C), but no cells expressed nestin or Sox2 (Fig. 6D).
Fig. 6.
NSCs can be recovered from injected glands after ductal development, pregnancy, lactation, and involution. Chimeric mammary glands composed of NSCs and wild-type MECs were dissociated, and the resulting cell suspension was placed in neural culture conditions. (A) Recovered cells from a mammary chimera in NSC culture conditions. The cells demonstrate a NSC morphology and also positive expression of nestin (red) and Sox2 (green). (B) Recovered cells from a second-generation transplant mammary chimera in NSC culture conditions. No cells display any NSC morphology or express Sox2; occasional cells express low levels of nestin. (C) Morphology of freshly isolated wild-type MECs grown in NSC culture conditions. (D) No fluorescent antibody detection of nestin and Sox2 was present in these cultures (C), indicating that no cells with NSC characteristics could be recovered from intact wild-type mammary glands. (Scale bars: 10 mm.)
Discussion
In 2002, we reported the discovery of an adjunct MEC population marked by activation of the Rosa26-LacZ reporter gene after Cre-Lox recombination as the result of WAP-Cre expression during pregnancy and lactation (6). This population was termed parity-induced MECs (PI-MECs). These cells survived postlactation involution and tissue remodeling of the mammary epithelium and were found to be associated primarily with ductal side branches in the involuted parous mammary tissue. In subsequent pregnancies, the PI-MECs proliferated and produced epithelial progeny to form secretory acini during early pregnancy. Further study demonstrated that these PI-MECs were long-lived and capable of proliferation through four serial transplant generations (7). During their expansion, the PI-MECs gave rise to luminal MECs (both ERα+ and PR+ and ERα− and PR− cells) during ductal morphogenesis and were found both within the body of active terminal end buds and along the subtending ducts. They did not give rise to the specialized cap cells found at the growing ends of the termini; thus, the duct-associated myoepithelial cells (which arise from cap cells) were not LacZ+. On impregnation of the transplant-bearing host, the PI-MECs proliferated and produced both luminal and myoepithelial progeny to the developing secretory acini (7). The PI-MECs maintained this property throughout four transplant generations. Subsequently, the PI-MECs were found to exist before pregnancy in the mammary epithelium (8) and to exhibit the same properties as those PI-MECs originally detected in postparous glands. Consequently, the PI-MECs were renamed parity-identified MECs. The PI-MECs express all of the properties of the lobule-limited mammary stem/progenitor cells originally described by Smith (9).
Consistent with the conclusion that they represent lobule-limited progenitor cells, PI-MECs are not capable of producing fully developed and functional mammary outgrowths without interacting with epithelial cells with an inactivated reporter gene (7), although they are able to form complete lobular-only structures in limiting dilution transplant experiments. In addition, the presence of PI-MECs among implanted cells appears to be essential for positive mammary epithelial growth (7, 10). Because PI-MECs are identified after pregnancy and survive involution, this model afforded us the opportunity to explore whether cells from nonmammary tissue would interact with wild-type MECs in the context of the mammary fat pad, proliferate, differentiate to the extent that their WAP-Cre gene became activated during pregnancy, and survive involution. As a result, their progeny after mammary growth and functional differentiation then could be detected by activation of the Rosa26-LacZ reporter gene.
In the present study, we carried out experiments using multipotent NSCs maintained as such under specific culture conditions and shown to be capable of differentiating into three distinct differentiated neural cell types in vitro. These NSCs were derived from both adult and embryonic brains of WAP-Cre/Rosa26R mice. Our findings demonstrate that they are capable of interacting with normal MECs and cooperate copiously in the generation of a fully differentiated functional mammary gland. NSCs alone without cooperation from coinjected MECs fail to produce mammary epithelium. This indicates that signals, both paracrine and juxtacrine, from bona fide MECs are indispensable to the active participation of NSCs in chimera formation. Once incorporated into mammary epithelial structures, NSC progeny behave similar to PI-MECs, producing both luminal and myoepithelial progeny and actively proliferating in secondary transplants. LacZ+ NSC luminal progeny synthesize milk protein in pregnant hosts and along ducts; some express PR and ERα.
Most LacZ+ NSC progeny among the mammary chimera no longer expressed NSC-specific genes; however, occasional cells continued to express Sox2 and nestin. The significance of this finding remains unclear at present, although this observation has been made in both primary chimeras and secondary transplants.
The redirected NSCs proliferated and contributed extensively to primary and secondary mammary outgrowth and produced both luminal epithelial progeny capable of synthesizing milk protein and myoepithelial cells in secretory acini. NSC progeny also included ER+ and PR+ epithelial offspring among the chimeric epithelium. MECs with these properties have been shown to be indispensable to complete duct and secretory alveolar development in murine mammary gland (11, 12). In the chimeric mammary tissue, NSC-derived mammary progeny, conditionally marked by LacZ expression due to WAP-Cre-initiated recombination, behaved exactly like the PI-MECs described earlier in intact WAP-Cre/Rosa26R female mammary glands after parity.
In these experiments, we were not able to ascertain whether NSC-derived mammary epithelial progeny contributed to other aspects of mammary development, because WAP-Cre activation of the reporter gene did not mark all mammary epithelia. Despite this, however, the widespread contribution of NSC-derived progeny to the MEC population before pregnancy (as determined by the uniform distribution of LacZ+ progeny along the mammary ducts in fully involuted, primiparous females) suggest that NSC-derived progeny contributed significantly to the total MEC content in the chimeric outgrowths. In addition, the finding that a specific tissue locale composed of stromal and epithelial factors can dictate the repertoire of bona fide somatic stem cells to its own purpose reinforces the concept of the inductive power of the tissue microenvironment in redirecting the intrinsic nature of a tissue-specific stem cell. We are presently engaged in studies designed to elucidate the essential characteristics of MECs composing the mammary stem cell niche and the indispensable signals required from these cells that enables acquisition of nonmammary cells and redirection of their cell fate(s).
Materials and Methods
Mice.
The transgenic WAP-Cre/Rosa26R mice were engineered and typed according to Wagner et al. (13). Female Nu/Nu mice were used as hosts for the transplantation studies. All mice were housed in Association and Accreditation of Laboratory Animal Care-accredited facilities in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The National Cancer Institute Animal Care and Use Committee approved all experimental procedure.
MEC Preparation.
MECs were collected from primary mammary cultures after 4–7 days on plastic culture flasks in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum, insulin (1.0 μg/ml), and epidermal growth factor (10 ng/ml). Fibroblasts were reduced before collection of the epithelial cells by differential trypsinization.
NSC Isolation.
We followed an established protocol, based on selective expansion, for the isolation and expansion of NSCs from the fetal and adult mice brains. After dissection, triturated tissue is plated in a culture medium that supports the expansion of stem cells but not other cells. The medium is free of serum and serum replacement and contains only three proteins: apotransferin (for iron transport), insulin as a pro-survival signal, and basic fibroblast growth factor, a mitogen for NSCs (2).
Fetal cultures are normally passaged approximately every 5 days. After the first passage (which removes most of the remaining contaminants), the cultures are composed of 95% NSCs. Clonal and real-time lineage analyses confirm their self-renewal properties and multipotential (2, 14). Adult cultures are composed of both multipotent stem cells and a glial-restricted progenitor, but their morphologies are distinct, and because they are cultured under clonal conditions, they are easily distinguished.
Fetal cultures are derived from E13.5 mouse embryo cortex, whereas adult cultures are derived from the subventricular zone of 2- to 3-month-old mice. After dissection, the culture conditions are identical for these two stem cell sources.
Fetal NSC Culture.
E13.5 cortical embryonic mouse central nervous system stem cells were cultured as described previously (2, 3). In brief, cells were expanded in serum-free DMEM/F12 medium with N2 supplemented with basic fibroblast growth factor (FGF2) (20 ng/ml) for 5 days under low-oxygen conditions (5%), after which they were replated from either fresh or frozen stocks at 1,000–10,000 cells/cm2. FGF2 was used in all our experiments unless stated otherwise.
Adult NSC Culture.
Adult NSCs were isolated from the subventricular zone (SVZ) of mice. SVZ tissue was microdissected from the lateral ventricles of the brain (5), triturated using a 1-ml pipette, and plated in serum-free DMEM/F12 medium with N2 supplement with FGF2 (20 ng/ml) for 5 days under low-oxygen conditions (5%). FGF2 was used in all of our culture experiments for NSCs unless stated otherwise.
Recovery of NSCs from the Mammary Fat Pads of Implanted Mice.
After implantation, mammary duct growth, pregnancy, lactation, and involution were allowed to occur (≈14 weeks). Then mammary fat pad tissue was dissected and triturated in N2 medium containing FGF2 (20 ng/ml). Dissociated cells were plated in serum-free DMEM/F12 medium with N2 supplemented with FGF2 (20 ng/ml) for 5 days under low-oxygen conditions (5%). FGF2 was used in all of our culture experiments unless stated otherwise. A soluble form of the Notch ligand Dll4 (R&D Systems; final concentration, 200 ng/ml) and a JAK kinase inhibitor (Calbiochem; final concentration, 200 nmol) also were included in the culture medium to support NSC survival (14).
Cell and Tissue Transplantation.
The surgical techniques used to clear the mammary epithelium from the fat pads of 3-week old host mice and the subsequent transplantation of tissue fragments or cell suspensions have been described in detail previously (15, 16). In brief, the mice were anesthetized, and the clearing procedure was performed immediately before the insertion of transplanted fragments or cell suspension. Cell suspensions were implanted in 10-μl volumes with a Hamilton syringe equipped with a 30-gauge needle. The implanted females were placed with males 4–6 weeks after implantation to initiate pregnancy and secretory development.
X-Gal and Immunocytochemical Staining of Mammary Tissues.
Whole mounts of the entire inguinal gland were fixed and stained as described previously (13). In brief, the glands were spread on glass slides, fixed in paraformaldehyde (4.0%) for 1–2 h, permeabilized in 0.01% Nonidet P-40 in phosphate-buffered saline overnight at 4°C, and then processed for X-gal as described earlier. For histological examinations, X-gal-stained whole mounts were embedded in paraffin, sectioned at 6.0 μm, and counterstained with Nuclear Fast Red. Immunocytochemistry was performed on deparaffinized sections. The primary antibodies used were against total purified mouse caseins, smooth muscle actin, nestin, Sox2, ERα, PR, and β-galactosidase. Antigen retrieval was performed when investigating ERα and PR by heating sections (in a microwave) in a citrate buffer (pH 6.0) for 3 min, followed by cooling for 30 min. Secondary detection was done through Alexa 488, Alexa 647, and Alexa 568 conjugated species-specific antibodies (Invitrogen). Slides were coverslipped using ProLong Gold antifade reagent with DAPI (Invitrogen). Known positive and negative tissue controls fixed similarly were used to confirm the specificity of staining. Fluorescent images were collected using a Zeiss NLO confocal microscope.
Determination of Ploidy by Propidium Iodide Staining.
Dispersed cell preparations were washed twice in DMEM without serum, resuspended at 1–2 × 107 cells/ml, and placed on ice for 15 min. Cells were fixed in 70% ethanol, treated with 100 units of RNase (Sigma Aldrich) at 37°C for 20 min, and then stained for 30 min at 4°C in a 50-μg/ml solution of propidium iodide (Molecular Probes) in phosphate-buffered saline. Cell clumps were removed by filtering through 40-μm nylon mesh before analysis on a Calibur flow cytometer running CellQuest software (Becton Dickinson). Subsequent cell cycle and DNA content analyses were performed using FlowJo software (Tree Star).
Acknowledgments.
The intramural research program of the Center for Cancer Research, National Cancer Institute supported all aspects of the research presented here, with the exception of the isolation and propagation of the neural stem cells.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci USA. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 3.Androutsellis-Theotokis A, et al. In: Methods in Molecular Biology. Weiner LP, editor. Vol 438. Totowa, NJ: Humana; 2008. pp. 31–38. [Google Scholar]
- 4.Williams JM, Daniel CW. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 5.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 6.Wagner KU, et al. An adjunct mammary epithelial population in parous females: Its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 7.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are multipotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 8.Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–736. doi: 10.1002/jcp.21071. [DOI] [PubMed] [Google Scholar]
- 9.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 10.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Brisken C, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner KU, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Androutsellis-Theotokis A, et al. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 15.Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 16.Daniel CW, Deome KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]