Abstract
Intratumoral innate immunity can play a significant role in blocking the effective therapeutic spread of a number of oncolytic viruses (OVs). Histone deacetylase inhibitors (HDIs) are known to influence epigenetic modifications of chromatin and can blunt the cellular antiviral response. We reasoned that pretreatment of tumors with HDIs could enhance the replication and spread of OVs within malignancies. Here, we show that HDIs markedly enhance the spread of vesicular stomatitis virus (VSV) in a variety of cancer cells in vitro, in primary tumor tissue explants and in multiple animal models. This increased oncolytic activity correlated with a dampening of cellular IFN responses and augmentation of virus-induced apoptosis. These results illustrate the general utility of HDIs as chemical switches to regulate cellular innate antiviral responses and to provide controlled growth of therapeutic viruses within malignancies. HDIs could have a profoundly positive impact on the clinical implementation of OV therapeutics.
Keywords: HDAC inhibitor, oncolytic virus, refractory tumors, combination therapy
Oncolytic virotherapy is an innovative alternative to conventional cancer therapies based on the concept that it is possible to select or engineer viruses to preferentially replicate in and kill tumor cells (1–5). A variety of strategies are being developed to restrict oncolytic virus (OV) growth to malignancies (4), but one common cellular characteristic that likely plays a role in the selectivity of a spectrum of OVs is an acquired, tumor specific defect in cellular innate antiviral responses (6). As an example, tumors often develop a diminished response to the antiviral IFN cytokines, perhaps because of strong selective pressure to avoid immune surveillance (6). Although aberrations in the cellular antiviral response occur frequently in tumors, the magnitude of the defect is quite variable and can be a barrier to effective OV spread through a malignancy (7–9). Indeed more potent OVs are being developed that express viral gene products to combat cellular innate immune responses (10, 11); however, this genetic approach may ultimately limit the safety of the therapeutic. We reasoned that combining a viral therapeutic with a compound that reversibly compromises host antiviral genetic programs could provide a means to enhance OV growth in tumor cells. Histone deacetylase inhibitors (HDIs) are small molecules currently in clinical development that have demonstrated potent anti-tumor activity but are also known to prevent the transcriptional activation of antiviral genes after IFN stimulation or virus infection (12–19). Here, we demonstrate that a variety of HDIs markedly enhance OV killing of tumor cells in vitro and in vivo but do not increase OV growth in normal tissues. HDIs, as tumor specific viral sensitizers, have the potential to significantly increase the spectrum of malignancies amenable to OV therapy.
Results
HDI Treatment Sensitizes PC3 Prostate Cancer Cells to VSV-Mediated Oncolysis.
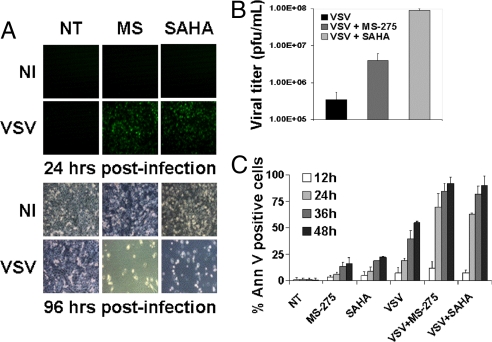
Vesicular stomatitis virus (VSV) is a prototypical rhabdovirus that grows poorly in normal tissues but replicates efficiently in cells lacking an intact IFN response (20), a property that prompted the development of VSV as an oncolytic agent for tumor cells with acquired defects in IFN signaling. We have shown that approximately 75% of tumor cell lines tested lack a normal IFN response (8); however, the extent of this defect is variable and, at low virus concentrations, IFN production may be sufficient to blunt VSV spread (21, 8, 9). As an example, the androgen independent prostate cancer cell line PC3 is partially responsive to IFN and at low multiplicities of infection, is refractory to VSV infection (21) (Fig. 1A). Because HDIs are known to interfere with the ability of cell lines to mount an IFN response, we examined the possibility that pretreatment of PC3 cells with the HDIs would sensitize them to VSV infection and subsequent virus-induced apoptosis. For these experiments, we used an attenuated strain of VSV encoding the GFP gene (VSV-Δ51-GFP) and 2 distinct HDI—MS-275 and SAHA (Vorinostat)—which have shown promising anti-cancer activity in preclinical (MS-275) and clinical (SAHA/Vorinostat) trials (22–31). Both HDIs dramatically increased VSV replication in PC3 cells as early as 24 h after infection, at which time robust GFP expression was detected by fluorescence microscopy and FACS analysis. Increased GFP expression correlated well with virus production from HDI treated cells [Fig. 1 A and B and supporting information (SI) Fig. S1A] and by 96 h, enhanced induction of apoptosis was observed in cells treated with HDI plus VSV, compared with cells treated with virus or HDI alone (Fig. 1 A and C).
Fig. 1.
HDIs enhance VSV oncolysis in partially resistant PC3 cancer cell line. PC3 cells were either not treated (NT) or pretreated with MS-275 or SAHA for 24 h, then infected or not (NI) with VSV-Δ51-GFP. (A) Viral replication was assessed by fluorescent microscopy for GFP expression after infection with VSV at 10−4 MOI. Phase-contrast microscopy at 96 h demonstrated massive cell death in combination-treated cells. (B) Viral titers as determined by standard plaque assay after VSV infection at 0.1 MOI. (C) Induction of apoptosis was established by Annexin-V staining after VSV infection at 0.1 MOI.
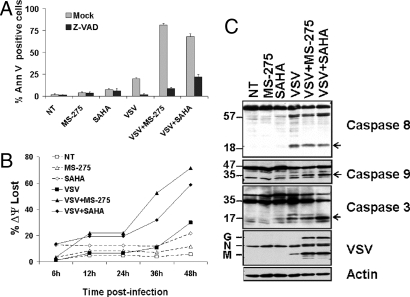
Addition of the caspase inhibitor z-VAD-fmk abrogated induction of apoptosis by VSV alone or in combination with MS-275 or SAHA, demonstrating that induction of cell death was caspase-dependent (Fig. 2A). Changes in mitochondrial membrane potential (JC-1 staining) were used as a measure of activation of the intrinsic apoptotic pathway (Fig. 2B). The combination of VSV and MS-275 or SAHA increased the number of cells exhibiting mitochondrial membrane depolarization to 72% and 59%, respectively, compared with VSV (30%), MS-275 (12%) or SAHA (21%) alone. Finally, the activation of apical and effector caspases was investigated by immunoblot analysis (Fig. 2C). Although VSV proteins were clearly expressed in the presence of HDAC inhibitors, the level of active caspase 8 was not affected, illustrating that HDIs did not affect the extrinsic apoptotic pathway. In contrast, activation of caspase 9 and downstream effector caspase 3 was observed after infection in the presence of MS-275 or SAHA (compared with VSV alone, Fig. 2C), indicating that combination treatment impacted at the level of the mitochondrial apoptotic pathway. These results reveal that HDIs act at 2 levels by increasing virus replication and spread, and affecting the intrinsic apoptotic pathway. The striking increase in oncolysis suggested the possibility that our virus pharmacophore and the HDIs interacted in a synergistic fashion. To test this idea, in vitro cytotoxicity of PC3 cells was assessed at varying concentrations of VSV and HDI in a fixed ratio design. Combination indices (CI) (10) were then used to qualify the interaction between VSV and MS-275 or SAHA. It is generally considered that CI values <0.7 indicate bona fide synergy. Both HDIs interacted with VSV in a highly synergistic fashion (CI < 0.4 at ED50). In addition, the decreasing CI values obtained with increasing cellular fractions affected (CI < 0.12 at ED90) indicate that the synergistic interaction between VSV and HDIs may be clinically relevant (Fig. S1B).
Fig. 2.
VSV plus HDI combination treatment synergistically increases apoptosis in PC3 cells. PC3 cells were either not treated (NT) or pretreated with MS-275 or SAHA for 24 h then infected with VSV at 0.1 MOI. (A) Induction of apoptosis was measured by Annexin V staining in the presence or absence of the pan-caspase inhibitor Z-VADfmk. (B) Mitochondrial membrane-depolarization was assessed by JC-1 staining. (C) Caspase 8, 3, and 9 cleavage was determined 48 h after VSV infection in the presence or absence of HDIs by immunoblot of whole cell lysates. Cleaved caspase forms are indicated by open arrows.
HDIs Enhance VSV Replication in PC3 Cells by Dampening the IFN Response.
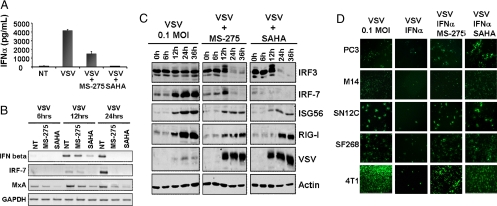
To gain an understanding of how MS-275 and SAHA enhanced VSV infection, virus activation of the IFN cascade in PC3 cells was examined in the presence or absence of HDIs. VSV infection of PC3 cells induced the expression of several gene products from the IFN cascade, including RIG-I, IFN alpha and beta, IRF-7, ISG56 and MxA, an IFN-inducible GTPase with direct involvement in the inhibition of VSV replication (32, 33) (Fig. 3 A–C, and Fig. S2A). HDI treatment led to the blunting of the cellular IFN response and robust virus protein production (Fig. 3C). RT-PCR revealed that infected PC3 cells treated with HDIs expressed less IFN-β mRNA at 12 h and essentially undetectable levels at 24 h after infection, in contrast to the infected untreated cultures (Fig. 3B). Similarly, the levels of MxA and IRF-7 mRNA were inhibited in HDI treated PC3 cells (Fig. 3B). However, HDIs did not impact on the IFN signaling cascade upstream of IRF-3. Indeed, virus-induced IRF-3 phosphorylation/degradation was easily detected in samples pretreated with HDIs but not in samples infected with VSV alone (Fig. 3C, first row). This absence of IRF-3 activation by VSV alone is in agreement with previous studies demonstrating that at low MOI, IRF-3 phosphorylation/degradation was not detected, yet was sufficient to induce expression of downstream ISGs that inhibited virus multiplication (34, 35). Treatment of PC3 cells with HDIs in the absence of virus infection did not affect IFN levels or IFN-inducible gene expression (data not shown).
Fig. 3.
HDIs augments VSV replication through inhibition of the IFN antiviral response. PC3 cells were either not treated (NT) or pretreated with MS-275 or SAHA for 24 h then infected with VSV at 0.1 MOI. (A) IFN-α levels in supernatants were assayed by ELISA at 24 h after infection. (B) Induction of antiviral genes IFN-beta, IRF-7 and Mxa was assessed at different times after infection by RT-PCR. NT denotes non-HDI treated cells (C) IFN-stimulated antiviral genes were analyzed by immunoblot at different times after VSV alone or VSV plus HDI treatment. (D) Different cell lines were pretreated with HDIs for 7 h and then infected with VSV-Δ51-GFP at 0.1 MOI in the presence or absence of recombinant IFNα. GFP expression was monitored 24 h after VSV inoculation.
Many tumor cells maintain a partial response to IFN that is sufficient to interfere with oncolytic virus spread in cultures (21). A collection of cancer cell lines with this phenotype was infected with VSV-Δ51-GFP in the presence or absence of IFNα (Fig. 3D) and GFP expression was used as a measure of virus infection and spread. As predicted, virus infection was severely impaired by addition of IFNα to the culture media, but this protective effect was reversed by pretreatment of cells with MS-275 or SAHA (Fig. 3D and Fig. S3). These data support the notion that HDIs act to inhibit expression of both IFN and IFN-inducible genes. The potentiation of virus spread was not restricted to oncolytic VSV, as we found that both vaccinia virus (36) and Semliki Forest virus also rapidly spread through tumor cell cultures exposed to HDIs (Fig. S2 B and C).
HDIs Specifically Enhance VSV Spread in Primary Human Tumor Specimens.
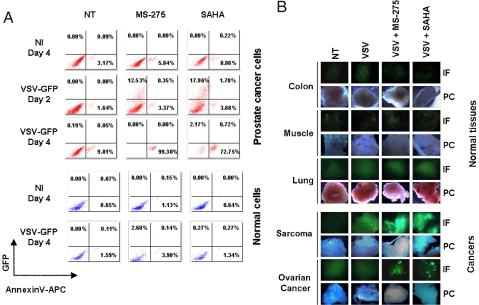
To determine whether HDI enhancement of VSV replication and tumor cell killing was effectively translated to primary human samples, malignant and adjacent normal prostate cell cultures were established from radical prostatectomy samples. Dissociated cultures infected with VSV-Δ51-GFP in the presence or absence of HDIs were analyzed for virally expressed GFP and Annexin-V staining by flow cytometry (Fig. 4A). No evidence of VSV replication or virus-induced apoptosis was observed in either normal or tumor cultures in the absence of HDIs. However, prostate cancer cells became GFP positive and were ultimately killed after VSV infection in the presence of either MS-275 or SAHA (Fig. 4A, upper panels). In contrast, normal prostate cells from the same patient remained refractory to VSV infection, even when exposed to HDIs (Fig. 4A, lower panels). In both normal and tumor tissue, the efficacy of HDI treatment was confirmed by monitoring acetylation of histone H3 by immunoblot in PC3 cells and normal PBMCs (Fig. S4A).
Fig. 4.
HDI pretreatment enhances VSV oncolytic activity in primary tumor specimens. (A) Ex-vivo primary cancer or normal prostate cells were subjected to 24 h HDI pretreatment followed by VSV-Δ51GFP infection (5 MOI). VSV replication (GFP, y axis) and apoptosis induction (AnnexinV-APC staining, x axis) were determined at 2 and 4 days after infection by FACS. NT denotes non-HDI treated cells, NI denotes non-VSV-infected samples. (B) Human ex-vivo cancer or normal tissue specimens were inoculated with VSV-Δ51-GFP in the absence or presence of HDI pretreatment for 7 h. GFP expression was monitored 48 h after viral inoculation by fluorescence microscopy (IF). Phase contrast (PC) images of tissue samples are shown. NT denotes non-HDI treated, non-VSV-infected cells.
Next, the ability of HDIs to stimulate virus growth in intact primary tumor samples was evaluated in human explants obtained from patients undergoing surgical resection. Tissue slices were incubated with VSV in the presence or absence of the drug; similar to the dispersed primary cultures, HDIs specifically enhanced VSV replication and spread only in primary tumor explants but not in normal tissue slices. For example, in both ovarian cancer and sarcoma samples, intense GFP fluorescence and virus replication were detected in the combination treated samples (Fig. 4B and Fig. S4B), whereas slices of normal colon, muscle, and lung tissue remained refractory to virus infection even in the presence of HDIs. Furthermore, PBMCs isolated from healthy donors (Fig. S4C) remained resistant to VSV infection and killing at high MOI even with HDI pretreatment. Taken together, these results demonstrate the ability of HDIs to specifically enhance virus replication in primary tumor tissues but not in normal human samples.
In vivo, Systemic Coadministration of MS-275 with VSV Augments Viral Oncolytic Activity Strictly at the Tumor Site.
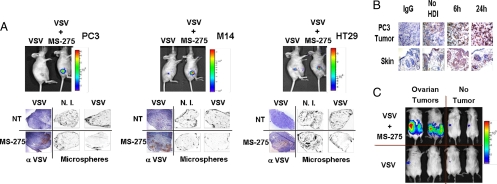
Five different in vivo cancer models were used to investigate the safety and anti-tumor efficacy profiles of HDI plus VSV combination therapy. These models included mice bearing PC3 (prostate), M14 (melanoma), HT29 (colon), 4T1 (breast), and SW620 (colon) s.c. tumors, and a spontaneous bilateral transgenic ovarian cancer model. MS-275 was chosen for the treatment of all models because of the noticeable enhancing effects demonstrated in vitro (Fig. S5 A and B), and the safety profile demonstrated in multiple phase I clinical trials (24, 37, 38). In all cases, the drug was administered i.p. (IP), while an assortment of virus delivery routes was tested. For these experiments, VSV-Δ51 was engineered to express firefly luciferase (VSV-Δ51-Luc) so that virus replication could be monitored in vivo using an In Vivo Imaging System (IVIS). In initial studies, mice bearing s.c. PC3, M14 or HT29 tumors were treated by direct intratumoral (IT) injection, along with daily drug (or vehicle) administration (Fig. S5C). In each tumor model, MS-275 therapy was able to promote VSV replication in vivo, as measured by firefly luciferase activity and by immunohistochemical (IHC) staining for VSV antigens (Fig. 5A). Hyperacetylation was furthermore confirmed in the PC3 tumors by immunohistochemistry at 6 and 24 h after treatment (Fig. 5B). Notably in these and subsequent experiments, the luciferase signal was restricted to the tumor mass (Figs. 5 and 6 and Fig. S6A) and infection of normal tissues in any of the mice receiving combination therapy was not detected (data not shown). Recently, we demonstrated that OV infection of tumors initiates a rapid and profound loss of blood flow to the tumor that can be measured by decreased uptake of intravenously administered fluorescent microspheres (39). This “vascular shutdown” phenomenon was clearly replicated in perfusion studies performed after coadministration of VSV and MS-275 in these 3 models (Fig. 5A).
Fig. 5.
HDI treatment specifically enhances VSV-Δ51-Luc replication at the tumor site. (A) PC3, M14, and HT29 s.c. xenograft tumor models were established in nude mice and treated with a single intratumoral VSV-Δ51-Luc injection (1 × 106 pfu) alone or in combination with MS-275 IP every 24 h. Viral replication at the tumor site was imaged using the IVIS system. Fluorescent orange microspheres were perfused to outline the tumor microvasculature (Microspheres). Frozen tumor sections were stained with anti-VSV antisera (α-VSV). NT = non-HDI treated, N.I. = non-VSV-infected. (B) Acetylation of histone H3 proteins was assessed in PC3 tumors by IHC at 6 h and 24 h after single IP delivery of MS-275. Skin sections were used as normal controls. (C) Transgenic mice bearing bilateral ovarian tumors were administered a single dose of VSV IP (1 × 108 pfu) alone or in combination with MS-275 IP. Viral replication in live animals was assessed 48 h after viral infection by IVIS imaging.
Fig. 6.
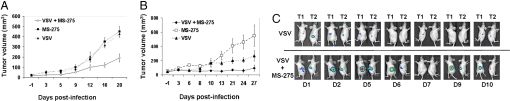
HDI plus VSV combination treatment augments tumor-specific viral replication and significantly reduces tumor size. (A) Immunocompetent BALB/c mice bearing s.c. 4T1 tumors were treated with VSV (1 × 108 pfu), alone or in combination with MS-275 IP. The efficacy of MS-275, VSV and VSV/MS-275 combination treatment in reducing tumor growth was assessed by tumor volume measurement over time. The average tumor size and standard error for each treatment group was calculated. (B) and (C) CD1 nude mice bearing SW620 tumors in hind flanks were treated with a single i.v. VSV injection (1 × 107 pfu) alone or in combination with MS-275. The efficacy of MS-275, VSV and VSV/MS-275 combination treatment in reducing tumor growth was assessed by tumor volume measurement over time (B). Virus replication at the tumor site was revealed in live animals by IVIS imaging (C). Time course of treatments are schematically presented in Figs. S5 and S6.
We next tested a spontaneous transgenic tumor model of ovarian cancer with the combination of MS-275 and VSV, both delivered by intraperitoneal injection. In Fig. 5C, IVIS imaging demonstrated that VSV replication was augmented by HDI treatment and restricted to the bilateral tumors arising on the ovaries of these mice. In both tumor bearing and tumor free mice treated with VSV alone, low level luciferase activity was occasionally observed in the spleen but this activity was transient and was not significantly enhanced by HDI treatment.
The 4T1 breast cancer model is highly metastatic when implanted s.c. and is refractory to VSV therapy (40) (Fig. 6A and Fig. S6A). In mice infused IV with VSV alone, a weak signal emanating from infected metastatic nodules was observed between 24 and 48 h but ultimately VSV was cleared (Fig. S6A) and treatment had no effect on disease progression. However, when combined with HDI therapy, virus replication was evident both in the primary s.c. lesion, and at multiple metastatic sites; replication persisted past 80 h and had a dramatic impact on tumor size (Figs. 6A and Fig. S6A). A more marked effect on tumor size was observed in the SW620 model of colon cancer, where i.v. VSV infection of s.c. SW620 tumors concomitant with daily MS-275 therapy resulted in a steady increase in luminescence at the tumor site and virtually no tumor growth in the combination treatment group (Fig. 6B).
Not only did MS-275 show tumor specific enhancement of VSV replication, but the HDI also behaved as a regulatory chemical switch that can be used to control virus replication within the tumor (Fig. 6C). This unique characteristic of the combination therapy was demonstrated in the SW620 model, where MS-275 therapy was halted on day 4 and the luciferase signal was lost from the tumor by day 7. Reinitiation of HDI therapy at day 7.5 resulted in a reemergence of the luciferase signal at day 9 and continued regression of the tumor (Fig. 6C and Fig. S6B). This observation demonstrates that VSV replication closely correlated with the pharmacokinetics of the HDI used and the combination strategy requires effective protein hyperacetylation within the tumor to reach maximal effective oncolytic activity.
Discussion
Oncolytic viruses are often engineered to exploit tumor specific genetic deficiencies or constitutively activated signaling pathways (1–5). Regardless of the mechanism of selective targeting, all oncolytic viruses must also overcome the arsenal of antiviral programs that the individual cell has at its disposal to resist infection and/or the spread of viruses. One strategy to generate a more potent OV is to arm the virus with genes that express products to circumvent or blunt the innate antiviral response of the individual cell (10, 11). A different approach is to engineer viruses that are unable to resist cellular antiviral programs (8, 41), thus increasing their safety profile because they cannot replicate in normal tissues but retain the ability to grow in malignant tissues. During the evolution of malignancies, genetic abnormalities accumulate that provide cancer cells with growth and survival advantages but at the same time compromise the ability of individual tumor cells to mount a robust antiviral response (8, 32, 42–44). The inability of a tumor cell to secrete or respond to IFN may facilitate tumor escape from immune surveillance (44). Although defects in cellular innate immunity are commonly found in tumor cells, the extent of the defect is quite variable (8, 21, 40). Because histone deacetylases have been implicated in modulating the IFN response in cell lines (12–19, 45), we hypothesized that HDIs could complement OVs and facilitate the infection and killing of tumors that had an impaired antiviral response. Our study design used relatively low mulitiplicities of infection (MOI) in vitro, to closely approximate the situation in vivo; furthermore we expected the effects of HDIs on virus spread to be most pronounced at low MOI. An unanticipated finding from this study was the effect of HDIs on the apoptotic program of infected cells; the intrinsic mitochondrial pathway appears to be preferentially targeted by the combination of HDIs and VSV, as demonstrated by an increase in mitochondrial depolarization and cleavage of caspases 9 and 3 in combination treated cells. The effect on cell death was synergistic and increased at higher effective doses of combined treatment, supporting a clinically relevant interaction between HDIs and VSV. In addition to a direct virus-mediated induction of tumor cell death, oncolysis may also be mediated by indirect mechanisms triggering apoptosis through the release of inflammatory cytokines and mediators from infected and dying tumor cells. In vivo, the effective dose of an OV that reaches a tumor is further compromised by the limitations imposed by tumor architecture and microenvironment. However, despite poor virus delivery, a significant portion of cells within OV-treated tumors undergoes apoptosis. We recently demonstrated that loss of blood flow to the interior of the tumor causes massive cellular apoptosis. Although the mechanisms remain to be defined, the absence of vascular perfusion within infected tumors was induced by neutrophil recruitment to the tumor bed (39). These current studies reinforce the involvement of “vascular shutdown” as an important mechanism of tumor cell killing, and further illustrate that the combination of OV and HDI augments vascular shutdown in the tumor bed.
Importantly, the combination of VSV and HDIs enhances tumor killing but does not significantly increase VSV infection of normal human or mouse tissues. Although the exact basis for this exquisite selectivity remains to be determined, the results are consistent with the idea that HDIs blunt the cellular IFN response in tumor cells. In combination treated PC3 cells, key antiviral genes such as IRF7, ISG56 and MXA were expressed at low levels and were poorly inducible by virus infection. Our results and those of others are consistent with the effects of HDIs occurring at the level of gene transcription (12–19, 45), but clearly HDAC activity is found in other cellular compartments besides the nucleus and has been implicated in the cellular response to a variety of stresses (46, 47). Although we favor a key role for HDIs in dampening IFN activity in tumor cells, the existence of additional stress responses affected by HDI that impact on virus replication cannot be excluded. Furthermore, different classes of HDIs may have distinct effects depending on the tumor or the oncolytic virus, in part because HDIs target different classes of HDACs (24, 27, 28).
A potential clinical advantage of this combination therapy approach is highlighted by the observation that continuous systemic administration of HDI is required to maintain robust virus replication within the tumor; it may thus be possible to regulate the magnitude of OV therapy by withdrawing or applying HDI. This strategy could be particularly advantageous when the OV therapeutic harbors a gene that facilitates imaging of the infection, or if the malignancy is located in an area (e.g., brain) where tumor swelling—as a consequence of virus infection—could lead to unwanted complications. HDI induced sensitization of tumor cells to viral oncolysis was not restricted to VSV, because both Semliki Forest virus and vaccinia virus also displayed increased oncolytic activity in the presence of HDIs. This observation is immediately relevant because vaccinia virus has entered into clinical trials and the effects of HDIs on the magnitude of oncolysis may be applicable to a wide spectrum of OVs under development. In conclusion, the diverse and safe applicability of HDI plus oncolytic virus combination therapy should facilitate rapid translation toward clinical application. Investigations are under way to delineate how HDIs may modulate transcriptional programs in tumor cells and how the host adaptive immune response may further enhance the anti-tumor effects of oncolytic viruses.
Methods
Viruses.
VSV-Δ51 expressing GFP and GFP-firefly luciferase fusion are recombinant derivatives of VSV-Δ51, a naturally occurring IFN-inducing mutant of VSV Indiana serotype (8). Viruses were propagated and purified as described (7, 8) in Vero cells (American Type Culture Collection).
Primary ex-Vivo Prostate Cancer Cell Cultures.
Radical prostatectomy specimens and their adjacent normal tissues (as histologically defined by a pathologist) were washed immediately in cold, sterile PBS (PBS). After removing excess, damaged epithelium and stromal tissue, specimens were cut into small pieces and incubated for 10 min at 37 °C in 0.05% trypsin/0.53 mM EDTA (Wisent). Surface epithelium was mechanically separated to dissociate cells into a single cell suspension. Prostate epithelial cells were grown in KSF medium supplemented with 5 mg/100 ml of bovine pituitary extract (BPE) (Gibco/BRL).
Flow Cytometry.
After staining with AnnexinV-APC (BD biosciences) or JC-1 stain (Invitrogen Canada Inc.) as per manufacturer's instructions, cells were subjected to flow cytometry analysis (104 events/measurement) on a FACS Calibur (Becton-Dickinson) and analyzed with FCS Express V3 software (8, 48, 49).
In Vivo Tumor Models.
All mice used were obtained from Charles River Laboratories. HT29 and M14 xenograft models (n = 2) were established in the hind flanks of 6–8-week-old female; nu/nu mice PC3 xenograft models (n = 2) were established in male nu/nu mice. After tumors became palpable, the double treated group received MS-275 i.p. at a concentration of 23 mg/kg/day. Four hours after administering the second HDI dose, all mice were injected intratumorally with 5 × 106 pfu of VSV-Luc. The double treated and MS-275 treated groups continued to receive 23 mg/kg of MS-275 i.p. every 24 h until killed (Fig. S5C). The ovarian transgenic tumor model tgMISIIRTAg564 (Garson et al., manuscript in preparation) is based on the transgenic model from Connolly et al. (50). At approx. 13 weeks of age, mice were administered MS-275 IP every 12h at 6 mg/kg, while VSV was administered IP at 1 × 108 pfu 4h after the initial HDI dose. Viral replication in live animals was assessed 48h after viral infection by IVIS imaging.
Tumor growth analysis was carried out in 2 models: human colon carcinoma SW620 xenografts in nude mice and 4T1 syngeneic breast carcinoma model in immuncompetent animals, all groups with n = 4–5. When tumors were palpable, MS-275 was administered i.p. at a concentration of 7 mg/kg every 12 h for 10 consecutive doses. VSV-Luc (1 × 107 pfu) was administered intravenously 4 h after the second MS-275 dose. This treatment cycle was repeated once more 72 h after the last MS-275 dose. Tumor sizes were measured every 3–4 days using an electronic caliper. The average tumor size from each treatment group was calculated for each time point and standard error was calculated to determine statistical significance.
IVIS Imaging.
Mice were injected i.p. with d-luciferin (200 ml at 10 mg/ml in PBS, Molecular Imaging Products Company), anesthesized <3% isofluorane (Baxter Corp.) and imaged with the In Vivo Imaging System 200 Series (Xenogen Corporation). Data acquistion and analysis was performed using Living Image v2.5 software.
Analysis of Tumor Perfusion.
Mice were injected intravenously with orange fluorescent microspheres and killed as described in ref. 39. Tumor perfusion was analyzed by visualizing fluorescent microspheres in the vasculature of 10 μm unfixed frozen sections using a ScanArray Express microarray scanner with a standard Cy3 laser (Packard Bioscience).
Supplementary Material
Acknowledgments.
This work was supported by grants from the National Cancer Institute of Canada, the Canadian Institutes for Health Research, and the Terry Fox Foundation (to J.H. and J.B.), the Cancer Research Society (to B.V.), a Fellowship from the Fonds de la Recherche en Santé du Québec (to T.L.-A.N. and J.-S.D.), a Studentship from the Natural Sciences and Engineering Research Council (to S.L.), a Joe and Amy Ip Fellowship (to J.-S.D.), and a Canadian Institutes of Health Research Senior Investigator award (to J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803988105/DCSupplemental.
References
- 1.Bell JC. Oncolytic viruses: What's next? Curr Cancer Drug Targets. 2007;7:127–131. doi: 10.2174/156800907780058844. [DOI] [PubMed] [Google Scholar]
- 2.Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: The education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7:133–139. doi: 10.2174/156800907780058862. [DOI] [PubMed] [Google Scholar]
- 3.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 4.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 5.Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: A new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 7.Cesaire R, et al. Oncolytic activity of vesicular stomatitis virus in primary adult T-cell leukemia. Oncogene. 2006;25:349–358. doi: 10.1038/sj.onc.1209055. [DOI] [PubMed] [Google Scholar]
- 8.Stojdl DF, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 9.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altomonte J, et al. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol Ther. 2008;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haralambieva I, et al. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang HM, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci USA. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genin P, Morin P, Civas A. Impairment of interferon-induced IRF-7 gene expression due to inhibition of ISGF3 formation by trichostatin A. J Virol. 2003;77:7113–7119. doi: 10.1128/JVI.77.12.7113-7119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph J, et al. Expression profiling of sodium butyrate (NaB)-treated cells: Identification of regulation of genes related to cytokine signaling and cancer metastasis by NaB. Oncogene. 2004;23:6304–6315. doi: 10.1038/sj.onc.1207852. [DOI] [PubMed] [Google Scholar]
- 15.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors-development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 16.Mehnert JM, Kelly WK. Histone deacetylase inhibitors: Biology and mechanism of action. Cancer J. 2007;13:23–29. doi: 10.1097/PPO.0b013e31803c72ba. [DOI] [PubMed] [Google Scholar]
- 17.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 18.Nusinzon I, Horvath CM. Positive and negative regulation of the innate antiviral response and beta interferon gene expression by deacetylation. Mol Cell Biol. 2006;26:3106–3113. doi: 10.1128/MCB.26.8.3106-3113.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlasakova J, et al. Histone deacetylase inhibitors suppress IFNalpha-induced up-regulation of promyelocytic leukemia protein. Blood. 2007;109:1373–1380. doi: 10.1182/blood-2006-02-003418. [DOI] [PubMed] [Google Scholar]
- 20.Lichty BD, et al. Vesicular stomatitis virus: A potential therapeutic virus for the treatment of hematologic malignancy. Hum Gene Ther. 2004;15:821–831. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed M, Cramer SD, Lyles DS. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. doi: 10.1016/j.virol.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Duvic M, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eyupoglu IY, et al. Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol Cancer Ther. 2006;5:1248–1255. doi: 10.1158/1535-7163.MCT-05-0533. [DOI] [PubMed] [Google Scholar]
- 24.Hess-Stumpp H, Bracker TU, Henderson D, Politz O. MS-275, a potent orally available inhibitor of histone deacetylases–the development of an anticancer agent. Int J Biochem Cell Biol. 2007;39:1388–1405. doi: 10.1016/j.biocel.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Mann BS, et al. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 26.Mann BS, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 27.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 28.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 29.Olsen EA, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 30.Qian DZ, et al. Antitumor activity of the histone deacetylase inhibitor MS-275 in prostate cancer models. Prostate. 2007 doi: 10.1002/pros.20611. [DOI] [PubMed] [Google Scholar]
- 31.Richardson P, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49:502–507. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 32.Noser JA, et al. The RAS/Raf1/MEK/ERK Signaling Pathway Facilitates VSV-mediated Oncolysis: Implication for the Defective Interferon Response in Cancer. Cells Mol Ther. 2007;15:1531–1536. doi: 10.1038/sj.mt.6300193. [DOI] [PubMed] [Google Scholar]
- 33.Schwemmle M, et al. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 34.tenOever BR, et al. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78:10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin R, et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem. 2006;281:2095–2103. doi: 10.1074/jbc.M510326200. [DOI] [PubMed] [Google Scholar]
- 36.McCart JA, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 37.Kummar S, et al. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res. 2007;13:5411–5417. doi: 10.1158/1078-0432.CCR-07-0791. [DOI] [PubMed] [Google Scholar]
- 38.Ryan QC, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 39.Breitbach CJ, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 40.Ebert O, Harbaran S, Shinozaki K, Woo SL. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 41.Kirn DH, et al. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040353. e353- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balachandran S, et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 44.Stojdl DF, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 45.Taplin ME. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 46.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008;90:306–312. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca Tumilasci V, et al. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary Chronic Lymphocytic Leukemia to VSV-induced oncolysis. J Virol. 2008;82:8487–8499. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharif-Askari E, et al. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology. 2007;365:20–33. doi: 10.1016/j.virol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Connolly DC, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.