Abstract
Significant anti-tumor responses have been reported in a small subset of cancer patients treated with the immunotherapeutic agent anti-CTLA-4 antibody. All clinical trials to date, comprising over 3,000 patients, have been conducted in the metastatic disease setting, which allows for correlation of drug administration with clinical outcome but has limited analyses of intermediate biomarkers to indicate whether the drug has impacted human immune responses within the tumor microenvironment. We conducted a pre-surgical clinical trial in six patients with localized bladder cancer, which allowed for correlation of drug administration with biomarkers in both blood and tumor tissues but did not permit correlation with clinical outcome. We found that CD4 T cells from peripheral blood and tumor tissues of all treated patients had markedly increased expression of inducible costimulator (ICOS). These CD4+ICOShi T cells produced IFN-gamma (IFNγ) and could recognize the tumor antigen NY-ESO-1. Increase in CD4+ICOShi cells led to an increase in the ratio of effector to regulatory T cells. To our knowledge, these are the first immunologic changes reported in both tumor tissues and peripheral blood as a result of treatment with anti-CTLA-4 antibody, and they may be used to guide dosing and scheduling of this agent to improve clinical responses.
Optimal T cell activation requires contemporaneous signals through the T cell receptor and costimulatory molecules (1). CD28, the prototypical costimulatory molecule, upon interaction with its ligands B7–1 and B7–2, plays a crucial role in initial T cell priming (2, 3). CD28-mediated T cell expansion is opposed by another B7–1,2 counter receptor, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), which attenuates the proliferation of recently activated T cells (4, 5). Blockade of the inhibitory signals mediated by CTLA-4 has been shown to enhance T cell responses and induce tumor rejection in a number of animal models (6, 7). Mouse studies have demonstrated that an increased ratio of effector to regulatory T cells (Teff/Treg) correlates with anti-tumor responses (8). A monoclonal antibody to human CTLA-4 has been found to elicit objective responses in clinical trials (9–11), including durable complete responses in a small subset of patients with metastatic disease.
The phenotypic and functional impact of anti-CTLA-4 therapy on human immune responses has not been established. Identification of biomarkers, such as changes in the Teff/Treg ratio identified in mouse studies, have been lacking for primarily two reasons: (i) There has not been a marker that clearly identifies effector T cells in patients treated with anti-CTLA-4 therapy, and (ii) access to tumor tissues has been limited in the metastatic disease setting, thus preventing identification of relevant biomarkers within the tumor microenvironment that can be correlated with biomarkers in peripheral blood. A few studies using only peripheral blood samples from patients have shown that treatment with anti-CTLA-4 antibody resulted in increased expression of markers associated with T cell activation such as HLA-DR or CD45RO, but these changes were not consistent in all treated patients, were not shown to have functional significance, nor did they correlate with clinical outcome (11–13). Knowledge of biomarkers that indicate biological changes accompanying drug administration or clinical outcome is necessary to understand the cellular and molecular mechanisms responsible for the effects induced by this agent to optimize its therapeutic benefits and minimize immune-related adverse events.
Inducible costimulator (ICOS) is a T-cell-specific surface molecule that is structurally related to CD28 and CTLA-4 (14, 15). The role of ICOS in immune responses has been strongly linked to production of Th2 cytokines. ICOS-deficient mice demonstrated decreased production of the Th2 cytokine interleukin 10 (IL-10) (16). IL-10 production by regulatory T cells has been associated with the suppression of effector T cell responses in a cell-extrinsic manner (17, 18), and it was reported that plasmacytoid dendritic cells with high expression of ICOS-ligand allowed for the differentiation of naïve CD4 T cells into IL-10-producing regulatory T cells as a result of ICOS/ICOS-ligand interactions (19). Based on these and other reports of IL-10 production by ICOS-expressing CD4 T cells (20), it has been thought that ICOS-expressing T cells might play a role in suppression of immune responses. However, very recent data suggested that ICOS-expressing T cells might also be involved in autoimmune responses. The sanroque mice, which have a defect that results in augmented stability of ICOS mRNA and thus have increased ICOS expression on CD4 T cells, exhibited an abnormally high accumulation of T lymphocytes and a lupus-like autoimmune syndrome (21). This finding suggests that increased ICOS expression may also play a role in enhancing immune responses. ICOS has also been shown to be associated with increased survival of both effector memory and regulatory T cells, demonstrating that its functional relevance is not restricted to regulatory T cells (22).
The role of ICOS-expressing T cells in cancer patients is currently unknown. In the course of examining the phenotypic and functional impact of anti-CLTA-4 treatment in patients with localized bladder cancer, we found that CTLA-4 blockade resulted in an increase in the number of CD4 T cells that express high levels of ICOS. This increase was detectable in both the peripheral blood and, even more strikingly, in the tumor tissues of treated patients. Total CD4 T cells as well as the CD4+ICOShi T cell population from treated patients had increased production of the Th1 cytokine IFN-gamma (IFNγ) after in vitro stimulation with anti-CD3 antibody. CD4+ICOShi T cells from three patients whose tumors expressed the cancer-testis tumor antigen NY-ESO-1 produced IFNγ in response to a set of overlapping NY-ESO-1 peptides. The ratio of effector (CD4+ICOShi) to regulatory (CD4+FOXP3+) T cells was increased in both the peripheral blood and tumors of anti-CTLA-4-treated patients. These data indicate that a major impact of CTLA-4 blockade in cancer patients was the expansion, both within the blood and the tumor itself, of a subpopulation of CD4 T cells that expressed high levels of ICOS and secreted IFNγ, a cytokine which that is known to play a role in antitumor responses (23, 24). Taken together, these data suggest that the ICOShi IFNγ-producing population includes tumor-reactive cells and may be involved in the clinical activity of anti-CTLA-4 antibody.
Results
Immunologic Consequences of CTLA-4 Blockade in Peripheral Blood: ICOS Expression by Peripheral Blood CD4 T Cells Is Increased by CTLA-4 Blockade.
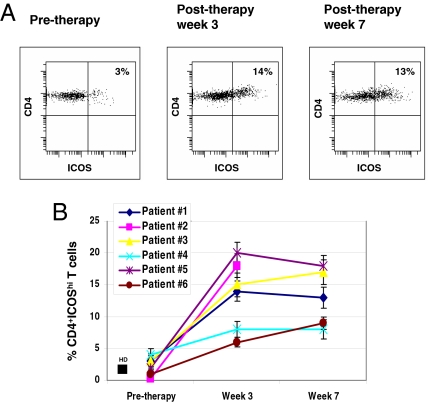
The schema for the neoadjuvant (pre-surgical) bladder cancer trial is shown in supporting information (SI) Fig. S1. We examined peripheral blood T cells from pre- and post-therapy samples for a number of potential markers of changes that might be associated with CTLA-4 blockade, including CD4, CD8, HLA-DR, CD69, CD45RO, and CD45RA. None of these showed consistent, significant changes after anti-CTLA-4 treatment in our group of patients with localized bladder cancer (data not shown). However, ICOS expression was considerably elevated, both in intensity and frequency, on CD4 T cells in the blood of all six patients after anti-CTLA-4 treatment. ICOS expression was also increased on CD8 T cells after anti-CTLA-4 treatment, but the magnitude of ICOS expression was much less on CD8 cells compared with CD4 T cells (data not shown). As shown in Fig. 1A, high levels of ICOS expression on CD4 T cells (CD4+ICOShi) from patient 1 increased from 3% pre-therapy to 14% at week 3 post-therapy and remained elevated at week 7. The presence of localized tumor by itself does not alter the frequency of CD4+ICOShi T cells in the systemic circulation, because their frequency (4 ± 3%, n = 10) in the peripheral blood of untreated bladder cancer patients was not significantly different from that of healthy donors (2 ± 1%, n = 10) (Figs. 1B and 2A). Increases of two- to sevenfold in ICOS expression were observed in all six bladder cancer patients after treatment with anti-CTLA-4 (Fig. 1B) (P < 0.05). Thus, an increase in the frequency of CD4+ICOShi T cells is a consistent and readily identifiable consequence of the administration of anti-CTLA-4 antibody to cancer patients.
Fig. 1.
ICOS expression by CD4 T cells in the blood of bladder cancer patients was increased after treatment with anti-CTLA-4. (A) ICOS expression on peripheral blood CD4 T cells was increased after anti-CTLA-4 treatment (patient 1). (B) Summary of the effect of anti-CTLA-4 therapy on CD4+ICOShi T cells in the blood of all six treated patients (with the exception of the week 7 sample for patient 2, who did not have blood drawn at week 7) as compared with the average frequency of CD4+ICOShi T cells in 10 healthy donors (HD).
Fig. 2.
Coexpression of ICOS and FOXP3 by CD4 T cells from peripheral blood. (A) The populations of CD4+ICOShi and CD4+ICOSlow T cells had subsets of both FOXP3+ and FOXP3− cells in healthy donors and untreated bladder cancer patients. Data shown are from one representative sample; numbers shown are mean percentages ± standard deviations calculated from samples from 10 healthy donors and 10 patients with untreated bladder cancer. (B) CD4+ICOShi and CD4+ICOSlow cells in pre- and post-therapy (weeks 3 and 7) samples from one patient (patient 1) treated with anti-CTLA-4 antibody also had subsets of both FOXP3+ and FOXP3− cells. (C) FOXP3 expression in total measured CD4 T cells (CD4+FOXP3+ cells) in pre- and post-therapy samples from one patient (patient 1) treated with anti-CTLA-4 therapy. (D) Variable effects of anti-CTLA-4 therapy on CD4+FOXP3+ T cells in the blood of all six treated patients (patient 2 did not have blood drawn at week 7).
FOXP3 Expression by Peripheral Blood CD4 T Cells Is Not Consistently Affected by CTLA-4 Blockade.
Because ICOS has been shown to be expressed by regulatory T cells as well as activated CD4 effector cells, we examined ICOShi cells for expression of the transcription factor FOXP3, which is strongly associated with regulatory T cell function (25). As shown in Fig. 2A, FOXP3+ cells comprised ≈5% of total measured CD4 T cells in normal healthy donors (Fig. 2A). Approximately 2% of CD4 T cells from the blood of normal healthy donors expressed high levels of ICOS, and of these, ≈23% expressed FOXP3 (Fig. 2A). Only ≈4% of CD4+ICOSlow T cells of normal healthy donors expressed FOXP3. In the blood of untreated cancer patients, the overall frequency of FOXP3+ cells among total CD4 T cells was variable but tended to be higher (21 ± 17%, n = 10) and was distributed between both the ICOShi and the ICOSlow compartments (Fig. 2A).
Similarly, CD4+ICOShi T cells from patients treated with anti-CTLA-4 antibody contained both FOXP3+ and FOXP3− subpopulations. Data for patient 1 are shown in Fig. 2B. FOXP3 expression in CD4+ICOShi T cells from patient 1 increased from 20% pretherapy to 33% at week 3 post-therapy but then decreased to 12% at week 7. Thus, the blood of normal healthy donors, untreated bladder cancer patients, and anti-CTLA-4-treated patients contained substantial populations of CD4 T cells that express high levels of ICOS but not FOXP3 and are therefore unlikely to be regulatory T cells.
Changes in the frequency of FOXP3+ cells among total CD4 T cells reflected changes in FOXP3 expression that occurred in the ICOShi and ICOSlow cells after treatment with anti-CTLA-4 therapy. For patient 1, FOXP3 expression as a fraction of total CD4 T cells (CD4+FOXP3+) increased from 4% pre-therapy to 9% at week 3 post-therapy but then decreased to 6% at week 7 (Fig. 2C). As shown in Fig. 2D, which shows the results from all six treated patients, anti-CTLA-4 therapy had inconsistent effects on FOXP3 expression. Patients 1, 3, and 5 had increased frequency of FOXP3 expression after the first dose of antibody, followed by decreases after the second dose. Patients 2 and 4 had decreases in FOXP3 expression after the first dose of antibody, and this remained low after the second dose of antibody, at least for patient 4 (patient 2 did not have blood drawn at week 7). Therefore, FOXP3 expression by CD4 T cells from peripheral blood of treated patients was not consistently altered by CTLA-4 blockade at doses of 3 mg/kg per dose of antibody.
CD4+ICOShi T Cells in the Peripheral Blood of Anti-CTLA-4 Treated Patients Contain IFNγ-Producing Effector Cells That Recognize the Tumor Antigen NY-ESO-1.
Because our data revealed that a substantial fraction of CD4+ICOShi T cells lacked FOXP3 expression and were therefore unlikely to be regulatory T cells, we examined the effect of CTLA-4 blockade on the function of circulating CD4 and CD4+ICOShi T cells. CD4 T cells from peripheral blood of healthy donors, patients with untreated bladder cancer (including pretherapy samples from patients), and patients who received treatment with anti-CTLA-4 antibody (post-therapy samples from weeks 3 and 7) were cultured in vitro for 3 days with anti-CD3 plus IL-2. All CD4 T cells proliferated equally well (Fig. S2). However, as shown in Fig. 3A, CD4 T cells from anti-CTLA-4-treated (post-therapy) patients produced approximately fivefold more IFNγ than T cells from untreated patients or healthy donors (P < 0.05). IL-10 production by CD4 T cells was not significantly changed after anti-CTLA-4 treatment (Fig. 3A), nor was IL-4 production noted to change (data not shown).
Fig. 3.
Increased production of IFNγ by CD4 and CD4+ICOShi T cells and recognition of tumor antigen NY-ESO-1 by CD4+ICOShi T cells. (A) Increased IFNγ production by CD4 T cells from the peripheral blood of patients treated with anti-CTLA-4 therapy. (B) Fold induction of IFNγ, IL-10, IL-4, IL-2, and FOXP3 mRNA levels relative to CD3-ε mRNA expression in CD4 T cells from peripheral blood. (C) Increased IFNγ production by CD4+ICOShi T cells from the peripheral blood of anti-CTLA-4-treated patients. (D) Tumor antigen NY-ESO-1 mRNA detected by RT-PCR and gel electrophoresis. Lane 1 shows the NY-ESO-1 mRNA detected in a positive control cell line, SK-Mel 37, which has high expression of NY-ESO-1; lane 2 shows the absence of NY-ESO-1 mRNA in the negative control cell line SK-Mel 23; lanes 3, 5, and 7 show the absence of NY-ESO-1 expression in tumor tissues from patients 1, 3, and 5, respectively; and lanes 4, 6, and 8 show the NY-ESO-1 expression in tumor tissues from patients 2, 4, and 6, respectively. B-actin is shown to be present in all samples. (E) CD4+ICOShi T cells from the pre-therapy blood sample of patient 6 and the post-therapy blood samples of patients 2, 4, and 6 produced IFNγ on recognition of APCs pulsed with overlapping NY-ESO-1 tumor antigen peptides encompassing the entire protein (all peptides) but did not respond to APCs without peptide (no peptide).
As shown in Fig. 3B, uncultured CD4 T cells analyzed ex vivo from post-therapy week 7 blood samples of anti-CTLA-4-treated patients had higher levels of mRNA for IFNγ (P < 0.05) and lower levels of FOXP3 mRNA (P < 0.05) than did CD4 T cells from untreated patients. There was no significant change in the levels of IL-4, (data not shown), IL-10, or IL-2 mRNA (Fig. 3B). The average Th1/Th2 cytokine ratio inferred from comparison of IFNγ and IL-10 mRNA levels increased from ≈1:1 in CD4 T cells from the blood of untreated (pre-therapy) bladder cancer patients to ≈3:1 in CD4 T cells from peripheral blood of patients treated with anti-CTLA-4 antibody (post-therapy), suggesting that anti-CTLA-4 therapy skewed CD4 effector cells toward a more Th1-like profile. These changes in the cytokine profiles were consistent with changes in levels of transcription factors related to functional differentiation of CD4 T cells. Levels of T-bet, a transcription factor responsible for regulation of IFNγ production and commitment to the Th1 lineage (26) was markedly increased (P < 0.05) after anti-CTLA-4 treatment, whereas GATA-3, a transcription factor associated with the production of IL-10 and other Th2 cytokines (27), was unaffected (Fig. S3).
CD4+ICOShi and CD4+ICOSlow T cells, sorted from uncultured CD4 T cells from the peripheral blood of healthy donors, patients with untreated bladder cancer (including pre-therapy samples from patients), and patients who received treatment with anti-CTLA-4 antibody (post-therapy samples from weeks 3 and 7) were assayed for cytokine production after stimulation with anti-CD3 plus IL-2 in vitro. CD4+ICOSlow T cells from treated patients did not produce significantly more IFNγ than CD4+ICOSlow cells from untreated patients or healthy donors. However, CD4+ICOShi T cells of all treated patients produced more IFNγ than CD4+ICOShi T cells from untreated patients or healthy donors (Fig. 3C) (P < 0.05), whereas IL-4 (data not shown) and IL-10 production consistently remained low. These data suggest that a major effect of CTLA-4 blockade is the induction of CD4+ICOShi effector T cells that produce IFNγ.
We next sought to determine whether the CD4+ICOShi T cells contained a population of cells capable of recognizing tumor antigens. We used RT-PCR to type the patients' tumors for expression of NY-ESO-1, a highly immunogenic cancer-testis tumor antigen known to be expressed by many bladder carcinomas (28). Patients whose tumors did not express the NY-ESO-1 antigen were not found to have NY-ESO-1-reactive T cells within peripheral blood samples (data not shown). However, three patients (patients 2, 4, and 6) were found to express NY-ESO-1 (Fig. 3D). CD4+ICOShi T cells from all three of these patients' post-therapy blood samples produced IFNγ in response to antigen-presenting cells (APCs) pulsed with NY-ESO-1 peptide antigens in a pool of overlapping peptides representing the entire sequence of NY-ESO-1, but not unpulsed APCs. CD4+ICOShi T cells obtained from the blood of two patients (patients 2 and 4) produced IFNγ in response to NY-ESO-1 only after treatment with anti-CTLA-4, whereas the third, patient 6, had NY-ESO-1 reactive CD4+ICOShi T cells even before therapy (Fig. 3E). Thus, in some patients, CD4+ICOShi effector T cells that recognize tumor antigens may exist before therapy, whereas in others CTLA-4 blockade allows for the expansion of these cells.
Immunologic Consequences of CTLA-4 Blockade in Tumor Tissues: ICOS Expression Is Higher on CD4 T Cells in Tumor Tissues After CTLA-4 Blockade.
We next sought to determine the effects of anti-CTLA-4 therapy on T cells in tumor tissues. As shown in Fig. 4A Left and Center for representative patients, the frequency of CD4+ICOShi T cells was higher in both nonmalignant urothelial (13%) and untreated cancer tissues (16%) than in the peripheral blood (compare Fig. 4A and Fig. 1 A and B). This difference may reflect activation of infiltrating effector or regulatory CD4 T cells by tissue- or tumor-specific antigens. More importantly, the frequency of CD4+ICOShi T cells was much higher in bladder tissues of all six anti-CTLA-4-treated patients as compared with tumor tissues from untreated patients (mean 45 ± 12% vs. 15 ± 8%) (Fig. 4 A Right and B) (n = 6, P < 0.05). Thus, CTLA-4 blockade resulted in a systemic increase in CD4+ICOShi T cells in both the tissues and the peripheral blood of treated patients.
Fig. 4.
Effects of anti-CTLA-4 therapy on tumor tissues. (A) ICOS expression on tumor-infiltrating CD4 T cells was increased after anti-CTLA-4 treatment. Data shown are from representative samples. (Left) Nonmalignant urothelial tissues from an untreated bladder cancer patient. (Center) Urothelial carcinoma tissues from an untreated bladder cancer patient. (Right) Urothelial carcinoma tissues from a bladder cancer patient treated with anti-CTLA-4 antibody. (B) Plot of percentage of CD4+ICOShi T cells from individual tissue samples including nonmalignant tissue samples (n = 4), untreated tumor tissues (n = 10), and anti-CTLA-4-treated tumor tissues (n = 6). (C) Plot of percentage of CD4+FOXP3+ T cells from individual tissue samples including nonmalignant tissue samples (n = 4), untreated tumor tissues (n = 10), and anti-CTLA-4-treated tumor tissues (n = 6). (D) Fold induction of CD3-ε, IFNγ, IL-10, IL-4, IL-2, T-bet, GATA-3, and FOXP3 mRNA levels relative to GAPDH mRNA expression in surgical samples of nonmalignant urothelial tissues and bladder cancer tissues from untreated and anti-CTLA-4-treated patients.
FOXP3 Expression Is Lower in CD4 T Cells in Tumor Tissues After CTLA-4 Blockade.
Next we examined the effects of CTLA-4 blockade on FOXP3 expression by CD4 T cells from tumor tissues. Within urothelial carcinoma tissues from bladder cancer patients, the frequency of CD4+FOXP3+ T cells was variable but was markedly higher (mean 67 ± 25%, n = 10) than in nonmalignant urothelial tissues (mean 8 ± 5%, n = 4) (Fig. 4C and Fig. S4). However, in all cases there was a lower frequency of CD4+FOXP3+ T cells in bladder-cancer tissues in anti-CTLA-4-treated tissues (mean 7 ± 4%, n = 6, P < 0.05) compared with untreated tumor tissues (Fig. 4C). Thus, although the effect of anti-CTLA-4 on FOXP3 expression in CD4 T cells from peripheral blood was variable, in tumor tissues their frequency was consistently decreased after anti-CTLA-4 therapy.
IFNγ Expression Is Increased in Tumor Tissues After CTLA-4 Blockade.
We analyzed mRNA from tissues for expression of relevant cytokines and transcription factors to assess the functional impact of anti-CTLA-4 antibody on tumor tissue. As shown in Fig. 4D, tumor tissue from anti-CTLA-4-treated patients consistently had higher levels of IFNγ, IL-2, and T-bet and lower levels of FOXP3 mRNA than tumor tissues from untreated patients (P < 0.05). IL-4, IL-10, and GATA-3 mRNA levels were not statistically significantly altered in tumor tissues from treated or untreated patients. The average ratio of Th1 to Th2 cytokines within the tumor increased from 0.1 in untreated samples to 1.0 in samples from anti-CTLA-4-treated patients. The magnitude of the rise in the ratio of Th1 to Th2 cytokines was much greater within the tumor than in circulating peripheral blood CD4 T cells. These data supported the notion that anti-CTLA-4 therapy leads to an increase in Th1 effector cells in both the systemic circulation and the tumor, with the effects more marked within the tumor, as would be expected for the major site of immunologic engagement during tumor rejection.
The Ratio of CD4+ICOShi Effector to CD4+FOXP3+ Regulatory T Cells Is Increased After Blockade of CTLA-4.
CD4+FOXP3+ Function as regulatory T cells after treatment with anti-CTLA-4 antibody.
As shown in Table 1, anti-CTLA-4 treatment resulted in an increase in the number of CD4+ICOShi T cells in all patients. As we have shown, the pool of CD4+ICOShi T cells, which is comprised of both FOXP3+ and FOXP3− cells, contains effector T cells that produce IFNγ, as opposed to IL-10, that increased after anti-CTLA-4 therapy. CD4+FOXP3+ T cells are thought to be predominantly regulatory T cells, but inference of function from expression data alone is confounded by the fact that transient expression of FOXP3 can occur on activation of human CD4 T cells without conferring regulatory T cell function (29, 30). We therefore sought to determine whether the CD4+FOXP3+ T cells of anti-CTLA-4-treated patients retained regulatory function. CD4+FOXP3+ T cells have high expression of CD25 (16) that allows for the isolation of these cells by cytometric flow sorting. We obtained a population of CD4+CD25+ T cells from normal donors and pre- and post-therapy patients' blood samples that typically contained >90% FOXP3+ cells (Fig. S5A). CD4+CD25+ cells from anti-CTLA-4-treated patients inhibited the proliferation of CD4+CD25− responder T cells in standard coculture assays at a level similar to that of cells from normal donors or pre-therapy patients (Fig. S5B). These results are consistent with a previous report demonstrating maintenance of suppressive activity of regulatory T cells after CTLA-4 blockade (12).
Table 1.
Ratio of effector CD4+ICOShi to regulatory CD4+FOXP3+ T cells in the peripheral blood of patients before and after treatment with anti-CTLA-4 antibody
| CD4+ | CD4+ICOShi | CD4+FOXP3+ | Ratio of CD4+ICOShi/CD4+FOXP3+ | |
|---|---|---|---|---|
| Patient #1 | ||||
| Pre-therapy | 210,413 | 6,312 | 8,417 | 0.8 |
| Week 3 | 190,976 | 26,736 | 17,188 | 1.6 |
| Week 7 | 191,467 | 24,891 | 11,488 | 2.2 |
| Patient #2 | ||||
| Pre-therapy | 139,483 | 558 | 43,324 | 0.0 |
| Week 3 | 176,359 | 31,745 | 15,688 | 2.0 |
| Patient #3 | ||||
| Pre-therapy | 135,549 | 4,066 | 23,706 | 0.2 |
| Week 3 | 130,560 | 19,584 | 53,112 | 0.4 |
| Week 7 | 156,792 | 26,655 | 12,939 | 2.1 |
| Patient #4 | ||||
| Pre-therapy | 143,975 | 5,759 | 10,078 | 0.6 |
| Week 3 | 134,259 | 10,741 | 4,028 | 2.7 |
| Week 7 | 164,543 | 13,163 | 4,936 | 2.7 |
| Patient #5 | ||||
| Pre-therapy | 209,441 | 4,189 | 58,644 | 0.1 |
| Week 3 | 272,621 | 54,524 | 119,953 | 0.5 |
| Week 7 | 238,434 | 42,918 | 21,459 | 2.0 |
| Patient #6 | ||||
| Pre-therapy | 15,6381 | 1,564 | 12,511 | 0.1 |
| Week 3 | 16,2389 | 9,743 | 11,367 | 0.9 |
| Week 7 | 15,9411 | 14,347 | 6,376 | 2.3 |
Values are numbers of cells per million collected events by flow cytometry.
CTLA-4 blockade shifts the balance of effector to regulatory T cells in peripheral blood and tumor tissues.
Our data show that changes in the frequency of regulatory CD4+FOXP3+ T cells in the systemic circulation after anti-CTLA-4 therapy at 3 mg/kg per dose of antibody do not provide a reliable marker of immunologic impact (Fig. 2D and Table 1). Similarly, total CD4 T cell numbers were not consistently altered and are not reliable indicators of anti-CTLA-4 treatment. However, anti-CTLA-4 treatment resulted in a consistent increase in CD4+ICOShi T cells above pre-therapy values, which resulted in an increase in the ratio of CD4+ICOShi to CD4+FOXP3+ T cells from pre-therapy values of 0–0.8:1 to post-therapy values of ≈2:1 in the peripheral blood of treated patients (Table 1). This increase was even more striking in the tumor tissues from treated vs. untreated patients, ranging from approximately 15- to 30-fold (Table S1). Given our data that the ICOShi population contains a significant population of IFNγ-producing cells, this change in the ratio of CD4+ICOShi to CD4+FOXP3+ cells reflects a relative increase in Teff/Treg.
Discussion
We have found that a consistent, major effect of treatment of patients with localized bladder cancer with anti-CTLA-4 antibody is an increase in the frequency of effector CD4 T cells that express high levels of ICOS and produce IFNγ. These ICOS-expressing CD4 T cells contain subsets of both FOXP3+ and FOXP3− cells, which suggests that ICOS expression is not restricted to a particular T cell subset. Isolated CD4+ICOShi T cells from anti-CTLA-4-treated patients, as compared with cells from healthy donors or untreated patients, have increased IFNγ production and respond to the NY-ESO-1 tumor antigen. The increased production of IFNγ by CD4+ICOShi T cells from treated patients may be a consequence of enhancement of CD28 costimulatory signals in the absence of the inhibitory effects mediated by CTLA-4. Mechanistic studies addressing this possibility and others will need to be investigated in future experiments.
Our current study also shows that CTLA-4 blockade in bladder-cancer patients resulted in an increase in the ratio of effector CD4+ICOShi to regulatory CD4+FOXP3+ T cells in both peripheral blood and tumor tissues. We should point out that our analyses between tumor tissues from untreated patients and tumor tissues from treated patients occurred in two different groups of patients, thus introducing possible confounding factors in our data, but because it is difficult to obtain sufficient fresh tumor tissues for flow cytometric analyses from the same patient at both pre-therapy and post-therapy timepoints, we relied on obtaining tissues at the time of surgery from untreated patients for comparison to tissues from treated patients, who had similar disease stage. Increased CD4+ICOShi T cells and the Teff/Treg ratio observed in treated tissues correlated with the increased values seen between pretherapy and post-therapy blood samples, which were matched samples obtained from each individual patient. Thus, our results from blood and tumor tissues consistently indicate an increase in CD4+ICOShi T cells and an increase in the Teff/Treg ratio after anti-CTLA-4 therapy. Previous data from preclinical studies have shown a correlation in the Teff/Treg ratio in tissues with successful anti-tumor responses in mice treated with anti-CTLA-4 antibody (8). Our data now establish immunologic markers within the human immune system that define a Teff/Treg ratio that may have correlation with clinical responses. It is interesting that all six patients with localized bladder cancer in our study had increased CD4+ICOShi T cells after treatment with the anti-CTLA-4 antibody; this may be a reflection of immunologic changes that occur with drug administration and does not reflect immunologic changes that will correlate with clinical benefit, or these changes may occur in patients with localized disease but only in patients with metastatic disease who will derive clinical benefit from treatment. It is also possible that not just the change in the ratio of Teff/Treg itself, but also the period for which this is sustained will be important in clinical outcome. All of these ideas will have to be addressed in future studies with anti-CTLA-4 therapy.
The immunologic markers identified in our small cohort will no doubt be examined in future clinical trials, including studies on patients with metastatic disease, patients who receive more than two doses or higher doses of anti-CTLA-4 antibody, and patients who receive other immune-modulating agents, to determine the biologic and clinical relevance of CD4+ICOShi T cells in cancer patients. In any event, the phenotypic and functional data presented here provide insight into the possible mechanisms of action of the anti-CTLA-4 antibody and provide biological markers to guide the development of this agent.
Materials and Methods
Patients.
Bladder cancer patients with diagnoses of urothelial carcinoma who were candidates for radical cystectomy were consented on an Institutional Review Board (IRB)-approved clinical trial. The trial allowed six patients to receive two doses of Ipilimumab anti-CTLA-4 antibody at 3 mg/kg before surgery, which was performed four weeks after the last dose of antibody. Healthy donor blood was obtained from volunteers (n = 10). Additional data regarding the clinical trial and informaion regarding blood and tissue processing is provided in supporting information (SI).
Flow Cytometry and Cell Culture.
Cells were stained and obtained according to standard procedures as described in SI Materials and Methods.
Real-Time PCR.
Total RNA samples from tissues and CD4 T cells were isolated and analyzed as described in the supporting information (SI).
RT-PCR Analyses for Detection of NY-ESO-1 mRNA.
Reverse transcription was performed by using 4 μg of mRNA from tumor tissues, and PCR was carried out by using 200 ng of cDNA with NY-ESO-1 gene-specific oligonucleotide primers as previously described (28).
ELISPOT Assay to Detect T Cell Responses to NY-ESO-1 Tumor Antigen.
CD4+ICOShi T cells were sorted from uncultured CD4 T cells as described above. ELISPOT assays were performed as previously described (30), with minor modifications listed in SI Text.
Statistical Analyses.
Statistical analyses were conducted by comparing two groups such that one group contained data from both pre-therapy and untreated patients, whereas the second group contained data only from post-therapy patients. The Wilcoxon rank-sum test was used to assess the differences in continuous variables between the two groups and calculated P values that were <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
We gratefully acknowledge the support and assistance of Drs. Jim Allison, Lloyd Old, Patrick Hwu, Laszlo Radvanyi, Eric Wieder, and Yong-Jun Liu. This work was supported in part by a Physician-Scientist Program Award and Institutional Research Grant from the University of Texas M.D. Anderson Cancer Center, a Career Development Award from the American Society of Clinical Oncology, a Gillson Longenbaugh Foundation Award, a Carl C. Anderson, Sr. & Marie Jo Anderson Charitable Foundation Award, and a Clinical Investigator Award from the Cancer Research Institute (to P.S.). Bristol-Myers Squibb sponsored and funded the clinical trial of neoadjuvant Ipilimumab for bladder cancer patients.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806075105/DCSupplemental.
References
- 1.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: Increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 2.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing affects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anticytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4714. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, et al. Cancer regression and autoimmunity induced by CTLA-4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Small EJ, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 14.Hutloff A, et al. ICOS is an inducible T cell co-stimulator structurally functionally related CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 15.Dong C, et al. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga SK, et al. T cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 17.Kohyama M, et al. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc Natl Acad Sci USA. 2004;101:4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohning M, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D, et al. Roquin represses autoimmunity by limiting inducible T cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 22.Burmeister Y, et al. ICOS controls the pool size of effector memory and regulatory T cells. J Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 23.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 24.Blankenstein T, Qin Z. The role of IFN-γ in tumor transplantation, immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–154. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 25.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 26.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 27.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, et al. Frequency of NY-ESO-1 and LAGE-1 expression in bladder cancer and evidence of a new NY-ESO-1 T cell epitope in a patient with bladder cancer. Cancer Immun. 2003;3:19. [PubMed] [Google Scholar]
- 29.Gavin MC, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2006;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.