Abstract
A recessive phenotype called spin (spontaneous inflammation) was induced by N-ethyl-N-nitrosourea (ENU) mutagenesis in C57BL/6J mice. Homozygotes display chronic inflammatory lesions affecting the feet, salivary glands and lungs, and antichromatin antibodies. They are immunocompetent and show enhanced resistance to infection by Listeria monocytogenes. TLR-induced TNF and IL-1 production are normal in macrophages derived from spin mice. The autoinflammatory phenotype of spin mice is fully suppressed by compound homozygosity for Myd88poc, Irak4otiose, and Il1r1-null mutations, but not Ticam1Lps2, Stat1m1Btlr, or Tnf-null mutations. Both autoimmune and autoinflammatory phenotypes are suppressed when spin homozygotes are derived into a germ-free environment. The spin phenotype was ascribed to a viable hypomorphic allele of Ptpn6, which encodes the tyrosine phosphatase SHP1, mutated in mice with the classical motheaten alleles me and me-v. Inflammation and autoimmunity caused by SHP1 deficiency are thus conditional. The SHP1-deficient phenotype is driven by microbes, which activate TLR signaling pathways to elicit IL-1 production. IL-1 signaling via MyD88 elicits inflammatory disease.
Keywords: Ptpn6, Toll-like receptors
A large body of evidence points to participation of the innate immune response in the pathogenesis of autoimmune disease (1, 2). The Toll-like receptors, Nod-like receptors, and RIG-I-like helicases sense microbial signature and endogenous molecules and initiate signaling leading to the production of proinflammatory cytokines. These cytokines, including types I and II IFN, TNF, IL-1, IL-6, and IL-12, mediate inflammatory responses to infection and enhance responses of the adaptive immune system. Moreover, their overproduction is often associated with autoimmune or autoinflammatory diseases. For example, type I IFN has been implicated in the development of systemic lupus erythematosus (SLE) (1), TNF contributes to the pathology of rheumatoid arthritis (3), and IL-1 contributes to neonatal onset multisystem inflammatory disease (NOMID) (4).
The motheaten mutants (me and viable motheaten, me-v) develop autoimmune disease and inflammation with features similar to SLE. Motheaten mice carry null or hypomorphic alleles of Ptpn6, encoding the hematopoietic protein tyrosine phosphatase SHP1 (5–7), which is thought to down-regulate signaling from cytokine receptors, B and T cell receptors, chemokine receptors, receptor tyrosine kinases, and integrins (8). Ptpn6me and Ptpn6me-v homozygotes or compound heterozygotes are immunodeficient and develop systemic inflammation and autoimmunity, marked by alopecia, glomerulonephritis, dermatitis, inflammation of the paws, and interstitial pneumonitis, which ultimately causes death (9, 10). In both strains, there is overproduction and accumulation of macrophages and neutrophils in the lungs, skin, and extremities, and elevated concentrations of serum immunoglobulins and autoantibodies (10–12).
In double mutant Ptpn6me-v/me-vRag1−/− mice, which lack B and T cells and cannot generate autoantibodies, severe inflammatory lesions still develop, in which macrophages and granulocytes infiltrate healthy tissues. This indicates that chronic inflammatory disease progression in Ptpn6me-v mutants does not require the adaptive immune response (13). However, mice lacking Ptpn6 expression only in B lymphocytes develop both autoimmunity and chronic inflammatory disease, as evidenced by increased levels of serum immunoglobulins reactive to single- and double-stranded DNA, lymphocytic infiltrates of the lung and liver, and glomerulonephritis (14). These data indicate that SHP1 deficiency need affect only B cells to cause autoimmunity featuring autoantibody production. Augmented cytokine production or signaling has been proposed to contribute to the development of disease in motheaten mutants (15, 16).
Here, we describe a hypomorphic allele of Ptpn6 (m1Btlr, designated spin) detected because it elicits chronic inflammatory and autoimmune disease. By testing the suppressive effects of mutations at other loci and deriving the mice into a germ-free (GF) environment, we have concluded that the inflammatory phenotype requires MyD88, IRAK4, and the IL-1 receptor, but not type I or type II IFNs, or TNF. Inflammation cannot be expressed in the absence of commensal microbes.
Results
spin Phenotype.
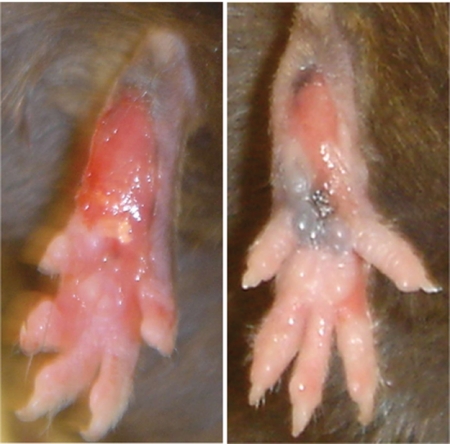
A recessive inflammatory phenotype (called spin to denote spontaneous inflammation) was observed in a G3 mouse homozygous for mutations induced by N-ethyl-N-nitrosourea (ENU) on a pure C57BL/6J background. The plantar surfaces of the feet develop inflamed, raw, weeping lesions (Fig. 1). By following this visible phenotype, a homozygous stock of spin mutants was established. On the C57BL/6 background, inflammation is 90% penetrant in both males and females scored between 10 and 25 weeks of age. Histological examination of the feet revealed thickening of the epidermis, microabscesses in the epidermal and dermal layers, bone marrow hyperplasia, and a neutrophilic infiltrate in the dermal layer [supporting information (SI) Fig. S1 A–D]. Mixed inflammatory infiltrates were also noted in the salivary glands (Fig. S1 E and F) and lungs (Fig. S1 G and H) of spin mice.
Fig. 1.
Chronic inflammation in homozygous spin mutants. Foot lesions develop in spin homozygotes from 6 weeks of age. Two representative foot lesions from spin homozygotes are shown.
Before 5 weeks of age, spin mice show no signs of foot inflammation and have normal frequencies and total numbers of myeloid and erythroid cells in the bone marrow, spleen, and peripheral blood (Table S1). However, the appearance of foot lesions by 6 weeks of age is associated with development of splenomegaly, an increased number of erythroid and myeloid cells in the spleen, and a paucity of mature B cells in the peripheral blood, spleen, and bone marrow (Tables S1 and S2). Elevated levels of serum polyclonal IgM, IgG, and antichromatin IgM and IgG are also evident in spin homozygotes (see Fig. 4), demonstrating an autoimmune component of the disease. The inflammatory phenotype is conferred by hematopoietic precursors, as proven by the outcome of reciprocal bone marrow transplantation between spin homozygotes and WT congenic C57BL/6J Ly5.1+ mice (data not shown).
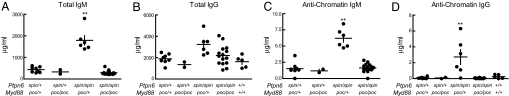
Fig. 4.
Autoimmune disease depends on MyD88 in spin mice. (A–D) The levels of serum polyclonal IgM (A) or IgG (B), and antichromatin IgM (C) or antichromatin IgG (D) were measured in 4- to 5-month-old mice of various genotypes. Compound homozygosity for Ptpn6spin and Myd88poc suppressed the elevated levels of serum immunoglobulins and antichromatin immunoglobulins found in Ptpn6spin/spin mice. **, one-way ANOVA; P < 0.0001 and posthoc Student–Newman–Keuls test, P < 0.05 for Ptpn6spin/spinMyd88poc/+ vs. all other genotypes.
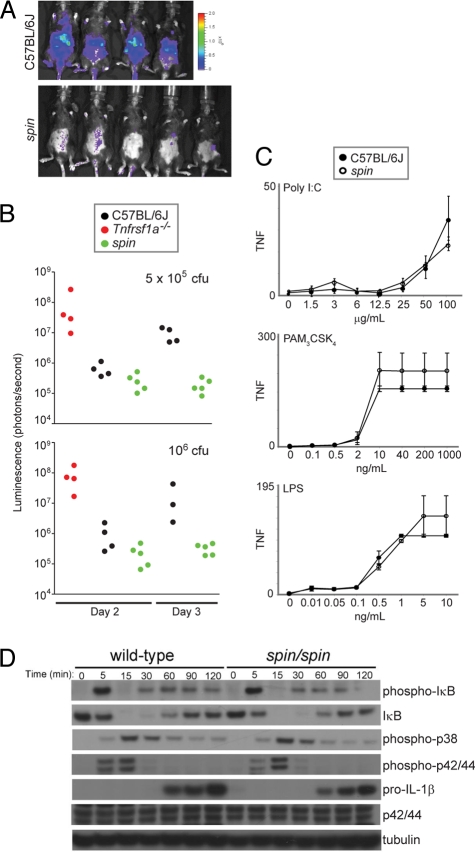
spin homozygotes showed augmented resistance to infection by Listeria monocytogenes. This was manifested by diminished bacterial burden in spin mutants on days 2 and 3 after inoculation with the bacteria at doses sublethal (105 cfu) or lethal (5 × 105 or 106 cfu) for WT mice (Fig. 2 A and B) and by enhanced survival (Table S3). spin mutants exhibited normal natural killer (NK) cell function in vivo and normal resistance to mouse cytomegalovirus (MCMV) (data not shown). Hence, the inflammatory and autoimmune phenotypes are not associated with demonstrable immunodeficiency. In addition, thioglycolate-elicited peritoneal macrophages derived from spin homozygotes showed normal TNF production in response to a range of TLR activating stimuli (Fig. 2C). Similarly, pro-IL-1β production, the degradation of IκB, and the phosphorylation of MAP kinases p38, p42, and p44 were all normal over time in bone marrow-derived macrophages stimulated with LPS (Fig. 2D). These observations suggest that microbe sensing via the TLR signaling pathways is not exaggerated in spin-derived macrophages.
Fig. 2.
spin mice display increased resistance to L. monocytogenes. (A) Bacterial load was visualized in spin homozygotes and C57BL/6J mice 3 days after challenge with 5 × 105 cfu luminescent L. monocytogenes. Visual inspection and quantitation of luminescence demonstrated that spin homozygotes had reduced levels of bacteria compared to controls. (B) C57BL/6J, homozygous spin, or Tnfrsf1a-deficient mice were challenged with 5 × 105 or 106 cfu L. monocytogenes. The bacterial load, depicted as luminescence in photons per second, was determined 2 and 3 days after infection. Tnfrsf1a-deficient mice succumbed within 3 days of infection. One-way ANOVA and post-hoc Student-Newman-Keuls test, P < 0.05 for C57BL/6J vs. spin homozygotes on day 3 after infection with 5 × 105 cfu, and P < 0.05 for spin homozygous or C57BL/6J vs. Tnfrsf1a−/− mice on day 2 after infection with 106 cfu. (C) TNF production by peritoneal macrophages isolated from C57BL/6J or spin homozygous mice was measured in vitro in response to treatment with the indicated concentrations of TLR3, TLR2/1, or TLR4 ligands (poly I:C, Pam3CSK4, or lipopolysaccharide, respectively). TNF production was comparable between C57BL6/J and spin macrophages. The average response of cells from seven C57BL6/J and seven spin homozygous mice is plotted; error bars represent SD. (D) LPS-stimulated pro-IL-1β production, IκB degradation, and phosphorylation of MAP kinases p38, p42, and p44 in bone marrow-derived macrophages from C57BL/6J or spin homozygous mice were compared by Western blotting of lysates made at the indicated timepoints after LPS treatment in vitro. WT and spin macrophages responded similarly to LPS stimulation.
Positional Cloning of spin.
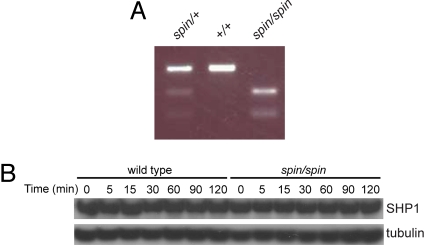
The spin phenotype was mapped on 155 meioses to a 6.2-Mb region of distal Chr. 6 (Fig. S2 A and B). Within the critical region was the Ptpn6 locus, a strong candidate, because plantar inflammation was reported in mice homozygous for the me-v allele (9, 10). The Ptpn6 cDNA was sequenced, and a T to A transversion predicting the amino acid substitution Y208N was identified (Fig. S2C). The mutation was confirmed at the genomic level by restriction enzyme digestion (Fig. 3) and resides in exon 5 of the 16-exon Ptpn6 gene. Allelism was tested by crossing Ptpn6spin heterozygotes to Ptpn6me-v heterozygotes, with absence of complementation in compound heterozygotes.
Fig. 3.
Detection of the spin mutation. (A) The mutation was predicted to introduce a HincII restriction enzyme site in genomic DNA from spin mutant mice. Amplification of Ptpn6 genomic DNA followed by HincII restriction enzyme digest confirmed the presence of the mutation. (B) SHP1 protein levels were normal in purified bone marrow-derived macrophages from spin homozygotes. The Western blot shown in Fig. 2D was reprobed with SHP1 antibodies, or tubulin antibodies as a loading control. Timepoints indicate minutes after stimulation with LPS in vitro.
Ptpn6 encodes the cytoplasmic protein tyrosine phosphatase SHP1, which contains two tandem N-terminal SH2 domains, a central catalytic domain, and a C-terminal tail. The binding specificity of phosphotyrosine-containing proteins for the SH2 domains of SHP proteins is known to depend critically on the three residues C-terminal to the phosphotyrosine (designated pY + 1 to pY + 3) (17, 18). The spin mutation occurs in the C-terminal SH2 domain, within the BG loop that forms part of the binding interface with pY + 1 and pY + 3 residues. The N-terminal SH2 domain is thought to undergo a conformational change that unblocks the catalytic domain upon engagement, allowing phosphatase activation (19–21). The C-terminal SH2 domain is not required for this disinhibition, but its presence is indispensable for optimal SHP signaling, and it has been proposed to aid in recruitment of binding partners.
spin mice exhibit the least severe phenotype in the existing Ptpn6 allelic series, where Ptpn6me is a null allele and Ptpn6me-v encodes a phosphatase with ≈20% of WT catalytic activity, respectively (5, 7). The Ptpn6me-v allele contains a splice site mutation that results in the use of two cryptic splice sites and likely yields some protein products with residual activity. Ptpn6spin/spin mice do not develop the immunodeficiency or lethal pneumonitis seen in either of the motheaten mutants. Additionally, although Ptpn6me/me and Ptpn6me-v/me-v mice are infertile and die by 3 and 9 weeks of age, respectively (10), Ptpn6spin/spin mice are fertile and survive for >1 year. Because Ptpn6me homozygotes have severe disease, and Ptpn6me-v heterozygotes have no disease, the Ptpn6spin allele likely encodes a protein with a specific activity that falls between 20% and 50% of the WT level. No differences in SHP1 protein levels were noted between C57BL/6J and spin macrophages when either untreated or stimulated with LPS (Fig. 3B).
MyD88, IRAK4, or IL-1R Mutations Suppress the spin Phenotype.
The constant exposure of the feet to microbial stimuli and the prominent plantar distribution of inflammation suggested a pathogenic mechanism in which minor trauma and infection might incite an exaggerated inflammatory response in spin mice. Microbe sensing occurs largely through TLRs, and we tested the hypothesis that TLR-mediated signals might initiate an inflammatory response in spin mutants. We created compound homozygous mutants, combining Myd88poc (22), Irak4otiose (a functionally null allele), or Ticam1Lps2 (23) with Ptpn6spin on a pure C57BL/6J background. Defective MyD88 signaling completely suppressed plantar inflammation in Ptpn6spin/spin mice (Table 1). Moreover, MyD88 deficiency suppressed elevation of total IgM and antichromatin IgM in the serum (Fig. 4 A and C). Whereas serum IgG levels are insignificantly elevated in Ptpn6spin/spin mice, antichromatin IgG is significantly elevated. Homozygosity for the Myd88poc mutation suppressed this phenotype (Fig. 4 B and D). IRAK4 is required for MyD88-dependent signaling, whereas TICAM1 (TRIF) is required for MyD88-independent signaling. IRAK4 deficiency suppressed inflammation in Ptpn6spin/spin mice, but TICAM1 deficiency did not (Table 1). MyD88-dependent (but not -independent) signaling is thus required for the development of chronic plantar inflammation caused by the Ptpn6spin mutation.
Table 1.
Homozygous spin mice with mutations in MyD88 are protected from foot inflammation
| Genotype | No. mice with inflamed feet/no. total mice | P value* vs. Ptpn6spin/spin | P value* vs. Ptpn6spin/spin Myd88poc/poc |
|---|---|---|---|
| Ptpn6spin/spin | 35/43 | – | <0.0001 |
| Ptpn6spin/spin Myd88poc/poc | 0/27 | <0.0001 | – |
| Ptpn6spin/spin Tnf−/− | 7/9 | 1 | <0.0001 |
| Ptpn6spin/spin Stat1m1Btlr/m1Btlr | 2/2 | 1 | 0.0025 |
| Ptpn6spin/spin Ticam1Lps2/Lps2 | 1/2 | 0.3636 | 0.0690 |
| Ptpn6spin/spin Irak4otiose/otiose | 0/2 | 0.0454 | 1 |
Mice were scored between the ages of 6 and 25 weeks for inflammatory lesions on the feet.
*Fisher's exact test, two-tail P value.
MyD88-dependent TLR signaling elicits the production of several proinflammatory cytokines, including IFNα, TNF, and IL-1. To determine whether signaling initiated by one or more of these cytokines is required for the inflammatory phenotype in spin mice, we generated compound homozygotes for spin and mutations blocking IFN, TNF, or IL-1 signaling.
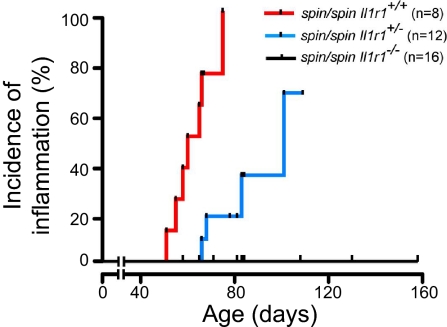
No suppression of the inflammatory phenotype was observed when a functionally null allele of Stat1 (m1Btlr or domino) was used to block both types I and II IFN signaling in spin homozygotes (Table 1). Consistent with this result, we found no defect in the magnitude or duration of STAT1 phosphorylation in response to IFNβ or IFNγ stimulation of homozygous spin macrophages (Fig. S3). Compound homozygosity for Ptpn6spin and a null allele of Tnf also failed to suppress inflammation (Table 1). In contrast, inflammation was fully suppressed in Ptpn6spin/spinIl1r1−/− mice that lack IL-1α and IL-1β signaling (Fig. 5). These data support the conclusion that SHP1 normally limits IL-1 signaling, and in the absence of SHP1, IL-1 signaling causes inflammatory disease.
Fig. 5.
Incidence curve showing age of onset of plantar inflammation in homozygous spin mice with or without null mutations in Il1r1. For difference between Il1r1+/+ and Il1r1−/−, P < 0.0001.
IL-1 signaling requires MyD88 and IRAK4. Although the MyD88-independent pathway contributes to IL-1 production, it is not primarily responsible for the inflammatory phenotype of spin mice, because TICAM1 deficiency fails to suppress disease in spin homozygotes. Our finding that SHP1 limits neither TNF nor IL-1 production in macrophages activated by TLR ligands (Fig. 2 C and D) may be interpreted in two ways, neither of which excludes the other. First, SHP1 may selectively dampen IL-1 signaling but not TLR signaling. Second, the IL-1-dependent process that causes inflammation may be limited by SHP1 in a cell type other than macrophages.
Normal Microbiota Required for Inflammation in spin Mutants.
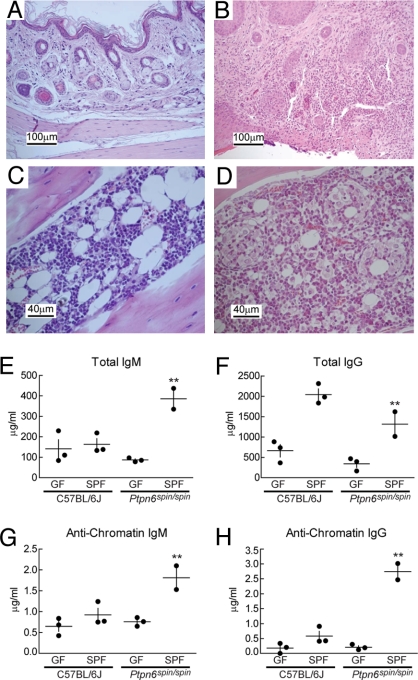
MyD88 signaling is prompted both by endogenous molecules (e.g., IL-1, IL-18, IL-33, extracellular matrix components, and/or DNA or RNA) and by microbial signature molecules detected via TLRs (2). To determine whether microbes drive inflammation in Ptpn6spin/spin mice, we derived these animals into a GF environment by Caesarian section of near-term females. In the absence of normal commensal flora, the phenotype was not expressed. Zero of three 20-week old GF Ptpn6spin/spin mice have developed plantar inflammation. Extensive histological analysis of the feet, kidney, liver, lung, lymph node, pancreas, and salivary gland from these mice revealed no lesions (Fig. 6 A and C and data not shown). Total serum IgM, IgG, and antichromatin antibody levels were also comparable between GF Ptpn6spin/spin and control C57BL/6 GF mice (Fig. 6 E–H). Two 20-week-old GF Ptpn6spin/spin mice were conventionalized by transfer into standard housing conditions. Both developed plantar inflammation, as evidenced by epidermal ulcerations and disruption of the dermis by cellular inflammatory exudate and a leukocyte infiltrate (Fig. 6B). Bone marrow hyperplasia was observed (Fig. 6D). Both conventionalized Ptpn6spin/spin mice also developed increased serum levels of total IgM, IgG, and antichromatin antibodies within 6–12 weeks (Fig. 6 E–H). Therefore, the chronic inflammation and autoimmunity of Ptpn6spin/spin mice are not endogenously driven but instead depend on the presence of microbes introduced during postnatal life.
Fig. 6.
Chronic inflammation and autoimmune disease in homozygous spin mice depends on microbes. (A–D) Haematoxylin/eosin-stained tissue sections from 6- to 20-week-old Ptpn6spin/spin mice derived and housed in GF conditions (A and C) and then conventionalized into normal SPF conditions (B and D). The feet of conventionalized mice are inflamed, with epidermal ulcerations displaying superficial necrosis and a leukocyte infiltrate (B), whereas GF mice display no foot inflammation (A). The cellularity of the bone marrow is increased in conventionalized mice, with numerous hematopoietic cells and granuloma-like structures (D). The bone marrow of GF mice appears normal (C). Sections are magnified ×20 (A and B) and ×40 (C and D). (E–H) The levels of serum polyclonal IgM (E) or IgG (F) and antichromatin IgM (G) or antichromatin IgG (H) were measured 6–12 weeks after mice housed in GF conditions were conventionalized by introduction into SPF conditions. GF conditions suppressed the elevated levels of immunoglobulins and anti-chromatin immunoglobulins found in Ptpn6spin/spin mice housed in SPF conditions. **, Student's t test, P < 0.05 for GF Ptpn6spin/spin mice vs. specific pathogen-free Ptpn6spin/spin mice.
Discussion
The development of disease in Ptpn6me-v/me-v mice has been suggested to stem from the increased production and/or signaling of proinflammatory cytokines. Elevated levels of TNF or TNF mRNA have been reported in the sera and cells of Ptpn6me-v/me-v relative to WT mice (15, 24–26). Anti-TNF antibody administration reduced lung tissue injury in Ptpn6me/me bone marrow chimeras (25), and treatment with soluble TNF receptor reduced dermatitis, pneumonitis, and inflammatory lesions of the extremities in Ptpn6me/me mice (27). SHP1 has also been implicated in the down-regulation of signaling elicited by type I IFN (e.g., JAK1 and STAT1 activation) (16). Strikingly, we found that neither STAT1 nor TNF deficiency attenuates disease in spin homozygotes, indicating that whatever inhibitory effect SHP1 may normally have on TNF and IFN production or signaling, these cytokines do not drive the pathologic effects of SHP1 deficiency. It remains possible that the milder spin mutation does not affect TNF or IFN production or activities, whereas the more severe me and me-v mutations do.
Here, we have implicated microbe-dependent production of IL-1 and subsequent IL-1 signaling in the pathogenesis of inflammation seen in SHP1-deficient mice. In part, microbe sensing may be TLR-dependent, and MyD88 deficiency may block the sequence of events that lead to inflammation at this level. However, other sensors, including the NOD/NALP family of cytoplasmic receptors and/or the RIG-I-like helicases, also stimulate production of IL-1. Because IL-1 (but not TNF or type I or II IFNs) appears to be essential for the development of inflammatory disease in spin homozygotes, MyD88 deficiency may suppress the spin phenotype by preventing IL-1 signaling. IL-1 signaling is known to be an essential element in the development of several severe inflammatory diseases, including NOMID, familial cold autoinflammatory syndrome (4), and certain cases of rheumatoid arthritis (28). We suggest here that it also mediates disease observed in mice with hypomorphic mutations affecting SHP1.
Our data suggest that SHP1 limits IL-1-dependent signaling events that cause both autoinflammatory and autoimmune disease. IL-1 signaling and signaling from many of the TLRs depend on MyD88, IRAK4, to some extent IRAK1, TRAF6, the E2 ligase complex Ubc13/Uev1A (29), TAK1, TAB1, and components of the IKK complex, all of which represent candidate substrates for SHP1. Inflammation in spin mice is suppressed by MyD88 but not TICAM1 deficiency. An et al. (26) recently reported that SHP1 inhibits IRAK1 in a phosphatase-independent manner by directly binding to the kinase domain of IRAK1, to promote TLR-dependent type I IFN production. However, we find no requirement for IFN signaling in the inflammatory phenotype of Ptpn6spin/spin mice or evidence that the phenotype is exaggerated in the absence of IFN signaling.
Previously, the motheaten phenotype was reported to occur under specific pathogen-free (SPF) conditions (12, 30). Our findings indicate that SPF conditions are insufficient to eliminate the relevant microbial drivers of the phenotype, and that authentic GF conditions are necessary. The dual requirement for a genetic lesion and a suitable microbial driver in the pathogenesis of inflammation has been observed in other settings. For example, the severe inflammation observed in hemophagocytic lymphohistiocytosis (HLH) has been shown to result from mutations that prevent exocytosis of toxic granules from lymphoid cells (e.g., in Unc13d, Rab27a) but is manifested only in the presence of an infectious driver such as lymphocytic choriomeningitis virus (LCMV) (31, 32). The sustained inflammatory disease observed in HLH caused by lesions affecting the exocytic machinery is presumed to result from failure to eradicate the causal microbe, which accumulates in vivo and stimulates expansion of antigen-specific lymphoid clones. These, in turn, drive the expansion of myeloid cells that both present antigen and cause tissue injury. In the case of Ptpn6spin, we suggest that instead, microbes initiate innate immune signaling via the TLR→MyD88/IRAK4 axis, which leads to IL-1 production. Signaling via the IL-1R→MyD88/IRAK4 axis causes chronic inflammation. It is likely that autoimmunity results from utilization of the same pathways. The cellular executors of inflammation and autoimmunity in SHP1 deficiency remain to be determined.
Materials and Methods
Mice and Genetic Mapping.
C57BL/6J mice bred at The Scripps Research Institute (TSRI) were injected with ENU, as described (33), to generate the Ptpn6spin [Mutant Mouse Regional Resource Centers (MMRRC): 015198-UCD], Stat1m1Btlr (MMRRC: 011917-UCD), Myd88poc (MMRRC: 010475-UCD), Ticam1Lps2 [The Jackson Laboratory: 005037] and Irak4otiose (MMRRC: 016983-UCD) strains. All strains are available from MMRRC or The Jackson Laboratory, as indicated. The spin strain (MGI Accession 3717648) is described at http://mutagenetix.scripps.edu. Il1r1−/− mice were obtained as the kind gift of Ian Wicks (The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia). Tnf−/− mice were from The Jackson Laboratory. Ptpn6me-v/me-v mice were provided by L. D. Shultz (The Jackson Laboratory). All experiments were carried out in accordance with the Institute Animal Care and Use Committee guidelines. Mice were housed under SPF conditions with unlimited access to food and water. Mice were derived into gnotobiotic conditions by Caesarian section of near-term females at Karolinska Institutet. Briefly, pregnant female mice were killed by cervical dislocation. Embryos were removed from the uterus and kept within their individual amnion sac and then transferred to surrogate GF foster mothers under sterile conditions.
Genetic Mapping Is Described in SI Materials and Methods.
For bone marrow transplantation studies, congenic B6.SJL-(Ptprca-Pepcb [Ly5.1+]) and C57BL/6J (PtprcbPepcb [Ly5.2+]) WT or homozygous spin mutant mice were reconstituted with 5 × 106 bone marrow cells after two 5.5-Gy doses of irradiation administered 4 hours apart.
Histology.
For histological analysis, tissues were fixed in 10% buffered formalin. Paraffin-embedded sections were stained with haematoxylin/eosin.
Analysis of Hematopoietic Cell Populations.
Hematopoietic cell populations were monitored by microscopic examination of cells after May–Grünwald Giemsa staining or by flow cytometry by using antibodies specific for CD3, CD4, CD8, B220, Gr1, Mac1, Ter119, and IgM (eBioscience).
Serology.
Total and antichromatin IgM and IgG serum antibody concentrations were calculated by ELISA, as described (34). Mouse serum with known concentrations of Ig (Nordic Immunological Laboratories) was used as a standard.
NK Cell Function Assay.
To test NK cell function in vivo, C57BL/6J control and b2m-deficient target splenocytes were labeled with low (20-μM) and high (200-μM) concentrations of carboxyfluorescein diacetate, succinimidyl ester, respectively, at room temperature for 10 min. Cells were washed and resuspended at 5 × 107 cells per ml. Control and target cell populations were mixed at a 1:1 ratio and injected intravenously. Mice were bled 36 h postinjection, and peripheral blood mononuclear cells were analyzed for CFSE staining by flow cytometry. The ratio of target:control was used to determine the killing efficiency.
MCMV and L. monocytogenes Infections and Measurement of Macrophage Responses to TLR Ligands.
MCMV (Smith strain, 105, 5 × 105, or 106 pfu) were injected i.p. to establish infection. A plaque assay using subconfluent NIH 3T3 cell layers was used to determine the viral titer in the spleen of infected animals. Serum IFNγ, IL-6, TNFα, IL-12/23 p40, and IL-12p70 levels were measured 36 h postinfection by ELISA (eBioscience). For in vivo challenge with L. monocytogenes (strain 10403S; Xenogen), 105, 5 × 105, or 106 cfu of bioluminescent bacteria were prepared and injected intravenously as described in ref. 35.
TNFα production by thioglycolate-elicited peritoneal macrophages was measured by L929 bioassay after stimulation with TLR-activating ligands for 4 h as described in ref. 35. Pro-IL-1β, MAPK phosphorylation, and MAPK proteins were measured in lysates derived from 4 × 106 bone marrow-derived macrophages grown by using L-929 cell conditioned medium and stimulated with 10 ng/ml LPS (Alexis). Blots were probed with antibodies against IL-1β (R&D Systems), tubulin (Sigma), phospho-IκB, IκB, phospho-p38, p38, phospho-p42/44, and p42/44 (Cell Signaling).
Immunoblotting.
Western blot analysis was performed by using standard methods as described in SI Materials and Methods.
PCR Primers Used for Genotyping.
Mice were genotyped by using PCR followed by either restriction enzyme digestion or sequencing as described in SI Materials and Methods.
Supplementary Material
Acknowledgments.
Annika Samuelsson and Karen Whitley gave excellent technical support. This work was supported by National Institutes of Health Grants CA31496, GM067759, and HHSN272200700038C. B.A.C. was supported by an National Health and Medical Research Council CJ Martin Fellowship. Funding was also provided by the Swedish Medical Research Council and the Swedish Strategic Foundation (IRIS).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806619105/DCSupplemental.
References
- 1.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 2.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 4.Shinkai K, McCalmont TH, Leslie KS. Cryopyrin-associated periodic syndromes and autoinflammation. Clin Exp Dermatol. 2008;33:1–9. doi: 10.1111/j.1365-2230.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 5.Shultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 6.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowski M, et al. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 9.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–258. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- 10.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am J Pathol. 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz LD, Green MC. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. J Immunol. 1976;116:936–943. [PubMed] [Google Scholar]
- 12.Shultz LD. Pleiotropic effects of deleterious alleles at the “motheaten” locus. Curr Top Microbiol Immunol. 1988;137:216–222. doi: 10.1007/978-3-642-50059-6_32. [DOI] [PubMed] [Google Scholar]
- 13.Yu CC, et al. B and T cells are not required for the viable motheaten phenotype. J Exp Med. 1996;183:371–380. doi: 10.1084/jem.183.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pao LI, et al. B Cell-Specific Deletion of Protein-Tyrosine Phosphatase Shp1 Promotes B-1a Cell Development and Causes Systemic Autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol. 1998;185:49–58. doi: 10.1006/cimm.1998.1272. [DOI] [PubMed] [Google Scholar]
- 16.David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Songyang Z, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, et al. Crystal structures of peptide complexes of the amino-terminal SH2 domain of the Syp tyrosine phosphatase. Structure (London) 1994;2:423–438. doi: 10.1016/s0969-2126(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, et al. Crystal structure of human protein-tyrosine phosphatase SHP-1. J Biol Chem. 2003;278:6516–6520. doi: 10.1074/jbc.M210430200. [DOI] [PubMed] [Google Scholar]
- 20.Pei D, Wang J, Walsh CT. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141–1145. doi: 10.1073/pnas.93.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure (London) 1998;6:249–254. doi: 10.1016/s0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Z, et al. Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis. Proc Natl Acad Sci USA. 2006;103:10961–10966. doi: 10.1073/pnas.0603804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 24.Wei G, et al. Activated Ets2 is required for persistent inflammatory responses in the motheaten viable model. J Immunol. 2004;173:1374–1379. doi: 10.4049/jimmunol.173.2.1374. [DOI] [PubMed] [Google Scholar]
- 25.Thrall RS, Vogel SN, Evans R, Shultz LD. Role of tumor necrosis factor-alpha in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am J Pathol. 1997;151:1303–1310. [PMC free article] [PubMed] [Google Scholar]
- 26.An H, et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 27.Su X, Zhou T, Yang P, Edwards CK, III, Mountz JD. Reduction of arthritis and pneumonitis in motheaten mice by soluble tumor necrosis factor receptor. Arthritis Rheum. 1998;41:139–149. doi: 10.1002/1529-0131(199801)41:1<139::AID-ART17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Dayer JM. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2003;42(Suppl 2):ii3–10. doi: 10.1093/rheumatology/keg326. [DOI] [PubMed] [Google Scholar]
- 29.Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 30.Lutzner MA, Hansen CT. Motheaten: An immunodeficient mouse with markedly less ability to survive that the nude mouse in a germfree environment. J Immunol. 1976;116:1496–1497. [PubMed] [Google Scholar]
- 31.Crozat K, et al. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d:a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. J Exp Med. 2007;204:853–863. doi: 10.1084/jem.20062447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder 7. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 33.Hoebe K, Du X, Goode J, Mann N, Beutler B. Lps2: A new locus required for responses to lipopolysaccharide, revealed by germline mutagenesis and phenotypic screening. J Endotoxin Res. 2003;9:250–255. doi: 10.1179/096805103225001459. [DOI] [PubMed] [Google Scholar]
- 34.Lawson BR, et al. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest. 2000;106:207–215. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutschmann S, et al. PanR1, a dominant negative missense allele of the gene encoding TNF-alpha (Tnf), does not impair lymphoid development. J Immunol. 2006;176:7525–7532. doi: 10.4049/jimmunol.176.12.7525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.