Abstract
At an early stage during Bacillus subtilis endospore development the bacterium divides asymmetrically to produce two daughter cells. The smaller cell (forespore) differentiates into the endospore, while the larger cell (mother cell) becomes a terminally differentiated cell that nurtures the developing forespore. During development the mother cell engulfs the forespore to produce a protoplast, surrounded by two bilayer membranes, which separate it from the cytoplasm of the mother cell. The activation of σG, which drives late gene expression in the forespore, follows forespore engulfment and requires expression of the spoIIIA locus in the mother cell. One of the spoIIIA-encoded proteins SpoIIIAH is targeted specifically to the membrane surrounding the forespore, through an interaction of its C-terminal extracellular domain with the C-terminal extracellular domain of the forespore membrane protein SpoIIQ. We identified a homologous relationship between the C-terminal domain of SpoIIIAH and the YscJ/FliF protein family, members of which form multimeric rings involved in type III secretion systems and flagella. If SpoIIIAH forms a similar ring structure, it may also form a channel between the mother cell and forespore membranes. To test this hypothesis we developed a compartmentalized biotinylation assay, which we used to show that the C-terminal extracellular domain of SpoIIIAH is accessible to enzymatic modification from the forespore cytoplasm. These and other results lead us to suggest that SpoIIIAH forms part of a channel between the forespore and mother cell that is required for the activation of σG.
Keywords: Bacillus subtilis, flagellar protein export apparatus, sporulation, type III secretion system
Endospore formation in the gram-positive bacterium Bacillus subtilis involves a series of morphological changes that culminate in the production of a dormant spore (reviewed in refs. 1–3). In response to nutrient depletion, vegetative cells undergo an asymmetric cell division that yields two dissimilar progeny cells, the mother cell and forespore. Remarkably, the mother cell engulfs the forespore and nurtures it to maturity. Ultimately, the mother cell lyses, releasing the mature spore. The differentiation of these two cell types is governed by two parallel programs of gene expression controlled by a cascade of alternative RNA polymerase sigma (σ) factors. To synchronize the differentiation of the mother cell and forespore, the two programs of gene expression are coordinated through a series of intercellular signaling pathways. Following asymmetric cell division, σF is activated in the forespore. σF then signals the activation of σE in the mother cell. Together, σF and σE control expression of the genes responsible for forespore engulfment. After the completion of forespore engulfment, σG is activated in the forespore and signals the activation of σK in the mother cell. σG and σK control expression of the genes necessary for forespore maturation. While the intercellular signaling pathways that trigger the activation of σE and σK in the mother cell are well understood, the mechanisms that trigger the activation of σG in the forespore are not known.
In addition to the completion of forespore engulfment, the eight-gene spoIIIA locus (spoIIIAA–AH) is also necessary for the activation of σG. The spoIIIA locus is expressed under the control of σE in the mother cell. With the exception of spoIIIAA, each of the spoIIIA genes (spoIIIAB–AH) encodes a predicted integral membrane protein. SpoIIIAH contains an N-terminal transmembrane segment (TMS) and a C-terminal extracellular domain. Although SpoIIIAH is inserted randomly into the mother cell membrane, it is targeted to the sporulation septum, separating the mother cell and forespore, through an interaction of its C-terminal domain with the forespore integral membrane protein SpoIIQ (4, 5). SpoIIIAH and SpoIIQ colocalize along the engulfing membrane and form discrete foci surrounding the forespore. However, it is unclear why SpoIIIAH is necessary for σG activity in the forespore.
In this study, we identified a homologous relationship between SpoIIIAH and the proteins that form the export channel and structural scaffold of type III secretion and flagellar export systems. We hypothesized that if SpoIIIAH also forms a channel, then its C-terminal extracellular domain may be accessible to modification by an enzyme produced in the forespore. To test this hypothesis, we developed a compartmentalized biotinylation assay. Using this assay, we demonstrated that the C-terminal domain of SpoIIIAH is accessible to biotin ligase produced in the forespore. These and other results suggest that SpoIIIAH and SpoIIQ may form a channel between the mother cell and forespore.
Results
C-Terminal Extracellular Domain of SpoIIIAH Is Homologous to the YscJ/FliF Protein Family.
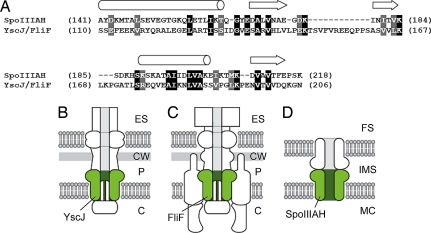
Although no significant relationship to a protein of known function could be established using conventional search methods, the HHpred server (6, 7) identified a homologous relationship between the C-terminal extracellular domain of SpoIIIAH and the YscJ/FliF protein family (PF01514) (Fig. 1A). The sequence similarity covers a 78-amino-acid stretch with 23% identity, corresponding to the C-terminal domain of the YscJ proteins and the central domain of the FliF proteins. The members of the YscJ family are involved in type III secretion of virulence factors in gram-negative bacteria (8). The structure of a representative member, the enteropathogenic Escherichia coli EscJ protein, indicated that these proteins probably form ring structures with a central channel that is 75Å in diameter (9). The dimensions of the 24-subunit EscJ ring were similar to those estimated by electron microscopy (10). The YscJ ring is thought to form the inner membrane channel of the type III secretion system (Fig. 1B) (11). The FliF protein forms the bacterial flagellar protein export channel and the structural scaffold for flagellar assembly (Fig. 1C) (12). Therefore, we hypothesized that the C-terminal extracellular domain of SpoIIIAH forms a large, hollow ring with the N-terminal TMS anchored in the mother cell membrane (Fig. 1D). The SpoIIIAH ring may be part of a channel between the mother cell and forespore. The similarity of SpoIIIAH with the YscJ/FliF protein family and the hypothesis that SpoIIIAH forms a channel were independently proposed by Camp and Losick (13).
Fig. 1.
Homology between SpoIIIAH and the YscJ/FliF protein family. HHpred alignment of SpoIIIAH and the YscJ/FliF family. (A) Identical (black boxes) and similar (gray boxes) amino acids are highlighted. Predicted secondary structure is indicated above the alignment. Cartoon representations of the type III secretion system (B), flagellar basal body (C), and SpoIIIAH channel model (D). YscJ, FliF, and SpoIIIAH are labeled and colored green. The extracellular space (ES), cell wall (CW), periplasm (P), and cytoplasm (C) of gram-negative bacteria, and mother cell (MC), intermembrane space (IMS), and forespore (FS) of bacterial endospores are indicated.
Compartmentalized Biotinylation Assay.
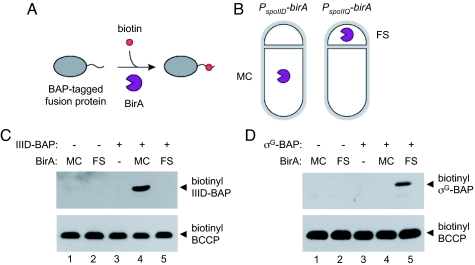
If SpoIIIAH forms part of a channel between the mother cell and forespore, then the C-terminal extracellular domain may be accessible to modification by an enzyme produced in the forespore. To test this prediction, we developed a compartmentalized biotinylation assay, involving the biotinylation of target proteins by the E. coli biotin ligase, BirA, produced in either the mother cell or forespore (Fig. 2 A and B). BirA transfers biotin to a specific lysine in biotin-accepting proteins (14). Biotinylated proteins can then be detected by Western blot with a streptavidin-horseradish peroxidase (HRP) conjugate (15). We tagged proteins with a 14-amino-acid biotin acceptor peptide (BAP), defined as the minimal substrate required for E. coli BirA-catalyzed biotinylation (16). Then we tested whether the fusion proteins were biotinylated in B. subtilis strains producing the E. coli BirA in either the mother cell or forespore, under the control of the spoIID and spoIIQ promoters, respectively (17, 18). Immunoblot analysis of BirA indicated that similar amounts of BirA accumulated after the onset of sporulation in both the mother cell- and forespore-producing strains [supporting information (SI) Fig. S1].
Fig. 2.
Compartmentalized biotinylation assay. Cartoon representations of (A) BirA-catalyzed transfer of biotin to biotin acceptor peptide (BAP)-tagged fusion proteins, and (B) strains producing E.coli BirA in the mother cell (MC) and the forespore (FS). Compartmentalized biotinylation of SpoIIID-BAP (C) and σG-BAP (D). Top in C and D are streptavidin-HRP Western blots with lysates prepared at t3 (SpoIIID-BAP) and t4 (σG-BAP). Lysates were prepared from strains producing different combinations of the indicated BAP-tagged fusion protein and BirA in the mother cell, the forespore, or not at all. C Lower and D Lower show streptavidin Western blots, with the same lysates shown in C Upper and D Upper, comparing levels of biotinyl-biotin carboxyl carrier protein (BCCP).
To test whether BirA activity was compartmentalized in these strains, we analyzed the biotinylation of BAP-tagged SpoIIID and σG, produced exclusively in the mother cell and forespore, respectively (19, 20). The biotinylated BAP-tagged fusion proteins were detected by Western blot with a streptavidin-HRP conjugate. SpoIIID-BAP was only biotinylated in the strain that produced BirA in the mother cell and not in the strain that produced BirA in the forespore (Fig. 2C, lanes 4 and 5). Conversely, σG-BAP was biotinylated only in the strain that produced BirA in the forespore and not the strain that produced BirA in the mother cell (Fig. 2D, lanes 4 and 5). Neither biotinyl-SpoIIID-BAP nor biotinyl-σG-BAP was detected in the control strains that produced BirA or the BAP-tagged fusion proteins alone (Fig. 2 C and D, lanes 1–3). B. subtilis BirA-catalyzed biotinylation of the biotin carboxyl carrier protein (21) served as an internal control, showing that each lane contained similar amounts of protein (Fig. 2 C and D, Bottom). Each of the strains was sporulation proficient, indicating that the BAP tag did not interfere with normal protein function (data not shown). These results demonstrate that the compartmentalized biotinylation assay can be used to determine whether a protein is accessible to either the mother cell or forespore cytoplasm.
The C-terminal Domain of SpoIIIAH Is Accessible to Forespore-Produced BirA.
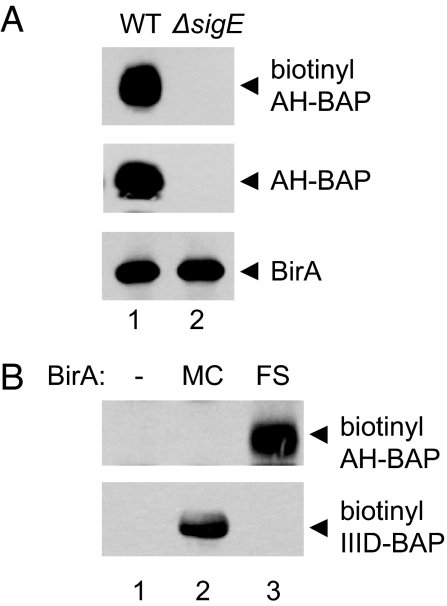
To test whether the C-terminal extracellular domain of SpoIIIAH is accessible to the forespore cytoplasm, we assessed compartmentalized biotinylation in strains producing a C-terminal BAP-tagged SpoIIIAH. The SpoIIIAH-BAP fusion protein was biotinylated when BirA was produced in the forespore (Fig. 3, lane 5). Biotinyl-SpoIIIAH-BAP was not detected in the absence of E. coli BirA (Fig. 3, lane 3) or when it was produced in the mother cell (Fig. 3, lane 4). Immunoblots with anti-SpoIIIAH antiserum showed that SpoIIIAH-BAP accumulated to levels similar to that of wild-type SpoIIIAH in each of the strains (Fig. 3 Lower). Furthermore, these strains supported the production of heat-resistant spores similar to that of the wild-type strain, indicating that the BAP-tagged fusion protein was functional (data not shown). These results suggest that the C-terminal extracellular domain of the mother cell protein SpoIIIAH is accessible to the forespore cytoplasm.
Fig. 3.
Forespore-specific biotinylation of C-terminal BAP-tagged SpoIIIAH. (Upper) A streptavidin-HRP Western blot with lysates prepared at t3 from strains producing combinations of SpoIIIAH-BAP and BirA produced in the mother cell, the forespore, or not at all, is shown. (Lower) An anti-SpoIIIAH Western blot with the same lysates as in Upper is shown.
Alternative explanations for the apparent forespore accessibility of the C-terminal BAP-tagged SpoIIIAH include noncompartmentalized production of SpoIIIAH-BAP in the forespore or noncompartmentalized BirA activity in the mother cell or intermembrane space. To test whether SpoIIIAH-BAP was produced in the forespore, we measured SpoIIIAH-BAP biotinylation in a mutant (sigE) that is defective in the mother cell but not forespore gene expression. If SpoIIIAH-BAP were produced in the forespore under the control of σF, then its expression would not be dependent on the mother cell specific activity of σE and therefore would be biotinylated even in a sigE mutant. However, we found that biotinyl-SpoIIIAH-BAP was not detected in the sigE mutant strain (Fig. 4A, Top). BirA production was unaffected in the mutant as expected, indicating that forespore gene expression was intact (Fig. 4A, Bottom). We also found that production of biotinyl-SpoIIIAH-BAP was not dependent on σG, the late forespore-specific factor (Fig. S2). Thus, SpoIIIAH-BAP production was completely dependent on σE and therefore restricted to the mother cell. To test for BirA activity in the mother cell in the strain that expressed BirA in the forespore, we analyzed biotinylation of SpoIIIAH-BAP and the mother cell protein SpoIIID-BAP in the same strain. As expected, SpoIIID-BAP was biotinylated only when BirA was produced in the mother cell but not in the strain that produced BirA in the forespore (Fig. 4B Lower). Conversely, SpoIIIAH-BAP was biotinylated only when BirA was produced in the forespore and not in the strain that produced BirA in the mother cell (Fig. 4B Upper). These results eliminate the possibility that biotinylation of SpoIIIAH-BAP occurred in the mother cell when BirA was produced in the forespore.
Fig. 4.
Compartmentalization of SpoIIIAH-BAP production and BirA activity. (A) SpoIIIAH-BAP biotinylation in WT (lane 1) and sigE (lane 2) strains producing BirA in the forespore. Streptavidin-HRP (Top), anti-SpoIIIAH (Middle), and anti-BirA (Bottom) Western blots with lysates prepared at t3. (B) Compartmentalized biotinylation of SpoIIIAH-BAP (Top) and SpoIIID-BAP (Bottom) in the same strains. Lysates were prepared at t3 and analyzed by streptavidin-HRP Western blots.
To test whether BirA produced in the forespore exhibited activity in the intermembrane space, we assessed biotinylation of C-terminal BAP-tagged control septal proteins SpoIID and SpoIVFA. SpoIID contains an extracellular peptidoglycan hydrolase domain that is necessary for septal thinning during forespore engulfment (22). SpoIVFA contains an extracellular domain that regulates late mother cell gene expression in response to a forespore protein that is secreted into the intermembrane space following engulfment (23). Neither SpoIID-BAP nor SpoIVFA-BAP was biotinylated (data not shown), while both supported the production of heat-resistant spores similar to wild type, indicating the fusion proteins were functional (data not shown). Therefore, BirA produced in the forespore is not active in the intermembrane space. This was not surprising because BirA-catalyzed biotinylation requires ATP (24), which is expected to be absent from the intermembrane space. Together these results indicate that SpoIIIAH-BAP is produced exclusively in the mother cell and accessible to biotinylation by BirA produced in the forespore cytoplasm. We note that the central diameter of the EscJ ring is large enough to accommodate BirA (9, 25). Therefore, these results are consistent with the hypothesis that SpoIIIAH forms a channel.
C-Terminal Extracellular Domain of SpoIIQ Is also Accessible to Forespore-Produced BirA.
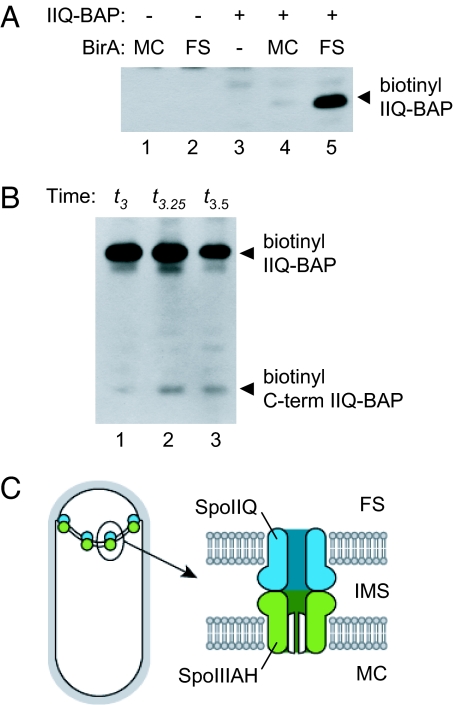
For the SpoIIIAH extracellular domain to be accessible to the forespore cytoplasm, it may interact with an integral membrane protein that forms a channel across the forespore membrane. Because SpoIIIAH interacts with the forepore protein SpoIIQ (4, 5), we tested whether the C-terminal extracellular domain of SpoIIQ was also accessible to the forespore cytoplasm. We introduced a C-terminal BAP-tagged spoIIQ allele into the compartmentalized biotinylation strains. These strains produced heat-resistant spores similar to wild type, indicating that the fusion protein was functional (data not shown). SpoIIQ-BAP was biotinylated only when BirA was produced in the forespore, suggesting that the C terminus of SpoIIQ is accessible to the forespore cytoplasm (Fig. 5A). After the completion of engulfment, SpoIIQ is processed by SpoIVB, a protease produced in the forespore and secreted into the intermembrane space, releasing the 30-kDa C-terminal domain from the full-length protein (26). To confirm that SpoIIQ-BAP was properly inserted into the membrane, we tested whether the biotinylated C-terminal proteolytic product accumulated after engulfment. Engulfment typically begins 2 h after the onset of sporulation (t2) and completes within 60 min (t3) (27, 28). We detected the expected 30-kDa biotinylated product beginning at ∼t3 (Fig. 5B), indicating that the biotinyl-SpoIIQ-BAP was correctly inserted in the membrane with the C-terminal domain in the intermembrane space. Because the insertion of bacterial integral membrane proteins is cotranslational [reviewed in (29)], it is unlikely that C-terminal BAP-tagged SpoIIQ is biotinylated in the cytoplasm before membrane insertion. These results are consistent with the idea that SpoIIIAH and SpoIIQ form a channel that is open on the forespore end and closed (or gated) on the mother cell end (Fig. 5C).
Fig. 5.
Forespore-specific biotinylation of C-terminal BAP-tagged SpoIIQ. (A) Streptavidin-HRP Western blot with lysates prepared at t3 from strains producing combinations of SpoIIQ-BAP and BirA produced in the mother cell, the forespore, or not at all. (B) Biotinyl-SpoIIQ-BAP proteolysis. Lysates prepared at t3 (lane 1), t3.25 (lane 2), and t3.5 (lane 3). (C) SpoIIIAH-SpoIIQ channel model. (Left) SpoIIIAH and SpoIIQ track along the engulfing membrane and colocalize in discrete foci surrounding the forespore. (Right) The C-terminal extracellular domains of SpoIIIAH and SpoIIQ interact in the intermembrane space and form a channel that is open at the forespore end and closed (or gated) at the mother cell end.
Degradation of the Putative Channel after Engulfment.
The interaction with SpoIIQ is necessary for the septal localization and stability of SpoIIIAH (4, 5, 30). This suggests that SpoIIQ should be necessary for the forespore accessibility and stability of SpoIIIAH-BAP. As expected, SpoIIIAH-BAP failed to accumulate in a spoIIQ mutant (data not shown). Septal thinning is necessary for the interaction of SpoIIIAH and SpoIIQ (4, 5). SpoIIIAH-BAP was undetectable (data not shown) in a mutant (spoIIP spoIID) that is completely defective in septal thinning (22).
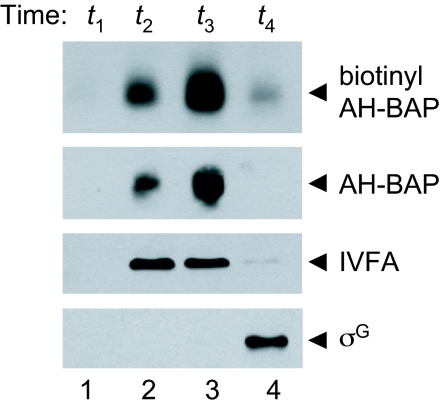
Because the interaction with SpoIIQ was necessary for SpoIIIAH-BAP stability, we hypothesized that biotinyl-SpoIIIAH-BAP would be degraded upon the completion of engulfment and initiation of SpoIIQ proteolysis. We found that biotinyl-SpoIIIAH-BAP accumulated until t3 and was degraded at t4 (Fig. 6 topmost). To confirm that the degradation of biotinyl-SpoIIIAH correlated with the completion of engulfment, we analyzed the control proteins SpoIVFA and σG. SpoIVB cleaves SpoIVFA after engulfment, resulting in SpoIVFA degradation (and activation of pro-σK processing) (31, 32). After engulfment, σG activates expression of its own gene, promoting the accumulation of σG. While SpoIVFA was present at t2–t3 and lost at t4 (Fig. 6 second from bottom), σG was detected by t4 (Fig. 6 bottommost). These results suggest that the channel forms during engulfment and is degraded shortly after its completion.
Fig. 6.
Degradation of biotinyl-SpoIIIAH-BAP after engulfment. Lysates prepared hourly between t1 and t4 were analyzed by streptavidin-HRP (topmost), anti-SpoIIIAH antibody (second from top), anti-SpoIVFA (second from bottom), and anti-σG (bottommost) Western blots.
Discussion
We identified a homologous relationship between the C-terminal extracellular domain of SpoIIIAH and the YscJ and FliF proteins, involved in type III secretion of virulence factors and flagellar protein export apparatus, respectively. We developed an in vivo compartmentalized biotinylation assay and showed that the C-terminal extracellular domain of the mother cell protein SpoIIIAH is accessible to biotinylation by BirA produced in the forespore, but not by BirA produced in the forespore. This result supports the hypothesis that SpoIIIAH forms a channel, analogous to those formed by YscJ and FliF, in the engulfing mother cell membrane. An alternative explanation is that the C terminus of SpoIIIAH extends across the forespore membrane. This is unlikely, however, because the C terminus of SpoIIIAH does not contain a predicted transmembrane segment or amphipathic α-helix. Furthermore, the C terminus of SpoIIIAH is predicted to form a globular mixed α/β domain that is topologically similar to the YscJ/FliF domain and unlikely to extend across the forespore membrane (9). The fraction of SpoIIIAH that is biotinylated is unknown. Therefore, we don't know whether all molecules of SpoIIIAH participate in channel formation or whether only a fraction of the SpoIIIAH C termini within a channel is accessible to BirA. We also showed that the C-terminal extracellular domain of the forespore protein SpoIIQ, with which SpoIIIAH interacts, is accessible to BirA produced in the forespore. Therefore, we propose that SpoIIIAH and SpoIIQ form a channel that is open on the forespore end and closed (or gated) on the mother cell end.
The core components of the flagellar protein export apparatus, including three soluble proteins (ATPase regulator FliH, ATPase FliI, and chaperone FliJ) and six integral membrane proteins (FlhA, FlhB, FliO, FliP, FliQ, and FliR), are homologous to the type III secretion system (33, 34). FlhA, FlhB, FliO, FliP, FliQ, and FliR are thought to be located within the FliF ring (35, 36). In the type III secretion system, the YscJ ring is thought to be functionally analogous to the FliF ring, providing a scaffold for the assembly of YscR, YscS, YscT, YscU, and YscV. FlhA and FlhB (YscV and YscU) act as a docking platform for the FliI (YscN) ATPase (35–38). Although the functions of FliO, FliP, FliQ, and FliR (YscR, YscS, and YscT) are unknown, together with FlhA and FlhB, they are thought to form a protein-conducting channel (36).
By analogy with the type III secretion system and flagellar protein export apparatus, we propose that SpoIIIAH acts as a scaffold upon which the components of a novel export apparatus is assembled and, together with SpoIIQ, forms the channel through which substrate translocation between the mother cell and forespore occurs. In addition to SpoIIIAH, the spoIIIA locus is predicted to encode a cytoplasmic protein (SpoIIIAA) and six integral membrane proteins (SpoIIIAB–AG), several of which share similarity with the components of several protein secretion systems (J.M., unpublished data). SpoIIIAA is similar to the secretion superfamily ATPases involved in type II secretion, type IV pilus assembly, type IV secretion, DNA uptake, and archaeal flagellar assembly systems (39). The predicted cytoplasmic domain of SpoIIIAB is similar to the type II secretion system protein GspF, a component of the inner membrane platform to which the ATPase docks (38). Although SpoIIIAC and SpoIIIAD do not share similarity with proteins of known function, they resemble FliQ and FliP (YscS and YscR), respectively, in size, and number, and orientation of transmembrane segments. SpoIIIAE is similar to various electrochemical potential-driven transporters. SpoIIIAF shows similarity with FlhB (YscU), while SpoIIIAG has limited similarity to YscJ and FliF. Therefore, we hypothesize that SpoIIIAB–AG are assembled within the SpoIIIAH ring and form a novel export apparatus that gates the SpoIIIAH–SpoIIQ channel at the mother cell end. SpoIIIAB, and possibly SpoIIIAF, provide a docking platform for SpoIIIAA, which couples substrate translocation with ATP hydrolysis. Although the roles of SpoIIIAA–AG are not known, our preliminary results indicate that SpoIIIAA is not required for formation of the SpoIIIAH–SpoIIQ channel (data not shown). This result is consistent with those of Camp and Losick in which they identified a mutation that partially bypasses the requirement for spoIIIAA–AG, but not spoIIIAH and spoIIQ, for σG activation (13). They postulated that, under certain conditions, SpoIIIAH and SpoIIQ can constitute a minimal pathway for σG activation. However, in an otherwise wild-type strain SpoIIIAA–AG are necessary for efficient σG activation via the SpoIIIAH–SpoIIQ channel.
The export apparatus model predicts that a substrate(s) is produced in the mother cell and translocated into the forespore, where it triggers the activation of σG. The nature of the substrate is unclear. On the basis of the similarities of the SpoIIIA proteins to various protein secretion systems, we suspect that the substrate is a protein. If this were the case, then a mutation in the gene encoding the protein substrate would block σG activation. No such mutant has been isolated in decades of selection and screening. As noted by Camp and Losick, it is possible that the gene is essential for viability or necessary for a process earlier in sporulation (13). The substrate may also be a peptide encoded by a small ORF (ORF) missed by mutagenesis and overlooked in microarray analyses. Another possibility is that the substrate is a small molecule or metabolite, without which the forespore cannot sustain essential processes such as transcription or translation (13). While we acknowledge that the substrate might be a metabolite, this model does not account for the fact that, before engulfment, σG remains inactive while σF is active in the forespore. This argues that σG inactivity is not exclusively because of a general metabolic deficiency but rather a specific inhibition of σG that must also be relieved. Although the function of the SpoIIIAH–SpoIIQ channel remains to be elucidated, all of the SpoIIIA-encoded products are highly conserved among evolutionarily diverse endospore-forming Bacillus and Clostridium species (data not shown). Therefore, this novel hybrid secretion system may be an ancient, but specialized and essential structure for mother cell–forespore communication.
Materials and Methods
To construct B. subtilis strains expressing E. coli birA in the mother cell and forespore, C-terminal His-tagged E. coli birA was amplified by PCR from strain MG1655 chromosomal DNA with a reverse primer containing the His6 coding sequence and the spoIID and spoIIQ promoters were amplified from B. subtilis strain JH642 chromosomal DNA. PspoIID-birA-his6 and PspoIIQ-birA-his6 were constructed by overlapping PCR, digested with BamHI and PstI, and ligated into the amyE gene replacement vector pDG1662. B. subtilis strain JH642 transformants were selected for chloramphenicol resistance and double-crossover recombinants were screened for spectinomycin sensitivity.
To construct strains expressing BAP-tagged alleles, the 3′ end of each coding sequence was amplified using gene-specific primers. Each reverse primer contained the BAP coding sequence followed by a stop codon. The spoIIID-BAP, sigG-BAP, spoIIIAH-BAP, spoIID-BAP, and spoIIQ-BAP fragments were digested with the appropriate restriction enzymes and ligated into an integration vector and used to transform B. subtilis. These strains contain the BAP-tagged alleles integrated at the native loci by Campbell recombination. spoIVFA-BAP was cloned into thrC gene replacement vector pDG1664 and used to transform B. subtilis. All B. subtilis strains, plasmids, and details of experimental procedures are described in SI Materials and Methods and Tables S1–S3.
Supplementary Material
Acknowledgments.
We thank Rich Losick and Amy Camp for communicating results before publication and helping with the manuscript. We also thank Kit Pogliano, Lee Kroos, and David Rudner for providing antibodies and strains, and Isabel Sa Nogueira for helpful suggestions. This work was supported by National Institute of General Medical Sciences Public Health Services Grant GM54395 (to C.P.M), and “Fundação para a Ciência e a Tecnologia” Grant POCTI/BIA-BCM/60855/2004 (to A.O.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806301105/DCSupplemental.
References
- 1.Piggot PJ, Coote JG. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Hilbert DW, Piggot PJ. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaylock B, Jiang X, Rubio A, Moran CP, Jr, Pogliano K. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev. 2004;18:2916–2928. doi: 10.1101/gad.1252704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doan T, Marquis KA, Rudner DZ. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol. 2005;55:1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- 6.Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 8.Galan JE, Collmer A. Type III secretion machines: Bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 9.Yip CK, et al. Structural characterization of the molecular platform for type III secretion system assembly. Nature. 2005;435:702–707. doi: 10.1038/nature03554. [DOI] [PubMed] [Google Scholar]
- 10.Sekiya K, et al. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc Natl Acad Sci USA. 2001;98:11638–11643. doi: 10.1073/pnas.191378598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimbrough TG, Miller SI. Assembly of the type III secretion needle complex of Salmonella typhimurium. Microbes Infect. 2002;4:75–82. doi: 10.1016/s1286-4579(01)01512-x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, et al. A structural feature in the central channel of the bacterial flagellar FliF ring complex is implicated in type III protein export. J Struct Biol. 1998;124:104–114. doi: 10.1006/jsbi.1998.4048. [DOI] [PubMed] [Google Scholar]
- 13.Camp AH, Losick R. A novel pathway of intercellular signaling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol Microbiol. 2008;69:402–417. doi: 10.1111/j.1365-2958.2008.06289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronan JE., Jr The E. coli bio operon: Transcriptional repression by an essential protein modification enzyme. Cell. 1989;58:427–429. doi: 10.1016/0092-8674(89)90421-2. [DOI] [PubMed] [Google Scholar]
- 15.Tian H, Boyd D, Beckwith J. A mutant hunt for defects in membrane protein assembly yields mutations affecting the bacterial signal recognition particle and Sec machinery. Proc Natl Acad Sci USA. 2000;97:4730–4735. doi: 10.1073/pnas.090087297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 1999;8:921–929. doi: 10.1110/ps.8.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driks A, Losick R. Compartmentalized expression of a gene under the control of sporulation transcription factor sigma E in Bacillus subtilis. Proc Natl Acad Sci USA. 1991;88:9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Londono-Vallejo JA, Frehel C, Stragier P. SpoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- 19.Kunkel B, Kroos L, Poth H, Youngman P, Losick R. Temporal and spatial control of the mother-cell regulatory gene spoIIID of Bacillus subtilis. Genes Dev. 1989;3:1735–1744. doi: 10.1101/gad.3.11.1735. [DOI] [PubMed] [Google Scholar]
- 20.Sun DX, Cabrera-Martinez RM, Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma G. J Bacteriol. 1991;173:2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marini P, Li SJ, Gardiol D, Cronan JE, Jr, de Mendoza D. The genes encoding the biotin carboxyl carrier protein and biotin carboxylase subunits of Bacillus subtilis acetyl coenzyme A carboxylase, the first enzyme of fatty acid synthesis. J Bacteriol. 1995;177:7003–7006. doi: 10.1128/jb.177.23.7003-7006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abanes-De Mello A, Sun YL, Aung S, Pogliano K. A cytoskeleton-like role for the bacterial cell wall during engulfment of the Bacillus subtilis forespore. Genes Dev. 2002;16:3253–3264. doi: 10.1101/gad.1039902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudner DZ, Losick R. Morphological coupling in development: Lessons from prokaryotes. Dev Cell. 2001;1:733–742. doi: 10.1016/s1534-5807(01)00094-6. [DOI] [PubMed] [Google Scholar]
- 24.Lane MD, Young DL, Lynen F. The enzymatic synthesis of holotranscarboxylase from apotranscarboxylase and (+)-biotin. I. Purification of the apoenzyme and synthetase; characteristics of the reaction. J Biol Chem. 1964;239:2858–2864. [PubMed] [Google Scholar]
- 25.Wilson KP, Shewchuk LM, Brennan RG, Otsuka AJ, Matthews BW. Escherichia coli biotin holoenzyme synthetase/bio repressor crystal structure delineates the biotin- and DNA-binding domains. Proc Natl Acad Sci USA. 1992;89:9257–9261. doi: 10.1073/pnas.89.19.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X, Rubio A, Chiba S, Pogliano K. Engulfment-regulated proteolysis of SpoIIQ: Evidence that dual checkpoints control sigma activity. Mol Microbiol. 2005;58:102–115. doi: 10.1111/j.1365-2958.2005.04811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogliano J. A vital stain for studying membrane dynamics in bacteria: A novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–1159. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp MD, Pogliano K. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc Natl Acad Sci USA. 1999;96:14553–14558. doi: 10.1073/pnas.96.25.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiba S, Coleman K, Pogliano K. Impact of membrane fusion and proteolysis on SpoIIQ dynamics and interaction with SpoIIIAH. J Biol Chem. 2007;282:2576–2586. doi: 10.1074/jbc.M606056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong TC, Cutting SM. SpoIVB-mediated cleavage of SpoIVFA could provide the intercellular signal to activate processing of Pro-sigmaK in Bacillus subtilis. Mol Microbiol. 2003;49:1425–1434. doi: 10.1046/j.1365-2958.2003.03651.x. [DOI] [PubMed] [Google Scholar]
- 32.Campo N, Rudner DZ. A branched pathway governing the activation of a developmental transcription factor by regulated intramembrane proteolysis. Mol Cell. 2006;23:25–35. doi: 10.1016/j.molcel.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.MacNab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 34.Cornelis GR. The type III secretion injectisome. Nat Rev. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 35.Minamino T, MacNab RM. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- 36.Minamino T, MacNab RM. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kihara M, Minamino T, Yamaguchi S, MacNab RM. Intergenic suppression between the flagellar MS ring protein FliF of Salmonella and FlhA, a membrane component of its export apparatus. J Bacteriol. 2001;183:1655–1662. doi: 10.1128/JB.183.5.1655-1662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Py B, Loiseau L, Barras F. An inner membrane platform in the type II secretion machinery of gram-negative bacteria. EMBO Rep. 2001;2:244–248. doi: 10.1093/embo-reports/kve042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Planet PJ, Kachlany SC, DeSalle R, Figurski DH. Phylogeny of genes for secretion NTPases: Identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc Natl Acad Sci USA. 2001;98:2503–2508. doi: 10.1073/pnas.051436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.