Abstract
The dopamine system, which plays a crucial role in reward processing, is particularly vulnerable to aging. Significant losses over a normal lifespan have been reported for dopamine receptors and transporters, but very little is known about the neurofunctional consequences of this age-related dopaminergic decline. In animals, a substantial body of data indicates that dopamine activity in the midbrain is tightly associated with reward processing. In humans, although indirect evidence from pharmacological and clinical studies also supports such an association, there has been no direct demonstration of a link between midbrain dopamine and reward-related neural response. Moreover, there are no in vivo data for alterations in this relationship in older humans. Here, by using 6-[18F]FluoroDOPA (FDOPA) positron emission tomography (PET) and event-related 3T functional magnetic resonance imaging (fMRI) in the same subjects, we directly demonstrate a link between midbrain dopamine synthesis and reward-related prefrontal activity in humans, show that healthy aging induces functional alterations in the reward system, and identify an age-related change in the direction of the relationship (from a positive to a negative correlation) between midbrain dopamine synthesis and prefrontal activity. These results indicate an age-dependent dopaminergic tuning mechanism for cortical reward processing and provide system-level information about alteration of a key neural circuit in healthy aging. Taken together, our findings provide an important characterization of the interactions between midbrain dopamine function and the reward system in healthy young humans and older subjects, and identify the changes in this regulatory circuit that accompany aging.
Keywords: aging, dopamine, fMRI, PET, reinforcement
Successful aging has become one of the most crucial public health challenges of our time. Achieving an understanding of age-related changes in the neurobiology of key brain circuits, such as the reward system, is an integral part of rising to this challenge. Detecting, predicting, and responding to reward information are fundamental capabilities of simple life forms that have evolved in humans into complex behavioral patterns, such as learning, motivation, and appetitive and hedonic activities, which remain essential as we age. A substantial body of data in animals indicates that dopamine is closely associated with reward processing (1–3), and that midbrain dopamine neurons send reward-related signals to postsynaptic sites, particularly the prefrontal cortex. In humans, although indirect evidence from pharmacological (4, 5) and clinical (6–8) studies also suggests a fundamental role of dopamine in reward processing, there has been no direct demonstration of a link between midbrain dopamine and reward-related neural response. Moreover, although the dopamine system is known to be particularly vulnerable to aging (9, 10), there has been no search for alterations in this predicted relationship in older humans. Here, by using 6-[18F]fluoroDOPA (FDOPA)—positron emission tomography (PET) and event-related 3T functional magnetic resonance imaging (fMRI) in the same subjects, we establish the link between midbrain presynaptic dopamine synthesis and activation of the reward circuit in humans and we identify age-related changes in the regulation of this system.
Fundamental electrophysiological experiments on nonhuman primates have demonstrated that midbrain dopamine cells fire both during anticipation of uncertain rewards and at the time of unexpected reward delivery (1, 11). In parallel with these fundamental results, fMRI studies in healthy, young subjects have documented that distinct reward anticipation- and outcome-processing phases are associated with differential patterns of specific midbrain dopaminergic postsynaptic targets, specifically, sustained ventral striatal and transient prefrontal cortex (PFC) activity, respectively (12, 13). Building on these findings, we investigated age-related changes in the reward system; first by determining whether projection sites of midbrain dopaminergic neurons respond differentially to the phasic midbrain dopaminergic signal and the sustained anticipatory signal in older participants, and second, by specifically testing the hypothesis that aging induces functional changes in the interactions of reward-related activity and midbrain dopaminergic function (assessed with 6-[18F]FDOPA PET).
Results
To disentangle brain response to anticipation of potential monetary rewards from brain response at the time of reward outcome, we first measured the blood-oxygen-level-dependent (BOLD) signal in healthy aging (66 ± 5 years old) and young subjects (25 ± 3.7 years old) during presentation of “slot machine” stimuli (see Materials and Methods). We found that healthy aging is accompanied by both distinct and common neurofunctional characteristics within specific components of the reward system [Fig. 1, Fig. 2, and supporting information (SI) Table S1].
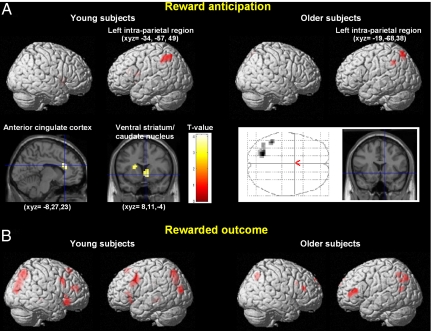
Fig. 1.
Statistical t maps of the within-groups effects in the different phases of the reward paradigm (P < 0.005, uncorrected). (A) (Left) Main effect of anticipating reward in young subjects during the delay period, showing activation in the left intraparietal cortex, ventral striatum, caudate nucleus, and anterior cingulate cortex. (Right) Main effect of anticipating reward in older subjects during the delay period, showing activation in the left intraparietal cortex only. The glass brain and the coronal slice indicate that no ventral striatum activity was observed in older subjects. (B) (Left) Main effect of reward receipt in young subjects at the time of the rewarded outcome showing activation in a large bilateral prefronto-parietal network. (Right) Main effect of reward receipt in older subjects at the time of the rewarded outcome showing bilateral prefronto-parietal activation.
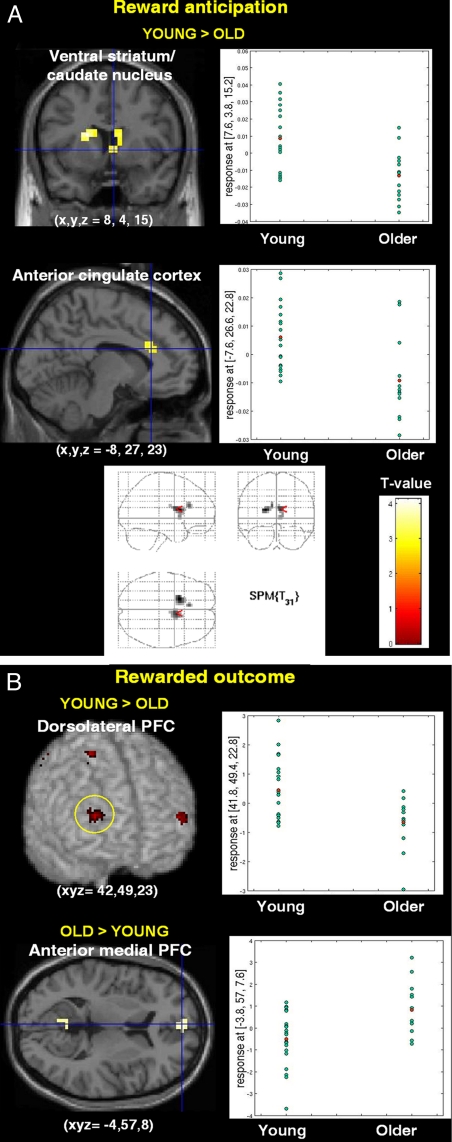
Fig. 2.
Statistical t maps showing between-group comparison of fMRI BOLD signal by task phase. (A) Between-group comparison during reward anticipation showing higher ventral striatum and anterior cingulate cortex activation in young subjects. The graphs show parameter estimates in these two brain regions in young and old subjects. The glass brain indicates that the ventral striatum and anterior cingulate cortex are the only two brain regions more activated in young subjects during reward anticipation. (B) Group-by-reward outcome interaction showing that young subjects activate the dorsolateral prefrontal and parietal cortices more robustly, whereas older subjects deactivated the medial PFC less than young subjects. Graphs show the parameter estimates in the right dorsolateral PFC and anterior medial PFC in young and old subjects.
During reward anticipation (Fig. 1A), young subjects recruited the ventral striatum, the anterior cingulate cortex, and the left intraparietal region. In contrast, older subjects recruited only the left intraparietal region and this was the sole brain area commonly activated by young and older subjects during reward anticipation (Fig. 1B). A formal between-group interaction analysis demonstrated that the ventral striatum and the anterior cingulate cortex were specifically and significantly recruited by the group of young subjects compared with the older participants (Fig. 2A).
At the time of reward delivery, young subjects recruited a large, bilateral fronto-parietal network that was also present, but to a lesser extent, in older subjects (Fig. 1B and Table S2). A formal group-by-reward outcome analysis confirmed a more robust activation of this brain network in young subjects (Fig. 2B Upper). Conversely, older subjects, when compared with young adults exhibited higher BOLD response in the anterior medial PFC, the posterior cingulate cortex, and the inferior parietal cortex (Fig. 2B Lower).
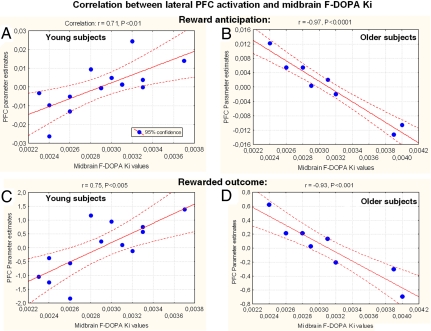
Of more primary import to our research questions, we also measured presynaptic dopamine synthesis within a subset of the fMRI cohort while subjects were in an awake, resting state with a series of 25 dynamic PET measurements acquired after injection of 8–16 millicuries (mCi) of 6-[18F]FDOPA, which measures the kinetics of amino acid decarboxylase, the rate-limiting enzyme in the transformation of 3,4-dihydroxyphenylalanine (DOPA) to dopamine. After voxel-based kinetic analysis, Ki was determined within a midbrain region of interest. Correlation between midbrain FDOPA uptake and reward-related BOLD signal during reward anticipation and outcome within all brain voxels was determined in each group separately with a voxelwise regression. Although no significant difference in midbrain FDOPA uptake between aging (0.0032 ± 0.0006; mean ± SD) and young adults (0.0029 ± 0.0004) was observed (P = 0.18), there was an interaction between midbrain dopamine synthesis and reward-related lateral PFC function in young versus older adults. In young subjects, midbrain FDOPA Ki values correlated positively with activation of the lateral PFC, both during reward anticipation (x, y, z = 42, 46, 19; Spearman's r = 0.71; P = 0.007) (Fig. 3A) and at the time of reward delivery (x, y, z = 49, 27, 11; r = 0.75; P < 0.005; x, y, z = −42, 30, −8; r = 0.7; P < 0.005) (Fig. 3C), whereas in older subjects a negative correlation was found, during both reward anticipation (x, y, z = 27, 27, 8; r = −0.85; P = 0.008; x, y, z = −23, 30, 15; r = −0.97; P < 0.0001) (Fig. 3B) and outcome (x, y, z = 53, 34, 8; r = −0.89; P < 0.005; x, y, z = −42, 34, 4; r = −0.93; P < 0.001) (Fig. 3D). The statistical significance of the observed between-groups differences in the directions of the slopes of the correlation of BOLD signal with midbrain Ki was confirmed both during the anticipatory period (right lateral PFC: Fisher Z test = 3.91, P < 0.0005) and at the time of the outcome (right PFC: Z = 4.37, P < 0.0001; left PFC: Z = 4.6, P < 0.0001).
Fig. 3.
Relationship between midbrain dopamine uptake (Ki) and lateral prefrontal BOLD signal in young and old adults during reward anticipation and at the time of reward delivery. Significant positive correlation of midbrain Ki with BOLD change during reward anticipation in young subjects (x, y, z = 42, 46, 19; Spearman's r = 0.71, P < 0.01; regression line with 95% confidence bands) (A) and significant negative correlation of midbrain Ki with BOLD change in older subjects (x, y, z = −23, 30, 15; r = −0.97, P < 0.0001) (B). Significant positive correlation of midbrain Ki with BOLD signal in lateral PFC at the time of rewarded outcome: in young subjects (x, y, z = 49, 27, 11; r = 0.75, P < 0.005) (C) and significant negative correlation of midbrain Ki with BOLD response in older subjects (x, y, z = −42, 34, 4; r = −0.93, P < 0.001) (D). Correlations were observed bilaterally in the prefrontal cortex in all comparisons (see Text), except during reward anticipation in young subjects, where right predominated.
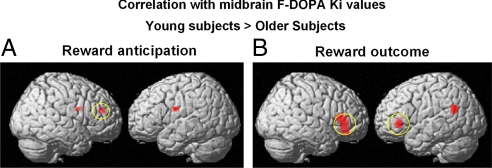
The regional specificity of these findings was examined with a formal between-group voxelwise comparison of the BOLD/midbrain FDOPA correlation maps for the older and younger subjects during reward anticipation and at the time of reward delivery. These analyses revealed that significant between-groups difference in correlation was observed predominantly in the lateral PFC (Fig. 4). These data provide direct in vivo evidence for a dopaminergic tuning mechanism of reward-related prefrontal function and for alteration of this tuning in aging.
Fig. 4.
Voxelwise comparisons between the correlation maps of F-DOPA Ki with BOLD signal in young versus older adults. (A) During reward anticipation, between-group voxelwise comparisons of the correlation maps revealed that the slopes of the correlations in young subjects were significantly greater than those in older subjects (in whom the correlations were negative; see Fig. 3) in the lateral PFC. (B) At the time of reward outcome, between-group voxelwise comparisons of the correlation maps showed that the slopes of the correlations in young subjects were significantly greater than the negative correlation in older subjects (see Fig. 3) in the bilateral PFC and left temporoparietal junction.
Discussion
This study directly characterizes the interactions between midbrain dopamine function and the reward system, both in healthy young humans and in older subjects, and thereby identifies changes in this regulatory circuit in healthy aging. Although predicted by animal results indicating that dopamine is closely associated with reward processing (2, 3, 14–18), such an association has not previously been shown directly in humans. Moreover, our multimodal imaging approach offers key insights into findings with fMRI, a technique that cannot by itself directly relate BOLD signal changes observed in the reward system with dopaminergic activity, a challenge met by our concurrent measurements of midbrain dopamine synthesis with FDOPA PET.
Although extensive investigations have been carried out on age-related neurofunctional changes in working memory and episodic memory circuits (19, 20), the majority of previously published fMRI studies of the reward system have been restricted to young subjects. Before addressing the question of the relationship between midbrain dopamine synthesis and reward-related neural activity, we disentangled brain response to anticipation of potential monetary rewards from that at the time of reward outcome by using event-related fMRI in healthy elders and young subjects.
During reward anticipation, young subjects recruited the ventral striatum and the anterior cingulate cortex (Fig. 1A and Table S1), confirming previous reports that anticipation of reward versus nonreward activated foci in the ventral striatum in healthy young subjects (12, 21). More importantly, this striatal activation was specific to young subjects (Fig. 2A). This result is in accordance with a recent report of decreased striatal activity in older subjects during reward association learning (22), although another study (23) failed to detect age-related differences in striatal activity during reward anticipation, and did not report results at the time of rewarded outcome. The discrepancy in the results between studies may be explained by differences in experimental paradigms. Specifically, one study used a probabilistic object reversal task including learning and search components (22), whereas the other study explored neural activity during a canonical monetary incentive delay task combining gains and losses (23). In addition to brain regions showing age-related differences during reward anticipation, we found a single brain area, the intraparietal region (Fig. 1A), commonly activated by young and older individuals during anticipation, likely reflecting a common attentional effect for potentially rewarded cues.
At the time of reward delivery, both young and older subjects recruited a large bilateral fronto-parietal network (Fig. 1B), but this network, and particularly the dorsolateral prefrontal cortex, was more robustly activated in young subjects (Fig. 2B and Table S2). This may reflect decreased neural sensitivity to salient rewards in older subjects, in agreement with cognitive studies demonstrating age-related dorsolateral PFC changes (19). Conversely, the anterior medial PFC, part of the default mode network, was less deactivated relative to baseline in older subjects compared with young adults. Although we cannot rule out the possibility that older individuals responded to nonrewarded events more robustly than younger participants, this finding extends to the reward domain previously observed age-related changes of functional properties within regions showing deactivations (24).
In addressing our primary research question, the relationship between midbrain dopamine synthesis and reward-related neural recruitment, we identified an age-related change in the direction of this relationship (Fig. 3). The age-related difference in the slope of the correlation between lateral prefrontal activity and dopamine synthesis rate demonstrates that for younger subjects, those with higher basal levels of dopamine have greater BOLD activity, both during reward anticipation and at the time of rewarded outcome, whereas older subjects show the opposite pattern, with greater reward-related BOLD activity for those with lower basal dopamine levels. Thus, the impact on dorsolateral prefrontal cortex (DLPFC) activation of a given level of midbrain dopamine was opposite in young and older subjects. A plausible cellular mechanism for the between-group difference in the direction of the relationship between prefrontal function and midbrain dopamine synthesis involves the marked loss of dopamine function in the aging PFC (25, 26), and the action of extracellular dopamine in the PFC (23, 27). A given rate of midbrain dopamine synthesis in young and old subjects would be predicted to lead, in the elderly, to less prefrontal dopamine stimulation and to prefrontal compensation for this reduction. This compensatory mechanism may involve complex and interactive effects between the BOLD response and the reduction of dopamine receptors in the PFC of older subjects (25, 26, 28–30). The opposing correlational results observed in young and older subjects are consistent with the well established inverted U-shaped relationship between dopaminergic activity and prefrontal function in which either reduced or excessive PFC dopamine receptor stimulation leads to suboptimal PFC function and where aged individuals have been posited to reside on the left limb of the curve where dopamine receptor stimulation is reduced (27, 31–34).
Several points concerning our multimodal approach should be made. First, because FDOPA uptake was assessed during a 90-min resting state, this measure presumably reflects a basal dopamine synthesis rate rather than dynamic variation of dopamine synthesis in response to the different stages of the reward paradigm. Thus, our findings likely reflect a primary role for tonic dopaminergic modulation of reward-related PFC activity (35). Second, comparing healthy elderly and healthy young control subjects, we found no between-group difference in midbrain dopamine synthesis, in accordance with several previous studies (36). It should also be noted that the resolution of the PET technique did not allow us to distinguish between ventral tegmental area and nigral dopamine neurons in the midbrain. Third, although FDOPA is primarily taken up and metabolized in dopamine neurons, a considerably lesser degree of FDOPA metabolism is also possible within serotonin and noradrenalin neurons (37). However, because of the predominance of FDOPA uptake in dopaminergic neurons, particularly in the midbrain, together with basic research documenting that the activity of midbrain dopamine neurons is the prime modulator of the reward system and the prefrontal cortex in particular (38), we believe that our results likely predominantly reflect dopaminergic regulation. Fourth, although correlational measures do not imply causality, our multimodal approach provides system-level information that reflects the mechanism of midbrain-prefrontal functional circuitry.
Finally, several special considerations concerning fMRI studies in aging deserve comment. The first consideration is that normal aging can affect the cerebrovascular system, which in turn affects the neurovascular coupling that is the basis of the BOLD signal (39). Thus, main effects of age in fMRI could be due to age differences in the coupling between neural activation and the BOLD signal rather than true age differences in neural activity. One way to mitigate this problem is the within-subject, across-event-types design and analysis adopted here, specifically first assessing within-group differences in task-related activity followed by tests of group-by-task interactions (40–43). We also minimized this concern by studying only individuals who were healthy, were receiving no medications, and had no signs of pathology on structural MRI. Second, older adults sometimes respond to cognitive activation paradigms with a smaller dynamic range of BOLD signal than do younger subjects; this was not the case in the brain regions showing group-by task-interactions in our study (Fig. 2). Third, although normal aging has been associated with a lag in the time-to-peak of the BOLD response, the overall shape of the BOLD response does not change with age (44, 45). Another potential confound is that brain volume is affected by aging, which could have influenced our PET data through partial volume effects. Our processing procedures, which include spatial normalization followed by voxelwise mapping, minimize these effects, as also seen in the largest FDOPA PET study to date (46). Future work will be necessary to evaluate whether additional measures to reduce partial volume effects might be beneficial in such multimodal experiments.
Our work offers important insights into the neurobiology of the reward system in healthy aging, pinpointing alterations across the adult lifespan and demonstrating the relationship of this functional circuit with midbrain dopamine. These findings may prompt further experiments in the identified circuit and may lead to studies of mechanistically targeted therapeutic interventions involving the dopamine system in individuals for whom, unlike our cohort, the neural aging process has not been successful. Our results are also relevant to several lines of investigation in clinical neuroscience because of the fundamental role of the dopaminergic reward system in key behavioral processes, because of the theoretical import of the findings (e.g., tuning mechanisms, dopaminergic influence on computational property of neural networks), and because of their potential clinical implications for dysfunctions of the dopaminergic system and pathologies of reward processing (e.g., parkinsonism, schizophrenia, drug addiction, and pathological gambling).
In summary, we directly demonstrate a tight coupling of midbrain dopamine synthesis and reward-related PFC activity, and provide direct evidence for an alteration of this regulatory relationship in older humans. Our multimodal neuroimaging approach, together with strong hypotheses derived from animal studies, provides mechanistic information about reward-related neural processing and its alteration in healthy aging.
Materials and Methods
Subjects.
Subjects provided written, informed consent as approved by the National Institute of Mental Health Institutional Review Board and the Radiation Safety Committee. First, we used an event-related fMRI paradigm designed to disentangle the brain regions activated in anticipation of potential monetary rewards from those responding at the time of rewarded outcome. Thirty-three healthy volunteers, 13 aging subjects (mean age = 66 ± 5 years, 6 women), and 20 young subjects (mean age = 25 ± 3.7 years, 10 women) were scanned during presentation of slot machine-type stimuli that systematically varied reward probability and magnitude (12, 13). We also used the tracer 6-[18F]FDOPA PET to measure the basal dopamine synthesis rate in 21 of the fMRI participants, 13 young (24.5 ± 3 years, 5 women) and 8 older subjects (65 ± 5 years, 2 females). The inclusion criteria were as follows: no central nervous system (CNS)-active medication or illicit drugs and no regular consumption of nicotine or alcohol. No subject had a history of gambling and all were free of past and present neurologic and psychiatric diseases as determined by normal medical history, physical examination, laboratory tests, and structured psychiatric diagnostic interview. All participants were additionally screened with structural MRI scans and were found to be free of brain abnormalities, including those common in older controls, such as atrophy and microvascular changes. The younger women were menstruating regularly and were randomly selected and distributed with regard to menstrual cycle phase. The older women were postmenopausal and had not received hormone replacement therapy for at least 5 years before the study. Subjects were paid for participating and earned extra money for performing the fMRI reward task described below. Subjects were told that they would earn a percentage of each of the $10/$20 bills presented on the screen, but were not told the exact percentage.
fMRI Methods.
Experimental paradigm.
Subjects viewed stimuli representing “slot machines” projected on a screen (13). Experimental trials were divided into two phases, reward anticipation and outcome. During reward anticipation, a slot machine was presented on the screen and the words: “Chance to win $XX” (where XX stands for $0, $10, and $20) remained visible on top of each slot machine with a pie chart displaying in red the probability of winning the indicated amount of money and in white the probability of receiving nothing. There were four slot machines (A, B, C, or D) designed to vary reward probability, magnitude, and expected reward value (reward probability·magnitude): Slot A: P = 1/4, $20; P = 3/4, $0; Slot B: P = 1/2, $20; P = 1/2, $0; Slot C: P = 1/2, $10; P = 1/2, $0; Slot D: P = 1, $0 (sure to get no reward).
During the delay phase, spinners from the slot machines rotated successively before stopping on a fixed image that was displayed until the end of the trial. The delay duration was fixed (15 s). During the outcome phase (2 s), pictures of “$10” and “$20” bills or “$0” were projected for 2 s, the former two surrounded, respectively, by a small and a large stack of gold pieces to visually reinforce the experience of distinct reward magnitudes. To equalize visual similarity between stimuli, the “$0” outcome was presented in a gray rectangle having the same dimensions as the bills. The intertrial interval between slot machines varied between 4 s and 16.5 s with a geometric distribution of mean = 6.8 s.
Subjects indicated which slot machine was presented by pressing a specific response button on a diamond-shaped four-response button device at the time of slot presentation and again at the time of the outcome (regardless of winning or not). The association between each slot machine and a specific response button was learned during a training session before scanning. These motor responses ensured that subjects were attending to the specific types of slot machines as well as their outcomes and enabled us to keep the motor component equal between slot presentation and outcome. Importantly, the stimulus presentation was not contingent on the subject's response. There were a total of six runs, each consisting of 16 trials (four trials for each type of slot machine). Each of the four possible slot machines occurred pseudo-randomly during each run. The exact probability of each potential outcome was reached at the end of each run for each slot machine. The order of the runs was randomized between subjects.
fMRI data acquisition.
Imaging was conducted on a GE 3-Tesla scanner with a real-time functional imaging upgrade. Series of 29 contiguous 3.3-mm axial slices per volume were collected, plus eight “dummy” volumes at the start of each run. These functional scans used an echo-planar single-shot real-time gradient echo T2* weighting (EPIRT) sequence [response time (RT) = 2,300 ms, echo time (TE) = 23 ms, field of view (FOV) = 24 cm, 64 × 64 matrix, voxel size = 3.75·3.75·3.3, flip angle = 90°). Signal dropout in orbitofrontal cortex from susceptibility artifact was reduced with local high-order z-shimming performed in the axial direction and by tilting subjects' heads 30° relative to the anterior and posterior commissures (AC–PC) line. High-resolution T1-weighted structural scans were acquired by using a magnetization-prepared gradient echo (MP-RAGE) sequence [180 sagittal slices of 1 mm; FOV = 256 mm, number of excitations (NEX) = 1, time of repetition (TR) = 11.4 ms, TE = 4.4 ms; matrix = 256 × 256; inversion time (TI) = 300 ms].
Image analysis.
Data were analyzed by using Statistical Parametric Mapping (SPM99). Preprocessing included slice timing and motion correction, coregistration to a standard template, alignment to the first volume for each subject, and spatial normalization to the Montreal Neurological Institute (MNI) T1-weighted template image. The data were then smoothed with a 10-mm FWHM Gaussian kernel. Head motion did not exceed 1.5 mm in any direction.
The BOLD response to each event type was modeled as a delta function at the appearance of the stimulus cue (1 s) and at the outcome (2 s), and as a rectangular pulse during the presence of the slot machine on the screen (15 s), and was convolved with a canonical hemodynamic response function. Within-subject time series modeling accounted for the following 15 regressors: four at the time of appearance of the slot machine (one for each stimulus type), four during the delay, and seven regressors at the outcome (rewarded vs. nonrewarded × 3, plus 100% chance of no reward). In the current analyses, only the anticipatory (delay) and outcome phases were assessed with two comparisons:
Anticipation of potential rewards (DelaySlot_A + DelaySlot_B + DelaySlot_C)/3 > DelaySlot_D);
Response at the time of rewarded outcome relative to no reward delivery: ($20Slot_A + $20Slot_B + $10Slot_C)/3 > $0Slot_D.
The default high-pass filter was applied to the time series. Condition-specific estimates of neural activity (betas) were computed independently at each voxel for each subject with the general linear model. We used random-effects models for within- and between-group analyses, and because of the strong a priori information and hypotheses about reward-related activity in the ventral striatum, prefrontal cortex, and anterior cingulate cortex, we set a threshold of P < 0.005, uncorrected. Brain regions outside the reward system are reported in the tables for completeness and to provide reference for future work in this domain.
PET Methods.
Data acquisition procedures and image data processing.
We used the tracer 6-[18F]FDOPA and scanned on a GE Advance 3D PET camera (32 planes, 6.5 mm FWHM). Uptake of labeled FDOPA was measured while subjects were in an awake, resting state after pretreatment with 200 mg of carbidopa (to reduce peripheral metabolism of FDOPA and increase tracer availability in the brain) with 25 images acquired over 90 min starting 90 s after injection of 8–16 mCi of FDOPA. PET data were attenuation-corrected, registered, and affine-normalized to an FDOPA template. The kinetic rate constant Ki for FDOPA uptake was calculated voxel-by-voxel using a linear fit based on the Patlak method (47), with a time activity curve in an occipital reference region as the input function. Both occipital and cerebellar reference regions are commonly used in FDOPA PET imaging. Here, we chose an occipital area because it is less susceptible to variation in head positioning in the scanner (46). Finally, FDOPA data were averaged within a midbrain template derived in normalized space from a publicly available probabilistic brain atlas (34).
Statistical analysis.
To test the hypothesis that prefrontal function was coupled to midbrain dopamine synthesis, midbrain Ki values were used as covariates and correlated separately for each group with BOLD signal during reward anticipation and at the time of reward delivery across the entire brain (voxelwise regression analysis). The resulting correlation maps were assessed for significance by using Gaussian random fields theory (P < 0.005, uncorrected). Between-group voxelwise comparisons of the correlation maps of the older and younger subjects were also performed during reward anticipation and at the time of reward delivery to assess the regional specificity of our findings in the lateral PFC.
Supplementary Material
Acknowledgments.
This work was supported by the National Institute of Mental Health intramural research program. J.-C.D. was also supported in part by a grant from the Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14751.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802127105/DCSupplemental.
References
- 1.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 2.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133(1):130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 4.Knutson B, et al. Amphetamine modulates human incentive processing. Neuron. 2004;43(2):261–269. doi: 10.1016/j.neuron.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: The role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 7.Reuter J, et al. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8(2):147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology Suppl 1. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Kaasinen V, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157(1):75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 12.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 13.Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cereb Cortex. 2006;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- 14.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 15.Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: Implications for intracranial self-stimulation. Proc Natl Acad Sci USA. 2005;102:19150–19155. doi: 10.1073/pnas.0509607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 18.Garris PA, et al. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- 19.Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 20.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 21.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(RC159) doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marschner A, et al. Reward-based decision-making and aging. Brain Res Bull. 2005;67:382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Samanez-Larkin GR, et al. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lustig C, et al. Functional deactivations: Change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- 26.Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- 27.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, et al. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- 29.Suhara T, et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl) 1991;103:41–45. doi: 10.1007/BF02244071. [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, et al. Dopamine transporters decrease with age. J Nucl Med. 1996;37:554–559. [PubMed] [Google Scholar]
- 31.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 32.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 33.Dreher JC, Guigon E, Burnod Y. A model of prefrontal cortex dopaminergic modulation during the delayed alternation task. J Cognit Neurosci. 2002;14(6):853–865. doi: 10.1162/089892902760191081. [DOI] [PubMed] [Google Scholar]
- 34.Meyer-Lindenberg A, et al. Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Lindenberg A, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 36.Kumakura Y, et al. PET studies of net blood-brain clearance of FDOPA to human brain: Age-dependent decline of [18F]fluorodopamine storage capacity. J Cereb Blood Flow Metab. 2005;25:807–819. doi: 10.1038/sj.jcbfm.9600079. [DOI] [PubMed] [Google Scholar]
- 37.Brown WD, et al. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999;53:1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- 38.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 39.D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 40.Hazlett EA, et al. Age-related shift in brain region activity during successful memory performance. Neurobiol Aging. 1998;19:437–445. doi: 10.1016/s0197-4580(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 41.Madden DJ, et al. Aging and recognition memory: Changes in regional cerebral blood flow associated with components of reaction time distributions. J Cognit Neurosci. 1999;11:511–520. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- 42.Reuter-Lorenz PA, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cognit Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 43.Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 44.D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- 45.Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 46.Whone AL, Bailey DL, Remy P, Pavese N, Brooks DJ. A technique for standardized central analysis of 6-(18)F-fluoro-L-DOPA PET data from a multicenter study. J Nucl Med. 2004;45:1135–1145. [PubMed] [Google Scholar]
- 47.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.