Abstract
We examined how the endogenous anticonvulsant adenosine might influence γ-aminobutyric acid type A (GABAA) receptor stability and which adenosine receptors (ARs) were involved. Upon repetitive activation (GABA 500 μM), GABAA receptors, microtransplanted into Xenopus oocytes from neurosurgically resected epileptic human nervous tissues, exhibited an obvious GABAA-current (IGABA) run-down, which was consistently and significantly reduced by treatment with the nonselective adenosine receptor antagonist CGS15943 (100 nM) or with adenosine deaminase (ADA) (1 units/ml), that inactivates adenosine. It was also found that selective antagonists of A2B (MRS1706, 10 nM) or A3 (MRS1334, 30 nM) receptors reduced IGABA run-down, whereas treatment with the specific A1 receptor antagonist DPCPX (10 nM) was ineffective. The selective A2A receptor antagonist SCH58261 (10 nM) reduced or potentiated IGABA run-down in ≈40% and ≈20% of tested oocytes, respectively. The ADA-resistant, AR agonist 2-chloroadenosine (2-CA) (10 μM) potentiated IGABA run-down but only in ≈20% of tested oocytes. CGS15943 administration again decreased IGABA run-down in patch-clamped neurons from either human or rat neocortex slices. IGABA run-down in pyramidal neurons was equivalent in A1 receptor-deficient and wt neurons but much larger in neurons from A2A receptor-deficient mice, indicating that, in mouse cortex, GABAA-receptor stability is tonically influenced by A2A but not by A1 receptors. IGABA run-down from wt mice was not affected by 2-CA, suggesting maximal ARs activity by endogenous adenosine. Our findings strongly suggest that cortical A2–A3 receptors alter the stability of GABAA receptors, which could offer therapeutic opportunities.
Keywords: A2A receptor, A3 receptor, microtransplantation into Xenopus oocyte, temporal lobe epilepsy
Repetitive activation of GABA receptor type A (GABAA) receptors causes a decrease the amplitude of the ionic current generated by the GABAA receptor, which provides a use-dependent run-down of GABA currents. It reflects GABAA-receptor instability, mostly due to receptor desensitization involving the shift of channels from the open to a desensitized state (1). It is a process that develops both in reconstituted systems and in native neurons. Noteworthy, in refractory human temporal lobe epilepsy (TLE), the GABAA run-down is larger, and its recovery is slower, compared with non-TLE tissues (2, 3).
In the brain, GABAA-receptor run-down reduces the efficacy of the GABA-ergic signal, which is inhibitory in the adult but excitatory in the immature brain (4). Thus, in the epileptic human brain the GABAA-receptor run-down may be pro-excitatory in the adult and pro-inhibitory in the early postnatal brain. Although the consequences of minor GABAA-receptor run-down in the “physiological” brain are damped by a relatively fast recovery of GABAA-receptor function, a long-lasting strong GABAA-receptor run-down becomes pathophysiologically relevant in the TLE brain, and in the adult this is expected to facilitate and reinforce seizures. Nevertheless, the picture in intractable epilepsy becomes more complicated with respect to GABAA-receptor function in the TLE hippocampal subiculum, a region considered to be responsible for the interictal discharges likely due to perturbed chloride homeostasis (5), where the inhibitory neurotransmitter GABA switches to immature excitatory role in at least 20% of the pyramidal neurons in human TLE (6, 7).
GABAA-receptor stability might therefore determine the efficacy of GABA-ergic neurotransmission in the human brain. Hence, our aim was to discover factors that modulate GABAA-receptor function that may help develop new antiepileptic treatments for refractory epilepsy. Here, we examined whether drugs targeting adenosine receptors could be added to the list of GABAA-receptor run-down modulators in the human brain, which until now includes brain-derived neurotrophic factor, phosphatase blockers, Zn2+, and levetiracetam (2, 8, 9).
The purine ribonucleoside adenosine is an essential component in living cells and an important messenger activating specific G protein-coupled adenosine receptors (ARs). Adenosine is considered an endogenous anticonvulsant in the brain where dysfunction of the adenosine-based neuromodulatory system may contribute to epileptogenesis (10, 11). However, despite a large body of literature on animal models emphasizing that ARs may play a role in epilepsy, and may represent a promising therapeutic target (10–13), data relevant to human refractory epilepsy are not yet available. In this work, we focused our experiments on Xenopus oocytes injected with membranes extracted from human TLE nervous tissues and on human epileptic slices obtained from neurosurgical resection of nervous tissues from patients afflicted with epileptic cortical dysplasia or TLE. For comparison, experiments were also performed on the temporal cortex of (i) pilocarpine-treated epileptic rats, and of (ii) A1 or A2A receptor-deficient mice. We report that the GABAA-receptor run-down is consistently altered by ARs antagonists, with the notable exception of the A1 receptor, suggesting a stringent functional association of ARs, with the GABAA-ergic system of the normal and epileptic brains.
Results and Discussion
Modulation of IGABA Run-Down by ARs Activity in Oocytes.
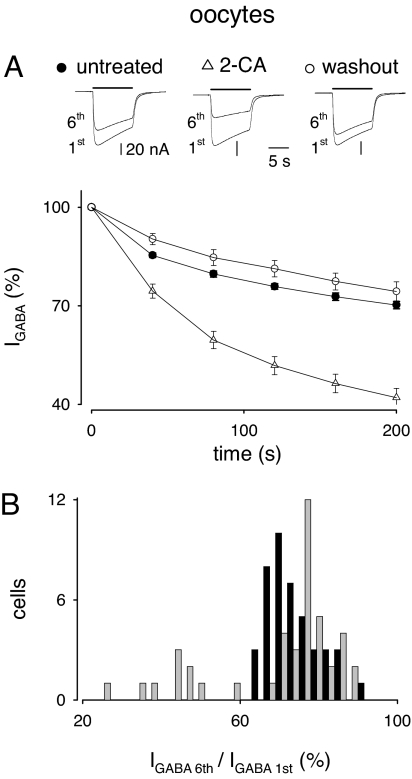
In agreement with our previous experiments (7–9), application of GABA (500 μM) to oocytes injected with membranes from the neocortex of five drug-resistant epileptic patients [nos. 1–5; listed in supporting information (SI) Table S1] elicited inward currents ranging from −30 to −700 nA (depending on both the oocyte and the donor human/frog, and on the lag between membrane injections and recordings). These currents were sensitive to the competitive GABAA-receptor antagonist bicuculline (100 μM, six oocytes, two frogs; not shown; see also refs. 7 and 8) and remained stable over time (1–2 h; GABA applications every 120 s). However, using IGABA run-down as a potent tool for revealing GABAA-receptor instability, the GABA currents elicited by receptors microtransplanted from epileptic patients nervous tissue exhibited a considerable run-down during repetitive applications (every 50 s) of the neurotransmitter [normalized IGABA (nIGABA) fall to 74.1 ± 0.6% at the sixth GABA application, range 54–90%; 138 oocytes, 20 frogs, 138/20; e.g., Fig. 1]. IGABA run-down was not related to changes in IGABA reversal potential, as proven in seven oocytes (one frog) by using voltage ramps during the first vs. the sixth GABA application (−22.0 ± 2.5 mV vs. −19 ± 3 mV, n = 7; P > 0.1; not shown). Application of a second run-down protocol 60 min after the first provided identical results (at the first test, nIGABA = 76.3 ± 1.1%; after 60 min, nIGABA = 78.8 ± 1.2%; P > 0.05; 34/8), providing an internal control for evaluation of drug effects.
Fig. 1.
Increase of IGABA run-down by 2-chloroadenosine (10 μM) in a subgroup (10 of 44) of oocytes microinjected with neocortical TLE membranes (patients #1 and #2). (A Upper) Superimposed currents elicited by the first and sixth GABA applications during run-down protocols before, during, and after treatment with 2-CA. Samples from one oocyte representative of 10 experiments. Time interval between run-down protocols: 1 h. (A Lower) Time course of IGABA run-down in 10 oocytes (two frogs, two TLE patients) treated as indicated by the symbols on the top. IGABA values were normalized to the first IGABA amplitude. IGABA amplitudes were not significantly affected by the 1-h treatment with 2-CA, varying from 110 ± 16 to 92 ± 17 nA in the 10 oocytes exhibiting increased run-down (P > 0.05), and from 143 ± 18 to 122 ± 14 nA in the remaining 34 oocytes (P > 0.05). (B) Histogram of the effects of 2-CA on IGABA run-down. Black bars, control run-down; gray bars, run-down after 1-h 2-CA treatment. Note the presence of a subpopulation of 10 cells showing increased IGABA run-down.
It has been reported that adenosine is tonically released by a wide variety of cells including Xenopus oocytes and brain cells, during normal as well as simulated pathophysiological circumstances (10, 11, 13–19). To investigate whether tonic activation of the ARs could influence GABAA-receptor stability, we inhibited AR action in oocytes using the broad spectrum antagonist CGS15943 and/or adenosine deaminase (ADA), which converts adenosine to inosine (20). In oocytes treated with either CGS15943 or ADA, we found a significantly reduced IGABA run-down (Table 1), indicating that inhibition of the overall tonic activity of ARs increased GABAA-receptor stability. Again IGABA run-down was not related to changes in IGABA reversal potential, as proven in five oocytes (one frog, patient #4), using voltage ramps during the first vs. the sixth GABA application in the presence of CGS15943 (not shown). Treatment of oocytes with erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA) (1 μM, 1-h incubation), a potent inhibitor of ADA, did not significantly affect IGABA run-down (nIGABA = 73 ± 3% before, and nIGABA = 73 ± 1% after EHNA hydrochloride; P > 0.2; nine cells, one frog, patient #4), suggesting that tonic activity exerted by endogenous adenosine on ARs is close to the maximal attainable levels.
Table 1.
IGABA stability in GABAA-receptor-microtransplanted oocytes from epileptic brains, treated with AR-related compounds
| Drug treatment (dose) | Tested oocytes (frogs) [patients] | IGABA, %, before treatment (n) | IGABA, %, after treatment | P |
|---|---|---|---|---|
| CGS 15943 (100 nM) [A1–A3 antagonist] | 15 (three) [#2, #4] | 70.2 ± 1.7 (11) | 81.9 ± 2.5 | <0.001 |
| ADA (1 unit/ml) | 20 (four) [#1, #2] | 76.8 ± 2.07 (18) | 91.1 ± 1.4 | <0.001 |
| DPCPX (10 nM) [A1 antagonist] | 13 (three) [#1, #4, #5] | *77.7 ± 2.0 (13) | *77.2 ± 2.4 | >0.5 |
| SCH 58261 (10 nM) [A2A antagonist] | 39 (four) [#1, #2] | 74.8 ± 2.2 (9) | 52.7 ± 3.7 | <0.001 |
| 39 (four) [#1, #2] | 66.8 ± 1.8 (15) | 78.0 ± 1.8 | <0.001 | |
| MRS 1706 (10 nM) [A2B antagonist] | 13 (two) [#1, #2, #5] | 71.1 ± 2.2 (5) | 80.3 ± 2.5 | <0.001 |
| MRS 1334 (30 nM) [A3 antagonist] | 20 (two) [#1, #4] | 77.2 ± 1.0 (13) | 82.9 ± 1.0 | <0.001 |
n, number of responsive, out of total tested, oocytes. Run-down potentiation, and n are in boldface. IGABA (%) values represent the tenth IGABA amplitude normalized to the first one of the run-down protocol.
*, not significantly different.
Because all ARs (A1, A2A, A2B, and A3) are expressed in the brain (15, 21), we used selective AR antagonists to determine which subtype is most important. The selective antagonists of A2A, A2B, and A3 receptors, SCH58261, MRS1706, and MRS1334 (21), respectively, altered the IGABA run-down as did the nonselective ARs antagonist CGS15943, albeit not in all cells. Whereas MRS1706 and MRS1334 reduced the IGABA run-down as effectively as CGS15943, SCH58261 also reduced the IGABA run-down but only in ≈40% of tested oocytes. Instead, in a subset (≈20%) of tested oocytes, SCH58261 actually increased the IGABA run-down. The selective A1 receptor antagonist DPCPX did not alter the IGABA run-down. Considered together, these findings indicate that the A1 receptor is not involved in run-down modulation, whereas A2A, A2B, and A3 receptors do modulate GABAA-receptor stability. The possible interactions between receptors and their signaling pathways are still unknown (10, 11, 22). Table 1 gives a summary of the effects of ARs antagonists on the GABAA-receptor run-down.
Subsequent experiments were made to examine whether the run-down of receptors microtransplanted from epileptic patients nervous tissue was also modulated by phasic activation of ARs, keeping in mind that these receptors displayed a significant endogenous activity as revealed by experiments with ARs antagonists (Table 1). To address this issue, oocytes injected with TLE membranes were exposed to the nonhydrolyzable agonist 2-chloroadenosine (2-CA), nonselective at a high dose (10 μM). In ≈80% of the oocytes tested (34 of 44 cells; six frogs, patients #1 and #2), 10- to 60-min treatment with 2-CA was ineffective (nIGABA = 74.5 ± 1.2% before, and nIGABA = 77.7 ± 0.9% after 2-CA; P > 0.05). The IGABA run-down in the remaining 10 oocytes exposed to 2-CA for 60 min fell from ≈70% to ≈42% and fully recovered after 2-CA withdrawal (Fig. 1), in agreement with the concept that ARs drive GABAA-receptor instability. Again, in the presence of 2-CA (10 μM), the IGABA reversal potential did not significantly change between the first and the sixth GABA application (−23.0 ± 1 mV vs. −20 ± 1 mV, n = 6; one donor; P > 0.1). These findings indicate that the application of exogenous adenosine was ineffective in modulating the GABAA-receptor run-down; with the exception of a small population of oocytes (≈20%) in which adenosine significantly increased the IGABA run-down. Thus, in the majority of the cells the endogenous adenosine exerts essentially a maximal effect on the ARs, thereby preventing any exogenous adenosine from having additional effects on GABAA-receptor stability.
Modulation of IGABA Run-Down in Human Pyramidal Neurons.
To determine whether the results from the oocytes were valid also in the native tissue, we performed experiments on pyramidal neurons from human cortical slices. The main issue was to estimate how the endogenous adenosine action on the ARs of human pyramidal neurons modulates GABAA-receptor stability.
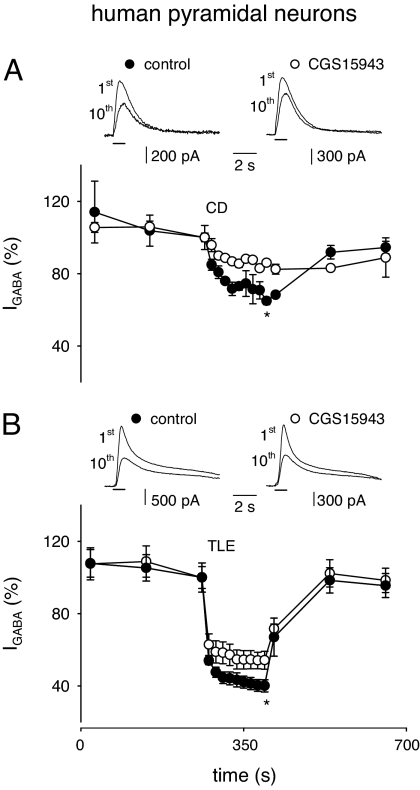
In human cortical slices from a frontal epileptic cortical dysplasia (ECD) patient (patient #9), repeated administrations of GABA (1-s pulses, 100 μM every 120 s) to five pyramidal neurons elicited stable outward currents, whose amplitudes ranged from 400 to 1,700 pA. More frequent applications of GABA (every 15 s) produced a run-down of IGABA to an nIGABA value of 65 ± 2% (Fig. 2A). The nonselective blockade of ARs by CGS15943 (100 nM for 15 min), in the presence of ADA (1 unit/ml) to fully avoid ARs activity, significantly reduced the IGABA run-down (nIGABA = 86 ± 1%; Fig. 2A; P < 0.01). In further experiments done on human pyramidal neurons of the temporal TLE cortex (patients #6–8), IGABA ranged from 600 to 2,700 pA. As reported in ref. 23, IGABA run-down was more marked in TLE than in ECD nervous tissue and was again reduced (in five of seven cells examined) by CGS15943 treatment, the nIGABA mean value shifting from 40 ± 3% to 54 ± 5% (Fig. 2B; P < 0.01). All of these data indicate that blocking the tonic activity of all ARs expressed in the human epileptic neurons reduced their IGABA run-down.
Fig. 2.
Decrease of IGABA run-down caused by CGS15943 in pyramidal neurons from human epileptic cortical slices. (A Upper) Superimposed sample currents elicited by the first and tenth applications of GABA (100 μM, horizontal bar) to one neuron. Holding potential, 0 mV. Indicated are the control condition and after a 15-min application of CGS15943 (100 nM). Slices were obtained from surgically resected ECD frontal cortex tissue (#9). (A Lower) Time course of the averaged run-down of five neurons in control condition (filled circles) and after CGS15943 plus ADA application (open circles). Data points after run-down protocol, significantly different (P < 0.05). Current amplitudes normalized to I1st of run-down protocol. Here and below, * = P < 0.01. (B Upper) Representative currents as in A. (B Lower) Time course averaged from five neurons. Slices were obtained from temporal cortical tissue surgically resected from four TLE patients (#6–#8 and #10).
Modulation of IGABA Run-Down in Rodent Pyramidal Neurons.
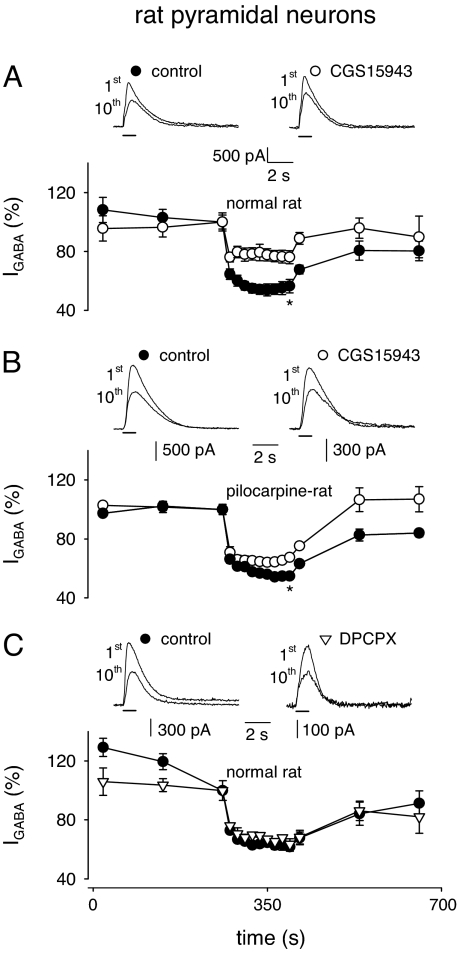
To see how general was the observation that blocking ARs increases the GABAA-receptor stability, further experiments were performed in slices from temporal cortex pyramidal neurons of untreated “healthy” or pilocarpine-treated “epileptic” rats. In control rats, CGS15943 strongly reduced IGABA (150 to 2,500 pA) run-down (Fig. 3A; the nIGABA value went from 57 ± 5% to 76 ± 4%; P < 0.01; 8 of 10 tested cells). Analogously, in pyramidal neurons of pilocarpine-treated rats (IGABA ranging from 1,300 to 2,800 pA), CGS15943 significantly reduced the current run-down (Fig. 3B; nIGABA from 55 ± 1% to 68 ± 2%; P < 0.01; five cells). To exclude the involvement of A1 receptors in the modulation of IGABA run-down, as happens in microtransplanted oocytes, we tested the effect of the A1 selective antagonist DPCPX (10 nM) in the presence of ADA (1 unit/ml) in normal rats. In five cells, DPCPX did not alter IGABA run-down (Fig. 3C), indicating that A1 receptors were not involved in the modulation of GABAA stability in rat brain, as seen in human brain (Table 1). All of these results indicate that the tonic activity of ARs, with the notable exception of the A1 receptor, affects the IGABA run-down throughout both human and rat brains.
Fig. 3.
Adenosine receptors and modulation of IGABA run-down of temporal pyramidal neurons in brain slices from control or pilocarpine-treated rats. (A Upper) Superimposed representative current traces (top) elicited by the first and tenth GABA applications (100 μM; horizontal bars; holding potential of 0 mV) from a single rat neuron in control condition and after 15 min of application of CGS15943 (100 nM) as indicated. (A Lower) Time courses averaged from nine neurons under control condition (filled circles) and after CGS15943 application (open circles). Current amplitudes were normalized to I1st. (B) Traces and time courses as in A. Points here and below represent means of five determinations (five neurons). (C) Traces and time courses as in A, with CGS15943 substituted by DPCPX (100 nM; inverted open triangles).
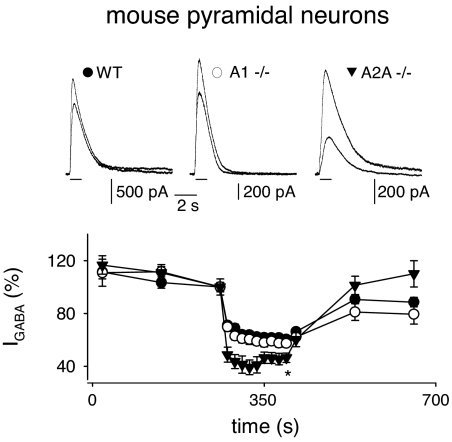
Further experiments were made on genetically modified mouse cortical neurons to gain more information about the types of ARs involved in modulating GABAA-receptor stability. Specifically, A1−/− neurons exhibited an IGABA run-down similar as wt neurons (Fig. 4), confirming that A1 receptor activity is not essential for the modulation of GABAA-receptor stability. In contrast, A2A−/− neurons showed an IGABA run-down significantly stronger (by ≈18%) compared with wt or A1−/− neurons (Fig. 4), indicating that A2A receptors are implicated in the stability of GABAA receptors. Interestingly, this increased IGABA run-down resembled the results obtained with the A2A receptor antagonist SCH58261 treatment, which in a subset of microinjected oocytes exhibited an enhanced run-down (see Table 1). Nevertheless, although it was clear that A1 receptors are not importantly involved in GABAA-receptor stability (Fig. 4), and that A2A receptors positively modulate the run-down, it was also clear that A2B and/or A3 receptors negatively modulate the GABAA-receptors stability. Actually, in A1−/− pyramidal neurons, there was a significant reduction by 18% of the IGABA run-down in the presence of the ARs antagonist CGS15943 (control, nIGABA = 54 ± 4%; 100 nM CGS15943, nIGABA = 66 ± 3%; n = 7; P < 0.02). Finally, it was found that a 15-min treatment of wt pyramidal neurons with 2-CA (10 μM) failed to alter the IGABA run-down (control, nIGABA = 54 ± 4%; 10 μM 2-CA, nIGABA = 56 ± 3%; n = 6; P > 0.05), likely because of saturation of AR activity by ambient adenosine. Considered together, these findings indicate that the A2A activity positively modulates GABAA-receptor stability in a limited subset of human membranes-microinjected oocytes, contrasting with A2B and/or A3 activity, which negatively modulates it.
Fig. 4.
IGABA run-down of temporal pyramidal neurons in slices obtained from control and A1−/− and A2A−/− mice. (Upper) Superimposed representative current traces elicited by the first and tenth GABA applications (100 μM; horizontal bars; holding potential of 0 mV), recorded from wt, A1−/−, and A2A−/− pyramidal neurons (filled circles, open circles, and inverted filled triangles, respectively). (Lower) Time courses averaged from 16 wt, 8 A1−/−, and 7 A2A−/− pyramidal cells. Experimental conditions are as in Fig. 3. Note the increased run-down observed in A2A−/− neurons.
The central finding in this work is that the tonic activity of ARs, with the notable exception of the A1 receptor, influences the use-dependent stability of GABAA receptors, thus altering the inhibitory efficacy of the neurotransmitter GABA during overstimulation of the GABAA-ergic system. This phenomenon seems to be fairly general in the brain because the GABAA-receptor run-down, which is particularly robust in the TLE brain, is a common event in the GABA-ergic system of both the human and rodent brains. Moreover, adenosine is an endogenous neuromodulator that is released not only during seizures, ischemia, and hypoxia (10, 11), but also during systemic inflammation (16) or simply at neuronal depolarization (18).
Beside our present findings, it is generally thought that adenosine is an inhibitory modulator of brain activity with anticonvulsant properties (10, 11). However, that role is mediated by the A1 receptor, which, as we report here, is not involved in the modulation of the GABA-receptor run-down. Therefore, it is possible that the modulation of AR tonic activity by ARs antagonists is beneficial against seizures reducing GABAA instability during hyperexcitability and influencing the GABA-ergic system, as elsewhere proposed (24). Thus, selective AR antagonists may display a therapeutic potential as antiepileptic drugs in the adult brain epilepsy, as in Parkinson's disease (25).
Many questions remain unanswered including the mechanisms by which A2A, A2B, and 8A3 receptors influence the use-dependent GABAA-receptor function. It is well known that GABAA-receptor function is regulated by protein kinase systems (26–28). Because all four subtypes of ARs can couple to mitogen-activated protein kinase (MAPK) activity (13, 21), whereas activation of A3 and A2 receptors inhibit and stimulate adenylyl cyclase activity, respectively, altering PKA activity (13, 21), we speculate that the effects of AR stimulation may be associated with MAPK and/or PKA activities, their variability depending on the activity of the different pathways. Furthermore, it is possible that the differential effects on GABAA-receptor stability induced by A2A or A2B inhibition are mediated through different G proteins (13, 21). Whatever the mechanism(s) responsible for the functional interactions between A2–A3 receptors and neuronal GABAA receptors, we present here conclusive evidence that blocking all together the human brain adenosine receptors in the human brain improves the stability of GABAA-ergic neurotransmission, and this may help in the investigation of new treatments targeted at increasing the inhibitory efficacy of GABA receptors.
Materials and Methods
Patients.
Surgical specimens were obtained from the temporal neocortex of nine patients afflicted with TLE (Table S1) or with epileptic cortical dysplasia (ECD), all operated at the Neuromed Neurosurgery Center for Epilepsy (Pozzilli-Isernia, Italy). Informed consent was obtained from all of the patients to use part of the biopsy material for our experiments; and the Ethics Committees of Neuromed and the University of Rome “Sapienza” approved the selection processes and procedures. For more details about patients and screening analysis, see ref. 23.
Membrane Preparation, Injection Procedures, and Electrophysiological Recordings from Oocytes.
Membranes were prepared as previously described, using tissues from human epileptic brain regions (temporal lobe). Preparation of Xenopus laevis oocytes and injection procedures were as detailed in ref. 29. From 12 to 48 h after injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (30). Determinations of the reversal potential of IGABA currents were performed by using the current-passing electrode filled with K+-acetate. We were unable to routinely record from oocytes injected with mouse cortical membranes because they frequently matured showing insensitivity to GABA. The oocytes were placed in a recording chamber (volume 0.1 ml) perfused continuously (9–10 ml/min) with oocyte Ringer at room temperature (21–23°C). The run-down of current elicited by GABA (GABAA-current) was defined as the decrease (in %) of the GABAA-current peak amplitude at the sixth GABA jet (current-plateau) after five applications of GABA (500 μM), 10 s in duration, at 40-s intervals. In all experiments the holding potential was −60 mV. IGABA reversal potential was monitored at first and sixth GABA applications by voltage ramps (from −100 to 40 mV, 1 s in duration) carried out during the current “steady state,” whereas control ramps were applied immediately before GABA applications. Agonists and antagonists of ARs diluted in oocyte Ringer were applied for 60 min after the control run-down protocol up to the end of the test run-down protocol.
Pilocarpine Model.
Male Sprague–Dawley rats (280–300 g; Charles River Laboratories) were used for the experiments. Animal were housed under standard conditions: constant temperature (22–24°C) and humidity (55–65%), 12-h dark/light cycle, and free access to food and water. All effort was made to minimize animal suffering. Procedures involving animals and their care were carried out in accordance with European Community and national laws and policies. Pilocarpine was administered i.p. (300 mg/kg; Sigma), and the rats' behavior was observed for several hours thereafter. Within the first hour after injection, all animals developed seizures evolving into recurrent generalized convulsions [status epilepticus (SE)]. SE was interrupted 3 h after onset by administration (i.p.) of diazepam (10 mg/kg). After a latent period of 2–3 weeks, SE animals begin to experience the spontaneous occurrence of seizures. In the present experimental series, spontaneous seizures began to occur 18 ± 2 days after SE, and rats were killed 30 days after SE.
AR1−/− and A2A−/− Mice.
A1 and A2A receptor knockout mice were generated as described in refs. 31 and 32. They were backcrossed onto C57BL/6 mice until essentially congenic, and genotyped by PCR.
Whole-Cell Recordings from Cortical Slices.
Neocortical slices were prepared from human frontal ECD cortex (Table S1), human temporal TLE cortex (Table S1), and temporal cortices of control or pilocarpine-treated rats, and A1 or A2A receptor-deficient mice. Transverse slices (300 μm) were cut in glycerol-based artificial cerebrospinal fluid (ACSF) with a vibratome (Leica VT 1000S) immediately after surgical resection. Slices were placed in a slice incubation chamber at room temperature with oxygenated ACSF and transferred to a recording chamber within 1–24 h after slice preparation. Whole-cell patch clamp recordings were performed as described in ref. 23 on pyramidal neurons at 21–23°C. Membrane currents were recorded by using glass electrodes (3–4 MΩ), at a 0-mV holding membrane potential, to avoid pressure-induced artifactual currents due to stretch-operated channels. Under these experimental conditions, with inactivated voltage-gated channels, cells were stable and healthy for 1–2 h. GABA was delivered to cells by pressure applications (10–20 psi, 1 s; General Valve Picospritzer II) from glass micropipettes positioned above whole-cell voltage-clamped neurons. In this way we obtained stable whole-cell currents and rapid drug wash before applying the run-down protocol. The current run-down protocol adopted was the following. After current amplitude stabilization with repetitive applications every 120 s, a sequence of 10 GABA applications, 1 s in duration, every 15 s were delivered, and the test pulse was resumed at the control rate (every 120 s) to monitor recovery of the GABA current. The reduction in peak amplitude current was expressed as percentage amplitude of current at the end of the run-down protocol (I10th) vs. control (I1st). For details, see ref. 23.
Chemicals and Solutions.
Oocyte Ringer had the following composition: 82.5 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 5 mM Hepes (adjusted to pH 7.4 with NaOH). ACSF had the following composition: 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1.25 mM NaH2PO4, 1 mM MgCl2, 26 mM NaHCO3, 10 mM glucose, 0.1 mM Na-pyruvate (pH 7.35). Glycerol-based ACSF solution contained 250 mM glycerol, 2.5 mM KCl, 2.4 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 26 mM NaHCO3, 11 mM glucose, and 0.1 mM Na-pyruvate (pH 7.35). Patch pipettes were filled with 140 mM K-gluconate, 10 mM HEPES, 5 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 2 mM MgCl2, and 2 mM Mg-ATP (pH 7.35, with KOH). All drugs were purchased from Sigma with the exception of GABA, CGS15943, DPCPX, SCH442416, MRS1706, MRS1334, and EHNA, which were purchased from Tocris, and 2-chloradenosine, which was purchased from Ascent Sci. Substances were freshly prepared before the experiment.
Statistics.
Data throughout the text represent means ± SEM. Differences among means were analyzed by one- or two-way ANOVA. Values were considered significantly different at P < 0.01. To obtain the averaged time course of IGABA, single time course data were normalized to the amplitude value recorded at the first GABA application of the run-down protocol.
Supplementary Material
Acknowledgments.
We are very grateful to Drs. K. Krnjević and E. Cherubini for critical reading of the manuscript, Dr. Jiang Fan Chen for allowing us to use the A2A knockout mice, and the patients whose brain tissue made this work possible. This work was supported by grants from Ministero della Salute (to F.E.), Ministero Università and Ricerca (PRIN grant to C.L.), and the Swedish Science Research Council (to B.B.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807277105/DCSupplemental.
References
- 1.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. pp. 140–169. [Google Scholar]
- 2.Palma E, et al. Phosphatase inhibitors remove the run-down of γ-aminobutyric acid type A receptors in the human epileptic brain. Proc Natl Acad Sci USA. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodkin H-P, Sun C, Yeh J-L, Mangan P-S, Kapur J. GABAA receptor internalization during seizures. Epilepsia. 2007;48(Suppl 5):109–113. doi: 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- 4.Cherubini E, Gaiarsa J-L, Ben-Ari Y. GABA: An excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 5.Huberfeld G, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 7.Palma E, et al. Abnormal GABAA receptors from the human epileptic hippocampal subiculum microtransplanted to Xenopus oocytes. Proc Natl Acad Sci USA. 2005;102:2514–2518. doi: 10.1073/pnas.0409687102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palma E, et al. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proc Natl Acad Sci USA. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma E, et al. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc Natl Acad Sci USA. 2007;104:20944–20948. doi: 10.1073/pnas.0710522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagonopoulou O, Efthimiadou A, Asimakoupoulos B, Nikolettos N-K. Modulatory role of adenosine and its receptors in epilepsy: Possible therapeutic approaches. Neurosci Res. 2006;56:14–20. doi: 10.1016/j.neures.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson K-A, Gao Z-G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forghani R, Krnjevic K. Adenosine antagonists have differential effects on induction of long-term potentiation in hippocampal slices. Hippocampus. 1995;5:71–77. doi: 10.1002/hipo.450050109. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro J-A, Sebastião A-M, De Mendonça A. Adenosine receptors in the nervous system: Pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- 16.Gourine A-V, et al. Release of ATP in the central nervous system during systemic inflammation: Real-time measurement in the hypothalamus of conscious rabbits. J Physiol. 2007;585:305–316. doi: 10.1113/jphysiol.2007.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredholm B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 18.Shock S-C, Leblanc D, Hakim A-M, Thompson C-S. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Bahima L, et al. Endogenous hemichannels play a role in the release of ATP from Xenopus oocytes. J Cell Physiol. 2006;206:95–102. doi: 10.1002/jcp.20440. [DOI] [PubMed] [Google Scholar]
- 20.Latini S, Pedata F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm B-B, IJzerman A-P, Jacobson K-A, Klotz K-N, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 22.Ciruela F, et al. Heterodimeric adenosine receptors: A device to regulate neurotransmitter release. Cell Mol Life Sci. 2006;63:2427–2431. doi: 10.1007/s00018-006-6216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragozzino D, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci USA. 2005;102:15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGaraughty S, Cowart M, Jarvis M-F, Berman R-F. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5:43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic J. Are adenosine antagonists, such as istradefylline, caffeine, and chocolate, useful in the treatment of Parkinson's disease? Ann Neurol. 2008;63:267–269. doi: 10.1002/ana.21348. [DOI] [PubMed] [Google Scholar]
- 26.McDonald B-J, et al. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 27.Song M, Messing R-O. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- 28.Bell-Horner C-L, Dohi A, Nguyen Q, Dillon GH, Singh M. ERK/MAPK pathway regulates GABAA receptors. J Neurobiol. 2006;66:1467–1474. doi: 10.1002/neu.20327. [DOI] [PubMed] [Google Scholar]
- 29.Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: New procedure for ion channel studies. Methods Mol Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 30.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc London Ser B. 1982;215:49l–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 31.Johansson B, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J-F, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.