Abstract
Inhibition of mitochondrial complex I is one of the leading hypotheses for dopaminergic neuron death associated with Parkinson's disease (PD). To test this hypothesis genetically, we used a mouse strain lacking functional Ndufs4, a gene encoding a subunit required for complete assembly and function of complex I. Deletion of the Ndufs4 gene abolished complex I activity in midbrain mesencephalic neurons cultured from embryonic day (E) 14 mice, but did not affect the survival of dopaminergic neurons in culture. Although dopaminergic neurons were more sensitive than other neurons in these cultures to cell death induced by rotenone, MPP+, or paraquat treatments, the absence of complex I activity did not protect the dopaminergic neurons, as would be expected if these compounds act by inhibiting complex 1. In fact, the dopaminergic neurons were more sensitive to rotenone. These data suggest that dopaminergic neuron death induced by treatment with rotenone, MPP+, or paraquat is independent of complex I inhibition.
Keywords: apoptosis, cell death, dopamine neurons, Ndufs4, Parkinson's disease
Parkinson's disease (PD) is the second most common aging-related neurodegenerative disorder (1). It is characterized by selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the brain. Although mechanisms underlying the selective dopaminergic neuron death are not well defined, mitochondrial complex I dysfunction has long been implicated in this process (2). The first evidence for complex I dysfunction in PD was the observation that drug abusers who were accidentally exposed to 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) developed PD (3). MPP+, the toxic metabolite of MPTP, is an inhibitor of complex I (4). Soon after the discovery of MPTP-induced PD, several studies showed that complex I activity is decreased in the substantia nigra, skeletal muscle, and platelets of patients with PD (5–7).
The mitochondrial complex I hypothesis for PD is also supported by the finding that chronic treatment of rodents with rotenone or paraquat induces many key features of PD, including motor deficits, loss of dopaminergic neurons, and accumulation of α-synuclein-containing inclusion bodies (8–15). Both rotenone and paraquat are widely used pesticides. Rotenone is an inhibitor of complex I (9). Because of its structural similarity to MPP+, paraquat has been hypothesized to inhibit complex I (16–21), although this hypothesis has been questioned (22). Finally, a recent report suggests that some of the subunits of complex I in human PD brains are oxidatively damaged, resulting in misassembling and functional impairment of complex I (23). Despite this correlative evidence, it has not been established that mitochondrial complex I inhibition is causally related to dopaminergic neuron loss in PD.
The Ndufs4 gene encodes an 18 kDa protein, one of the 46 subunits comprising mitochondrial complex I. Ndufs4 is required for complete assembly and function of complex I (24, 25). To test the role of complex I inhibition in the selective dopaminergic neuron toxicity induced by rotenone, MPP+, and paraquat, we used a mouse line in which exon 2 of the Ndufs4 gene was deleted (26). This causes a frame shift that precludes synthesis of the mature Ndufs4. Similar deletion mutations in the human NDUFS4 gene cause deficiencies in complex I activity and infant lethality (27, 28). If complex I inhibition is obligatory for selective dopaminergic neuron loss associated with PD, and more specifically in the rotenone, MPP+, and paraquat models of PD, one might expect greater baseline apoptosis in cultured dopaminergic neurons from Ndufs4−/− mice. In addition, Ndufs4−/− dopaminergic neurons should be less sensitive to toxicity induced by rotenone, MPP+, or paraquat.
Results
Complex I Activity Is Lost in Primary Mesencephalic Cultures and Purified Mitochondria Prepared from Ndufs4-Deficient Mice.
To investigate the role of complex I in dopaminergic neuron death, mesencephalic cultures were prepared from individual embryonic day (E) 14 embryos obtained from breeding Ndufs4+/− mice. The genotype of each culture was determined by PCR using residual tissue, but was unknown until the experiments were completed. To obtain healthy mesencephalic cultures, the neurons were dissociated and plated immediately after dissection. We were able to obtain 9–12 wells of cultured cells from each embryo. The initial plating density was 3 to 5 × 104 cells/well. Approximately 100–150 cells in each well, which accounted for 1%–3% of the total population, stained positive for tyrosine hydroxylase (TH) after 6 days in vitro (DIV 6). Tyrosine hydroxylase is the rate-limiting enzyme in dopamine synthesis and was used as a marker for dopaminergic neurons.
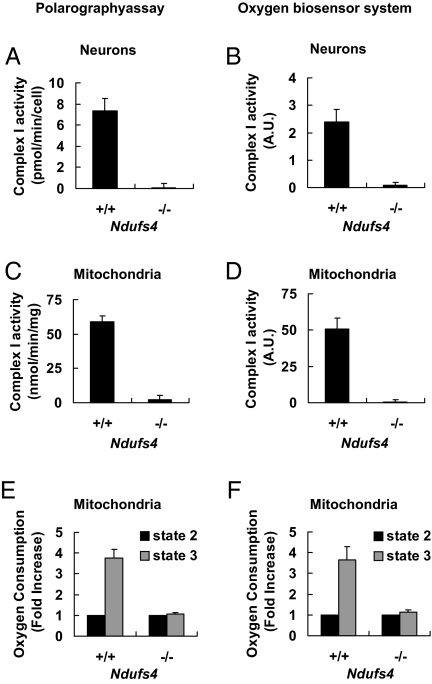
We assayed mitochondrial complex I activity by measuring the complex I inhibitor-sensitive oxygen consumption rate using cultured E14 mesencephalic neurons (Fig. 1 A and B) or purified mitochondria from these neurons (Fig. 1 C and D). Oxygen consumption was measured using the polarography assay (Fig. 1 A and C) or a 96-well oxygen biosensor system (Fig. 1 B and D). Though there was no statistically significant difference between wild-type and heterozygote cultures (data not shown), the cells and purified mitochondria from Ndufs4−/− mice had no detectable complex I activity. Similar results were obtained when complex I activity was measured in cultured neurons by a modified NADH dehydrogenase activity assay [see supporting information (SI) Fig. S1]. In contrast to the loss of complex I activity, oxygen consumption from complex II was not compromised in neurons cultured from Ndufs4−/− mice; if anything, it was slightly higher in Ndufs4−/−cultures than in the Ndufs4+/+ littermates (Fig. S1D). Thus, Ndufs4 gene deletion resulted in a complete loss of complex I activity without disrupting the function of the rest of the respiratory chain in cultured neurons and purified mitochondria.
Fig. 1.
Deletion of the Ndufs4 gene abolishes mitochondrial complex I activity in cultured mesencephalic neurons. Ndufs4 heterozygotes were mated and primary mesencephalic neurons were cultured separately from each mouse E14 fetus for 6 days before treatment or analysis. (A) Complex I activity in intact neurons measured by the polarography method. (B) Complex I activity in neurons measured by the oxygen biosensor system. (C) Complex I activity in purified mitochondrial measured by the polarography assay and is expressed per milligram protein. (D) Complex I activity in purified mitochondrial measured by the oxygen biosensor system. (E) State 2 and state 3 oxygen consumption rates in purified mitochondria were measured by the polarography method. (F) State 2 and state 3 oxygen consumption rates in purified mitochondria were measured by the oxygen biosensor system. The oxygen consumption rate of state 3 was calculated as fold increase over that of state 2. Values represent means (n = 3). Error bars represent SEM.
Alternatively, complex I-driven mitochondrial respiration can be assessed by monitoring oxygen consumption in the presence of complex I substrates before (state 2 respiration) and after addition of ADP (state 3). ADP induces a burst of oxygen consumption (state 3) until all of the added ADP is converted to ATP (state 4). Complex I-driven mitochondrial respiration is quantified as the ratio of state 3/state 4 respiration rates (29–31). This method does not involve the use of rotenone to inhibit complex I and thus complements the assays in Fig. 1 A–D. There was no apparent increase in oxygen consumption upon addition of ADP into the Ndufs4−/− mitochondrial preparation; thus no state 4 respiration rate could be measured. Because state 2 respiration rate was similar to state 4 respiration rate in the wild-type mitochondrial preparation, we used the ratio of state 3/state 2 instead to measure complex I-driven oxidative phosphorylation (30). Purified mitochondria prepared from Ndufs4−/− mesencephalic cells lacked complex I-driven mitochondrial respiration determined by the polarography assay (Fig. 1E) or the oxygen biosensor system (Fig. 1F).
In contrast to wild-type cultures, the culture media for Ndufs4−/− cells turned yellow after 6 days of incubation if the media was not changed. This was probably due to lactate accumulation caused by absence of complex I activity. Therefore, fresh culture media was routinely added at 24 h after plating, and half of the medium was changed every 48 h thereafter.
Interestingly, when we measured oxygen consumption by the polarography assay using mechanically chopped whole-brain tissue fragments from postnatal day 1 (P1) Ndufs4−/− mice, ≈37% of the total oxygen consumption was rotenone sensitive (Fig. S1). However, purified mitochondria from this same tissue preparation had no complex I activity. The basis for this differential activity depending on the mitochondrial purity is unclear. Nevertheless, Ndufs4 gene deletion results in a loss of mitochondrial complex I activity in cultured neurons and in purified mitochondria used in this study. This is consistent with reports that mutations in the human NDUFS4 gene inhibit complex I activity (24, 32–34).
Loss of Complex I Activity in Ndufs4−/− Mice Does Not Increase Basal Rate of Cell Death of Cultured Dopaminergic Neurons.
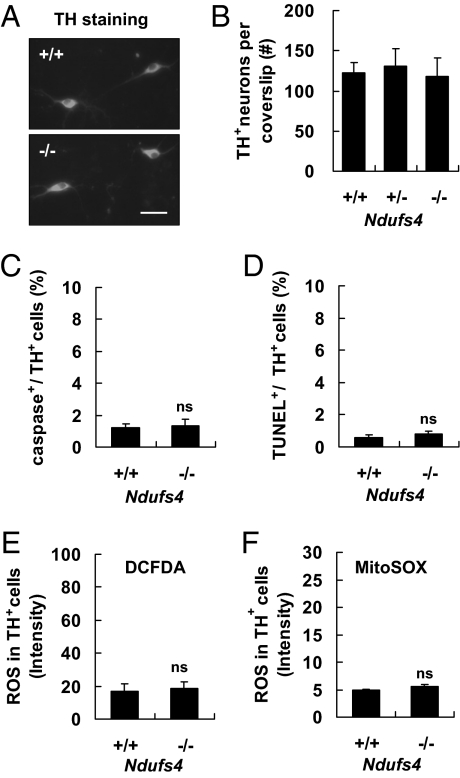
Mesencephalic neurons cultured from Ndufs4−/− mice looked similar to those from Ndufs4+/+ mice (Fig. 2A). There were equal numbers of TH+ neurons in cultures prepared from Ndufs4+/+ and Ndufs4−/− mice after 6 days in culture (Fig. 2B). In addition, morphometric parameters were not affected by Ndufs4 deletion; the length of the longest neurites, the soma size, the number of neurites on each neuron, and the number of branch points on the longest neurites were similar for TH+ neurons prepared from Ndufs4−/− or Ndufs4+/+ littermates (Table S1). Ndufs4 gene deletion did not change the proportion of GABAergic neurons (GABA+), total neurons (NeuN+), astroglia (GFAP+), microglia (IBA1+), or oligodendrocytes (Olig2+) in the cultures (Fig. S2). Furthermore, there was no increase in basal levels of active caspase 3 (Fig. 2C), terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) (Fig. 2D), total intracellular reactive oxygen species (ROS) (Fig. 2E), or ROS in the mitochondrial compartment (Fig. 2F) in TH+ dopaminergic neurons cultured from Ndufs4−/− mice compared with Ndufs4+/+ mice. The normal survival of dopaminergic neurons from Ndufs4−/− mice in culture was confirmed in mice in which the Ndufs4 gene was selectively inactivated in cells that express TH (by crossing the conditional Ndufs4 mice with Th-Cre). Although only a small group of these mice was generated, they did not manifest behavioral or biochemical deficiencies typically associated with PD over a period of 9 months (W.C. Watt, N.A. Sorscher, and R.D.P., unpublished observations). These data suggest that loss of complex I activity alone is not sufficient to induce dopaminergic neuron death, consistent with an earlier report (29).
Fig. 2.
Ndufs4 gene deletion does not increase basal apoptosis in TH+ dopaminergic neurons. Cells were analyzed at DIV 6. (A) Representative photomicrographs of TH immunostaining of mesencephalic neurons cultured from Ndufs4+/+ and Ndufs4−/− mice. (Scale bar: 30 μm.) (B) Ndufs4 deletion does not affect the number of TH+ neurons surviving in culture (n = 4). (C–F) Ndufs4 deletion does not change the number of caspase 3+ cells (C), the number of TUNEL+ cells (D), the level of total intracellular ROS measured by CM-DCFDA staining (E), or the level of mitochondrial ROS measured by MitoSOX staining (F) in TH+ neuron population. Values represent means (n = 3; ns: not statistically significant). Error bars represent SEM.
TH+ Dopaminergic Neurons Cultured from Ndufs4−/− Mice Are Not Protected from Rotenone Toxicity.
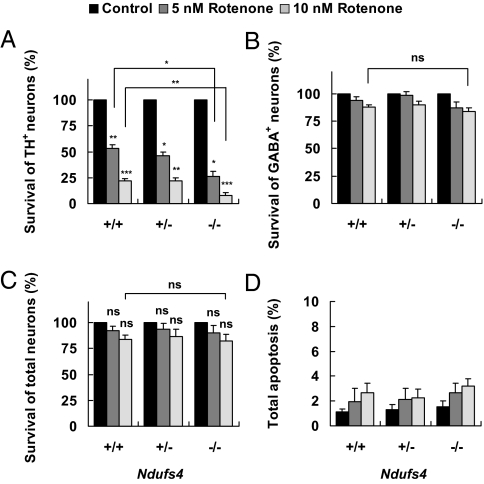
Cultures were exposed to 5 or 10 nM rotenone for 24 h. Surprisingly, the Ndufs4 gene deletion did not protect dopaminergic neurons against rotenone-induced cell death; rather, Ndufs4−/− dopaminergic neurons were even more sensitive to rotenone than Ndufs4+/+ neurons (Fig. 3A). Rotenone toxicity was specific to dopaminergic neurons in all three Ndufs4 genotypes and did not occur at a statistically significant level in the GABAergic (Fig. 3B) or total neuron population (Fig. 3C). There was no difference in the extent of apoptosis (Fig. 3D) or cell death (Fig. S3A) in the entire cell population, associated with the genotype of the cells. These results indicate that loss of complex I activity does not protect TH+ dopaminergic neurons against rotenone-induced toxicity.
Fig. 3.
Ndufs4 deletion does not protect TH+ dopaminergic neurons from rotenone toxicity. Cell cultures were treated with 5 or 10 nM rotenone or vehicle control on DIV 5 and fixed for analysis 24 h later. (A) Survival of TH+ neurons. (B) Survival of GABAergic neurons. (C) Survival of total neurons (NeuN+). (D) Apoptosis in the entire population measured by nuclear condensation/fragmentation after Hoechst staining. Values represent means (n = 3). Error bars represent SEM.
Sensitivity of Mitochondrial Complex I Inhibition by Rotenone.
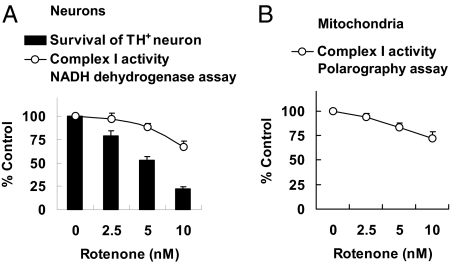
Rotenone has been reported to be a specific and potent mitochondrial complex I inhibitor; IC50 varies from 0.1 nM to 100 nM depending on the system and methods used (8, 35–38). Because the data in Fig. 3 indicate that rotenone toxicity to dopaminergic neurons does not depend on complex I, we investigated the dose–response relationship between rotenone inhibition of complex I and induction of dopaminergic neuron death. E14 mesencephalic cultures prepared from C57/BL6 mice were treated with 0, 2.5, 5, or 10 nM rotenone for 24 h (Fig. 4A), the same conditions used in Fig. 3 to stimulate apoptosis. Although treatments with 5 or 10 nM rotenone killed ∼50% or ∼75% dopaminergic neurons (Fig. 3A), they only inhibited complex I activity ∼11% or ∼33%, respectively (Fig. 4A). Similarly, rotenone only partially inhibited complex I to the same degree when applied to freshly purified mitochondria (Fig. 4B). Together with the data in Figs. 1 and 2 showing that a complete loss of complex I activity in Ndufs4−/− cells has no effect on basal cell death of dopaminergic neurons, these results indicate lack of correlation between complex I inhibition and dopaminergic neuron death.
Fig. 4.
Rotenone inhibition of complex I activity. Mesencephalic neurons were cultured from E14 C57/BL6 mouse embryos for 6 d. (A) Comparison of dose response of rotenone inhibition of complex I and induction of TH+ neuron death. Cells were treated on DIV6 with rotenone for 24 h, and complex I activity in intact cells was measured by a NADH dehydrogenase activity assay (n = 7). (B) Mitochondria were freshly purified from cells on DIV6, and complex I activity in mitochondria was measured by the polarography method. Values represent means (n = 3). Error bars represent SEM.
Ndufs4 Deletion Does Not Affect Loss of Cultured TH+ Dopaminergic Neurons Induced by MPP+ or Paraquat.
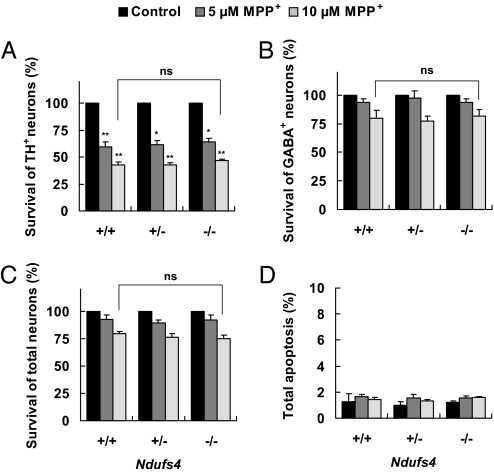
To examine the effect of loss of complex I activity on MPP+-induced dopaminergic neuron death, primary mesencephalic cultures were treated with 5 μM or 10 μM MPP+ for 48 h. Treatment with MPP+ caused a dose-dependent loss of TH+ neurons; the degree of TH+ neuron loss was the same in cultures prepared from all three Ndufs4 genotypes (Fig. 5A). Furthermore, loss of complex I activity in Ndufs4−/− neurons did not have any effect on the survival of the GABAergic neurons (Fig. 5B) or total neurons (Fig. 5C). Total apoptosis (Fig. 5D) or cell death (Fig. S3B) in the entire cell population was unaffected by Ndufs4 gene deletion.
Fig. 5.
TH+ dopaminergic neurons from Ndufs4−/− mice exhibit the same sensitivity to MPP+ toxicity as those from the wild-type mice. Cells were treated with 5 μM or 10 μM MPP+ or vehicle control on DIV 5, and fixed for analysis 48 h later. (A) Survival of TH+ neurons. (B) Survival of GABAergic neurons. (C) Survival of total neurons (NeuN+). (D) Apoptosis in the entire population. Values represent means (n = 3). Error bars represent SEM.
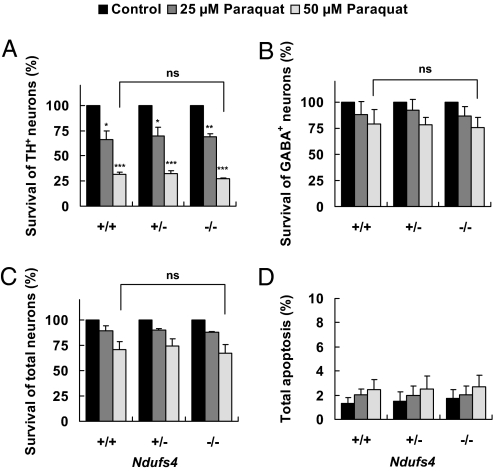
Treatment with 25 or 50 μM paraquat also selectively killed dopaminergic neurons, but deletion of the Ndufs4 gene did not change the sensitivity to paraquat (Fig. 6 and Fig. S3C). These data indicate that dopaminergic neuron death induced by MPP+ or paraquat does not require complex I inhibition.
Fig. 6.
Paraquat induces the same degree of TH+ dopaminergic neuron loss regardless of Ndufs4 genotype. Cell cultures were treated with 25 μM or 50 μM paraquat or vehicle control on DIV 5 for 24 h. (A) Survival of TH+ neurons. (B) Survival of GABAergic neurons. (C) Survival of total neurons (NeuN+). (D) Apoptosis in the entire population. Values represent means (n = 3). Error bars represent SEM.
Discussion
The objective of this study was to examine the role of complex I inhibition in dopaminergic neuron death induced by rotenone, MPP+, and paraquat—treatments that have been used to selectively kill dopaminergic neurons. Although mitochondrial complex I dysfunction has long been implicated in dopaminergic neuron death and pathogenesis of PD, a causal relationship has not been established (2). Mesencephalic neurons cultured for 1 week from E14 Ndufs4−/− mice have no significant complex I activity. However, dopaminergic neurons in these cultures appeared normal and healthy with no decrease in survival during culture compared with neurons from wild-type mice. Likewise, selective loss of Ndufs4 in catecholaminergic neurons did not appear to have any deleterious effect in mice up to 9 months of age; thus, the normal survival of dopaminergic neurons in culture reflects survival in vivo.
The most surprising result from these studies is that lack of complex I activity did not affect the sensitivity of dopaminergic neurons to MPP+ or paraquat, and actually increased the sensitivity to rotenone. One would expect that the dopaminergic neurons would be resistant to these agents if they act by inhibiting complex I. Nevertheless, it is striking that dopaminergic neurons are more vulnerable to all three chemicals than the other neurons, suggesting that there is a complex I-independent, intrinsic property of dopaminergic neurons that makes them more susceptible.
The increased sensitivity of Ndufs4−/− dopaminergic neurons to rotenone is intriguing. Although one could argue that the loss of complex I activity in Ndufs4−/− cells leads to altered production of ROS, which sensitizes them to other death stimuli or to oxidative stress, there was no apparent change in ROS production or basal cell death in the Ndufs4−/− cells under normal conditions. The fact that loss of complex I activity does not have any effect on MPP+- or paraquat-induced TH+ neuron loss argues against a generic increase in sensitivity to death stimuli. In addition, paraquat is known as an ROS generator (39, 40). Sodium arsenite, which has no known effect on complex I activity and induces cell death through oxidative stress (41, 42), also caused the same extent of cell death in Ndufs4+/+ and Ndufs4−/− cells (Fig. S4). Thus loss of complex I activity does not change the overall sensitivity of dopaminergic neurons to oxidative stress. Our data indicate that rotenone has a greater effect on dopamine neuron toxicity than on complex I activity, suggesting that there may alternative targets of rotenone in dopamine neurons. Other studies have implicated microtubule destabilization, vesicle accumulation, and oxidative stress in rotenone-induced loss of dopaminergic neurons (43, 44). In agreement with those studies, we have data indicating that rotenone toxicity involves destabilization of microtubules and accumulation of cytoplasmic dopamine (W.-S.C., S.E.K., R.D.P., and Z.X., unpublished work).
Although it is accepted that the main target of MPP+ neurotoxicity is through complex I inhibition, alternative mechanisms have been proposed, including microtubule depolymerization, oxidative damage, and inhibition of glycolysis (45–48). Our data illustrate that loss of complex I activity has no effect on MPP+-induced dopaminergic neuron loss, strongly suggesting that complex I inhibition is not primarily responsible for MPP+ toxicity within dopaminergic neurons.
Mechanisms underlying paraquat-induced dopaminergic neuron death are not well defined. Early studies suggested that paraquat may inhibit the activity of isolated mitochondrial complex I in vitro (16, 17). Furthermore, because of its structural similarity to MPP+, prominent reviews and textbooks have concluded that paraquat is an inhibitor of mitochondrial complex I (16, 18–21). However, others have questioned whether paraquat can reach the internal mitochondrial membrane and inhibit complex I in intact cells (49), and one recent study suggests that paraquat is not a complex I inhibitor (22). Moreover, oxidative stress, independent of complex I inhibition, has been suggested to be critical in paraquat cytotoxicity (4, 39, 40, 50–56). Data presented here support the argument that paraquat does not act as a complex I inhibitor (22).
Although blue native gel electrophoresis showed a marked loss of fully assembled complex I, other components of complex I were expressed in Ndufs4−/− cells at a similar level as in Ndufs4+/+ cells (26). Thus, one may argue that partially assembled complex I, although lacking complex I activity and the ability to generate NAD+, could still transfer electrons. This may explain the toxicity of rotenone and MPP+ in Ndufs4−/− neurons. However, if electrons can flow through partially assembled complex I and pass to complex IV, there should be complex I inhibitor-sensitive oxygen consumption in cultured cells or purified mitochondria from Ndufs4−/− mice. Alternatively, if electrons pass through partially assembled complex I but do not continue on to complex IV and do not contribute to oxygen consumption and ATP generation, one would expect increased ROS production under basal conditions. However, our data showed that basal levels of ROS in Ndufs4−/− cells were not significantly higher than those in Ndufs4+/+ cells, and there was no complex I inhibitor-sensitive oxygen consumption in Ndufs4−/− cells. Thus, our data argue against the idea that partially assembled complex I can still transfer electrons in Ndufs4−/− cells. Nevertheless, further experimentation is needed to exclude the possibility that electrons flow through partially assembled complex I in a way that does not contribute to the generation of either energy or ROS.
Mutations in NDUFS4 have been linked to Leigh syndrome, a fatal human disorder caused by complex I deficiency. All NDUFS4 mutations in the human disease cause impaired assembly and function of complex I, and early postnatal lethality (24, 32–34). In this study, we used Ndufs4−/− mice as a genetic model to study mitochondrial complex I deficiency and dopaminergic neuron death. Like humans, deletion in Ndufs4 gene in mice caused Leigh-like syndrome and lethality at postnatal week 7 (26). Neurons cultured from Ndufs4−/− mice may therefore provide a useful model to study molecular and cellular mechanisms for Leigh syndrome and other neurodegenerative diseases where mitochondrial complex I impairment has been implicated.
Materials and Methods
Generation of Ndufs4-Null (Ndufs4−/−) Mice and Primary Mesencephalic Neuron Cultures.
Generation and characterization of the Ndufs4−/− mice has been described (26). To prepare primary cultured dopaminergic neurons from single embryos, we modified the cell culture protocol of Gille et al. (57). See SI Text for details.
Isolation of Mitochondria and Mitochondria Complex Activity Assays.
Isolation of mitochondria was performed as described (58). Polarography assay to monitor mitochondrial oxygen consumption was performed as described (58). Oxygen consumption was also measured using the BD Oxygen Biosensor System (BD Biosciences) as described per manufacturer's instructions (59–61). Alternatively, we modified published protocols for NADH dehydrogenase activity assay in intact cells (58, 62). See SI Text for details.
Drug Treatments, Immunocytochemistry, and Quantification of TH+, GABAergic, or Total Neurons.
All drug (Sigma) treatments were performed in defined serum-free N2 medium. Neuron cultures were fixed and stained with antibody as indicated. Cells immunostained positive for TH antibody (Sigma) and having neurites twice the length of the soma were scored as TH+ cells. Cells immunostained positive for GABA (Sigma) or NeuN (Chemicon) antibody were counted and scored as the GABAergic or total neuron population. See SI Text for details.
Quantification of ROS, Terminal Deoxynucleotidyl Transferase-Mediated Biotinylated UTP Nick End Labeling (TUNEL), and Total Apoptosis.
ROS level in TH+ neurons was measured using 5 μM 5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-DCFDA; Molecular Probes), and MitoSOX (Molecular Probes). TUNEL staining was performed using TDT (Promega) as described (63). The number of TUNEL-positive cells in the TH+ neuron population was quantified. Apoptosis was determined for the entire cell population by nuclear condensation and/or fragmentation after Hoechst staining (63). See SI Text for details.
Statistical Analysis.
Data were from at least three independent experiments, each with at least duplicate or triplicate determinations. Statistical analysis of data was performed using two-way ANOVA and post hoc Student t test (*P < 0.05; **P < 0.01; ***P < 0.005; ns, not statistically significant).
Supplementary Material
Acknowledgments.
We thank Dr. John Alberta (Dana-Farber Cancer Institute, Boston, MA) for the anti-Olig2 antibody. This work was supported by National Institutes of Health Grant ES012215, Postdoctoral Fellowship Program of the Korea Science and Engineering Foundation (to W.S.C.), and the Howard Hughes Medical Institute (S.E.K. and R.D.P.), and facilitated by National Institute of Child Health and Human Development Grant P30 HD02274 and University of Washington National Institute of Environmental Health Sciences Center for Ecogenetics and Environmental Health Grant P30ES07033.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807581105/DCSupplemental.
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 3.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 4.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno Y, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989;163:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- 6.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 7.Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 8.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 9.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 10.Manning-Bog AB, et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 11.McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem. 2003;85:82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- 12.Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- 14.Thiruchelvam M, et al. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima T, et al. Mechanism of cytotoxicity of paraquat. III. The effects of acute paraquat exposure on the electron transport system in rat mitochondria. Exp Toxicol Pathol. 1994;46:437–441. doi: 10.1016/S0940-2993(11)80056-4. [DOI] [PubMed] [Google Scholar]
- 17.Tawara T, et al. Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Arch Toxicol. 1996;70:585–589. doi: 10.1007/s002040050316. [DOI] [PubMed] [Google Scholar]
- 18.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Cookson MR. Mitochondria and dopamine: New insights into recessive parkinsonism. Neuron. 2004;43:301–304. doi: 10.1016/j.neuron.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Gregus Z, Klaassen CD. In: Casarett and Doull's Toxicology: The Basic Science of Poisons. Klaassen CD, editor. New York: McGraw–Hill; 2001. pp. 35–81. [Google Scholar]
- 21.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 22.Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88:193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 23.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scacco S, et al. Pathological mutations of the human NDUFS4 gene of the 18-kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J Biol Chem. 2003;278:44161–44167. doi: 10.1074/jbc.M307615200. [DOI] [PubMed] [Google Scholar]
- 25.Petruzzella V, Papa S. Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: The NDUFS4 gene. Gene. 2002;286:149–154. doi: 10.1016/s0378-1119(01)00810-1. [DOI] [PubMed] [Google Scholar]
- 26.Kruse SE, et al. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budde SM, et al. Clinical heterogeneity in patients with mutations in the NDUFS4 gene of mitochondrial complex I. J Inherit Metab Dis. 2003;26:813–815. doi: 10.1023/b:boli.0000010003.14113.af. [DOI] [PubMed] [Google Scholar]
- 28.Iuso A, et al. Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. J Biol Chem. 2006;281:10374–10380. doi: 10.1074/jbc.M513387200. [DOI] [PubMed] [Google Scholar]
- 29.Perier C, et al. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem J. 1996;315(Pt 1):295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 32.van den Heuvel L, et al. Demonstration of a new pathogenic mutation in human complex I deficiency: A 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet. 1998;62:262–268. doi: 10.1086/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budde SM, et al. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- 34.Petruzzella V, et al. A nonsense mutation in the NDUFS4 gene encoding the 18 kDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum Mol Genet. 2001;10:529–535. doi: 10.1093/hmg/10.5.529. [DOI] [PubMed] [Google Scholar]
- 35.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 36.Murai M, Ichimaru N, Abe M, Nishioka T, Miyoshi H. Mode of inhibitory action of Deltalac-acetogenins, a new class of inhibitors of bovine heart mitochondrial complex I. Biochemistry. 2006;45:9778–9787. doi: 10.1021/bi060713f. [DOI] [PubMed] [Google Scholar]
- 37.Chinopoulos C, Adam-Vizi V. Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: Relevance to Parkinson's disease. J Neurochem. 2001;76:302–306. doi: 10.1046/j.1471-4159.2001.00060.x. [DOI] [PubMed] [Google Scholar]
- 38.Beretta S, et al. Partial mitochondrial complex I inhibition induces oxidative damage and perturbs glutamate transport in primary retinal cultures. Relevance to Leber Hereditary Optic Neuropathy (LHON) Neurobiol Dis. 2006;24:308–317. doi: 10.1016/j.nbd.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 39.McCormack AL, et al. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- 40.McCormack AL, Atienza JG, Langston JW, Di Monte DA. Decreased susceptibility to oxidative stress underlies the resistance of specific dopaminergic cell populations to paraquat-induced degeneration. Neuroscience. 2006;141:929–937. doi: 10.1016/j.neuroscience.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 41.Bernstam L, Nriagu J. Molecular aspects of arsenic stress. J Toxicol Environ Health B Crit Rev. 2000;3:293–322. doi: 10.1080/109374000436355. [DOI] [PubMed] [Google Scholar]
- 42.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 43.Sherer TB, et al. An in vitro model of Parkinson's disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Y, Liu W, Jiang H, Jiang Q, Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280:34105–34112. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 45.Cappelletti G, Surrey T, Maci R. The parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as a destabilising factor. FEBS Lett. 2005;579:4781–4786. doi: 10.1016/j.febslet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 46.Andersen JK. Iron dysregulation and Parkinson's disease. J Alzheimers Dis. 2004;6:S47–S52. doi: 10.3233/jad-2004-6s602. [DOI] [PubMed] [Google Scholar]
- 47.Mandel S, Maor G, Youdim MB. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: Effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)-epigallocatechin-3-gallate. J Mol Neurosci. 2004;24:401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- 48.Mazzio E, Yoon KJ, Soliman KF. Acetyl-L-carnitine cytoprotection against 1-methyl-4-phenylpyridinium toxicity in neuroblastoma cells. Biochem Pharmacol. 2003;66:297–306. doi: 10.1016/s0006-2952(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 49.Shimada H, Hirai K, Simamura E, Pan J. Mitochondrial NADH-quinone oxidoreductase of the outer membrane is responsible for paraquat cytotoxicity in rat livers. Arch Biochem Biophys. 1998;351:75–81. doi: 10.1006/abbi.1997.0557. [DOI] [PubMed] [Google Scholar]
- 50.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: A review. J Toxicol Clin Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- 52.Yumino K, Kawakami I, Tamura M, Hayashi T, Nakamura M. Paraquat- and diquat-induced oxygen radical generation and lipid peroxidation in rat brain microsomes. J Biochem. 2002;131:565–570. doi: 10.1093/oxfordjournals.jbchem.a003135. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu K, Matsubara K, Ohtaki K, Shiono H. Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res. 2003;46:523–532. doi: 10.1016/s0168-0102(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu K, et al. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 2003;976:243–252. doi: 10.1016/s0006-8993(03)02750-1. [DOI] [PubMed] [Google Scholar]
- 55.Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 56.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Gille G, et al. Pergolide protects dopaminergic neurons in primary culture under stress conditions. J Neural Transm. 2002;109:633–643. doi: 10.1007/s007020200052. [DOI] [PubMed] [Google Scholar]
- 58.Tieu K, et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraker C, et al. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: Oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15:745–758. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- 60.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci USA. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo BB, Nakamaru-Ogiso E, Flotte TR, Matsuno-Yagi A, Yagi T. In vivo complementation of complex I by the yeast Ndi1 enzyme. Possible application for treatment of Parkinson disease. J Biol Chem. 2006;281:14250–14255. doi: 10.1074/jbc.M600922200. [DOI] [PubMed] [Google Scholar]
- 62.Heidenrich KA, Gilmore PR, Garvey WT. Glucose transport in primary cultured neurons. J Neurosci Res. 1989;22:397–407. doi: 10.1002/jnr.490220405. [DOI] [PubMed] [Google Scholar]
- 63.Hsuan SL, Klintworth HM, Xia Z. Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways. J Neurosci. 2006;26:4481–4491. doi: 10.1523/JNEUROSCI.4922-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.