Abstract
Chloroplast biogenesis involves careful coordination of both plastid and nuclear gene expression, which is achieved in part by retrograde signaling from the chloroplast to the nucleus. This can be demonstrated by the fact that the herbicide, Norflurazon (NF), which causes bleaching of chloroplasts, prevents the light induction of photosynthesis-related genes in the nucleus. It has been proposed that the tetrapyrrole pathway intermediate Mg-protoporphyrin IX acts as the signaling molecule in this pathway and accumulates in the chloroplasts and cytosol of the cell after NF treatment. Here we present data that demonstrate that this model is too simplistic. We have developed a sensitive liquid chromatography-mass spectrometry (LC/MS) method to measure tetrapyrrole intermediates and have shown that no Mg-protoporphyrin IX, nor indeed any other chlorophyll-biosynthesis intermediate, can be detected in NF-treated plants under conditions in which nuclear gene expression is repressed. Conversely when endogenous Mg-protoporphyrin IX levels are artificially increased by supplementation with the tetrapyrrole precursor, 5-aminolevulinic acid, the expression of nuclear-encoded photosynthetic genes is induced, not repressed. We also demonstrate that NF-treatment leads to a strong down-regulation of tetrapyrrole biosynthesis genes, consistent with the absence of an accumulation of tetrapyrrole intermediates. Finally, there is no correlation between nuclear-gene expression and any of the chlorophyll biosynthetic intermediates over a range of growth conditions and treatments. Instead, it is possible that a perturbation of tetrapyrrole synthesis may lead to localized ROS production or an altered redox state of the plastid, which could mediate retrograde signaling.
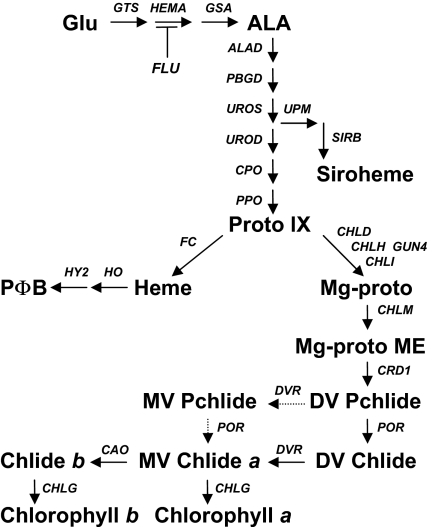
The tetrapyrrole biosynthetic pathway leads to the synthesis of a number of important products including the chlorophylls and hemes. The enzymatic steps of the pathway are well characterized (Fig. 1) (1–3), and genes for virtually all of the enzymes have been identified in higher plants. The pathway is tightly regulated to ensure a continuous cofactor supply to the cognate apoproteins whilst avoiding the phototoxic accumulation of intermediates (3,4). This is exemplified by the coordination of chlorophyll synthesis with the production of light-harvesting chlorophyll proteins (LHCs) encoded by the nucleus, which is in part mediated by retrograde signals from the chloroplast to the nucleus. These signals can be observed following treatment with the herbicide Norflurazon (NF), which causes photooxidative damage of chloroplasts in white light (WL) and leads to a dramatic reduction in the expression of Lhcb and other nuclear-encoded photosynthesis-related genes (5,6). A screen for Arabidopsis mutants defective in the response to NF revealed the involvement of the tetrapyrrole pathway. In the original screen a total of five nonallelic gun (genomes uncoupled) mutants were identified, in which Lhcb1.2 was up-regulated in the light in the presence of NF. The gun1 mutant lacks a chloroplast-localized pentatricopeptide repeat protein (7), whereas the other four mutants are defective in the tetrapyrrole pathway: gun5 has a mutation in CHLH, a subunit of Mg-chelatase (8), gun4 lacks a regulator of Mg-chelatase (9), and gun2 and gun3 (allelic to hy1 and hy2) are deficient in heme oxygenase and phytochromobilin synthase respectively (8) (Fig. 1). Additional tetrapyrrole-related gun mutants have also been identified (10, 11).
Fig. 1.
The tetrapyrrole pathway in plants showing intermediates and genes analyzed in this study. Intermediates: Glu, glutamate; ALA, 5-aminolevulinic acid; Proto IX, protoporphyrin IX; Mg-proto, Mg-protoporphyrin; Mg-proto ME, Mg-protoporphyrin monomethyl ester; DV Pchlide, divinyl protochlorophyllide; MV Chlide, monovinyl chlorophyllide. Table S1 lists the enzymes that correspond to the gene names.
The observation that many gun mutants are impaired in tetrapyrrole biosynthesis led to the suggestion that one or more intermediates in the pathway might themselves act as signaling molecules in plastid-nucleus signaling. Lesions in the pathway that give rise to a gun phenotype would all be expected to be compromised in the accumulation of Mg-protoporphyrin IX (Mg-proto). Strand, et al. (11) reported that Mg-proto accumulated in wild-type (WT) plants after an NF treatment, but accumulation was reduced in gun mutants. In addition, application of exogenous Mg-proto to protoplasts inhibited Lhcb1.2 expression, whereas porphobilinogen, protoporphyrin IX (Proto IX) and heme had no effect. These results supported previous observations that manipulation of Mg-proto levels using inhibitors affected expression of photosynthesis-related genes (12, 13). Similarly, barley Mg-chelatase mutants xantha-f, -g and -h expressed Lhcb genes in the presence of NF in the light, whereas the barley xantha-l mutant, defective in a later enzyme, Mg-proto monomethylester cyclase (CRD1) (Fig. 1), did not (14). The chlm mutant of Arabidopsis, lacking Mg-proto methyltransferase, accumulated high levels of Mg-proto and also showed severe repression of Lhcb expression in the light (15). Most recently a report by Ankele, et al. (16) described in vivo visualization of Mg-proto and its export from chloroplasts to the cytosol following NF treatment.
However, there are difficulties with a model that proposes elevated Mg-proto as a signaling molecule leading to the repression of nuclear genes, including that this intermediate is phototoxic. Additionally, levels of Mg-proto and the repression of Lhcb expression are not always correlated. For example chlI mutants of Arabidopsis do not have a gun phenotype (8), even though they are compromised in Mg-proto production. Moreover, in barley seedlings treated with NF, where Lhcb expression is reduced, no porphyrins accumulated (14). One explanation for these discrepancies is the difficulty in measuring tetrapyrrole intermediates quantitatively in plant material. To overcome this, we developed a novel liquid chromatography/mass spectrometry (LC/MS) method that enables targeted metabolite profiling of tetrapyrroles in seedlings. Each tetrapyrrole can be distinguished by retention time and fragmentation signature, thus allowing unambiguous identification (17). Using this method, we have re-examined the proposed role of Mg-proto (and other chlorophyll intermediates) as signaling molecules. Our results call into question the simple model of Mg-proto accumulation acting to repress nuclear gene expression.
Results
Measurement of Tetrapyrrole Intermediates by LC/MS in Arabidopsis Seedlings.
To establish the profile of the tetrapyrrole intermediates in WT seedlings after NF treatment, it was important to use a method that could identify the compounds unambiguously, and that was both reproducible and sensitive. The analysis of tetrapyrroles has conventionally been carried out by HPLC separation followed by either spectrophotometric or fluorimetric detection, but the work-ups necessary for this analysis can introduce errors in quantification, and often the different intermediates coelute so that they cannot easily be distinguished from one another (18). We have developed a method for detection by tandem MS with an ion-trap instrument, which generates unique fragmentation patterns that are diagnostic for the individual molecules, so permitting unequivocal identification (17). All of the intermediates from Proto IX to monovinyl chlorophyllide (MV-Chlide) can be detected [supporting information (SI) Fig. S1], and quantified by reference to standard curves of known amounts of the compound (Fig. S2). The threshold of sensitivity is 200 fmol injected, although it is possible to detect the presence of much lower levels from the diagnostic fragmentation pattern.
Table 1 (treatment 1) shows the level of tetrapyrrole intermediates present in aerial tissues of 4-d-old dark-grown Arabidopsis seedlings incubated in 1 mM ALA for 16 h, a treatment that causes their accumulation. All of the chlorophyll branch intermediates from Proto IX were detectable, including both divinyl (DV)- and MV-protochlorophyllide (Pchlide), indicating that the order of enzymic steps is not fixed (18) (see Fig. 1). The presence of low levels of Chlide in this tissue was most likely because the sampling and extraction was carried out under green safelight, which allowed some photoconversion, but the amount present was always less than 15% that in tissue harvested after 2 min exposure to WL (data not shown). The same observation has been previously observed but never quantified (e.g., ref. 19). In gun5 seedlings grown under the same conditions, Proto IX was elevated compared to WT, whereas the levels of Mg-porphyrins were reduced, as expected for a Mg-chelatase mutant (8). In 4d-old etiolated seedlings grown in the absence of ALA (Table 1, treatment 2) neither Mg-proto nor its methylester were detectable, but the other intermediates were all present, albeit at lower levels than in the ALA-treated plants. Illumination of 3-d-old untreated seedlings with WL for 24h (treatment 3) led to a rapid photoconversion of Pchlide to Chlide, levels of which then increased steadily, mirroring the increase in chlorophyll (data not shown). In illuminated gun5 seedlings there were detectable amounts of Proto IX, whereas in WT there was none.
Table 1.
Estimation of tetrapyrroles in aerial tissue of Arabidopsis WT and gun5 seedlings determined by LC/MS
| Treatment | pmol·g−1 FW |
||||||
|---|---|---|---|---|---|---|---|
| Proto IX | Mg-proto | Mg-proto ME | DV-Pchilde | MV-Pchlide | DV-Chlide | MV-Childe | |
| 1 + ALA Dk | |||||||
| WT | 165 ± 74 | 47 ± 22 | 280 ± 181 | 2450 ± 1394 | 2116 ± 1362 | 200 ± 35 | 287 ± 124 |
| gun5 | 313 ± 115 | 21 ± 5 | 97 ± 45 | 1996 ± 1272 | 1170 ± 845 | 318 ± 82 | 294 ± 141 |
| 2 Dk | |||||||
| WT | 32 ± 9 | ND | ND | 510 ± 218 | 1172 ± 600 | 79 ± 14 | 172 ± 45 |
| gun5 | 39 ± 11 | ND | ND | 354 ± 121 | 151 ± 72 | 109 ± 15 | 99 ± 12 |
| 3 24 h WL | |||||||
| WT | ND | ND | ND | 137 ± 49 | 358 ± 175 | 2098 ± 1155 | 3421 ± 878 |
| gun5 | Present | ND | ND | 51 ± 16 | 201 ± 114 | 1436 ± 812 | 654 ± 105 |
| 4 + ALA + NF Dk | |||||||
| WT | 101 ± 53 | 47 ± 22 | 108 ± 54 | 2355 ± 1318 | 1390 ± 889 | 58 ± 25 | 243 ± 131 |
| gun5 | 410 | Present | 71 | 2614 | 1565 | 136 | 401 |
| 5 + NF Dk | |||||||
| WT | Present | ND | ND | 481 ± 217 | 689 ± 357 | 219 ± 56 | 250 ± 73 |
| gun5 | Present | ND | ND | 536 ± 335 | 226 ± 144 | 79 ± 10 | 184 ± 110 |
| 6 + NF 24 h WL | |||||||
| WT | ND | ND | ND | 7 ± 1 | 5 ± 1 | 13 ± 5 | 73 ± 36 |
| gun5 | ND | ND | ND | 14 ± 8 | 5 ± 1 | 25 ± 9 | 11 ± 5 |
Seedlings were grown for 4 d in the dark (Dk) or 3 d in the dark then 24 h in light (WL). For treatments 4–6, seedlings were grown with 5 μM NF. ALA (1 mM) was applied to the seedlings 16 h before harvest for treatments 1 and 4. Values are the mean of at least four independent experiments ± standard deviation, except for gun5 under treatment 4. The limit of sensitivity for accurate quantification is equivalent to 20 pmol·g−1 FW. Present, compound identified unambiguously by the mass of the major ion and its fragmentation pattern but below the quantification threshold; ND, correct mass/fragmentation pattern not detected.
Effect of NF Treatment on Tetrapyrrole Pathway Intermediates.
The LC/MS method described above can accurately and reproducibly measure Proto IX and Mg-proto in plant tissue corresponding to 20 pmol/gFW, much less than the 6 nmol/gFW reported by Strand, et al. (11) to accumulate after NF treatment of Arabidopsis seedlings. We therefore used this method to analyze the porphyrin content of seedlings grown in the presence of 5 μM NF in the dark for 3 d, then illuminated with WL for 24 h, conditions under which nuclear gene expression is repressed (see later). Fig. 2A compares the photodiode array (PDA) trace after HPLC of extracts from treated and untreated tissue. Note that the PDA scale for the NF-treated sample has been expanded greatly. As expected, carotenoids, which elute at about 25–35 min, were absent in the NF-treated tissue, since NF inhibits the carotenoid biosynthesis enzyme phytoene desaturase. However, porphyrins were also severely reduced. In Fig. 2B the mass spectrum in the range m/z 200-1000 is shown for the two samples. Again there is nothing in the region corresponding to porphyrins (18–26 min) (Fig. 2 B and Inset). Specific query of the mass spectrum for individual tetrapyrroles found no Mg-proto or Proto IX present. Although small peaks at the corresponding elution times were seen, these were below threshold and did not give the correct fragmentation pattern (Fig. S3 D and G). Similarly, Fig. 2C shows the fluorescence trace from the HPLC, with the excitation and emission wavelengths for Mg-proto; there is virtually no signal in the NF-treated tissue. In fact, the only tetrapyrroles that could be measured with confidence were DV-Pchlide and MV-Chlide, at 2–5% of those seen in untreated seedlings (Table 1, treatment 6). In contrast, in dark-grown NF-treated seedlings the tetrapyrrole profile was similar to that in untreated seedlings, both with and without ALA feeding (Table 1, treatments 4 and 5), demonstrating that NF does not inhibit tetrapyrrole synthesis per se, but rather the absence of carotenoids causes photodestruction of the tetrapyrroles.
Fig. 2.
Analysis of tetrapyrroles by LC/MS. WT Arabidopsis seedlings were grown for 3 d in complete darkness with or without 5 μM NF, followed by 24 h in WL. Pigments were extracted from aerial tissue and analyzed by LC/MS as described in Experimental Procedures. (A) PDA trace across the complete spectrum (200–800 nm) for the two samples (offset for clarity). Note that the scale for the 5 μM NF sample has been greatly expanded compared to the control (no NF). The regions corresponding to the elution range for the majority of porphyrins and carotenoids are indicated. (B) Total ion current (TIC) chromatogram of the same traces sampled from m/z 200-1000. The inset shows an enlargement of the region corresponding to the elution times for porphyrins. (C) Fluorescence spectra for the same traces at the excitation and emission wavelengths specific for Mg-proto (410 nm and 595 nm respectively). Also shown is the emission spectrum of 1 pmol Mg-proto standard.
The inability to measure tetrapyrroles in the tissue was not due to an artifact during extraction, because when plant samples were spiked with 50 pmol/gFW of Proto IX or Mg-proto standard (Fig. S3E), most of the standard was recovered. Nor was there a transient accumulation of porphyrins that had disappeared by the time of sampling: within 15 min of illumination of dark-grown seedlings, the tissues photobleached due to the absence of photoprotective carotenoids. At the same time Proto IX disappeared, and we could not detect this intermediate, Mg-proto or Mg-protoME over the following 24 h by either MS or fluorescence (data not shown). To ensure that growth conditions were not responsible for our inability to detect tetrapyrroles in NF-treated material, we analyzed material that had been grown under different conditions, including replicating those used by Strand et al. (11), namely growth in continuous light in NF for 6 d. We also tested whether inclusion of sucrose in the medium had any effect. In all cases there was no accumulation of Mg-proto or any other tetrapyrrole intermediate (data not shown). Finally, when using the slightly different HPLC conditions of Strand et al. (11) we were still unable to detect porphyrins or carotenoids by absorbance or in the mass spectrum in NF-treated seedlings (Fig. S4).
Norflurazon Treatment Has a Dramatic Effect on Tetrapyrrole Biosynthesis Genes.
To characterize further the effect of NF treatment on the tetrapyrrole pathway we analyzed the expression of tetrapyrrole biosynthesis-related genes using Affymetrix full genome microarrays through the GARNet/NASC Affymetrix facility (see Experimental Procedures). Seedlings were germinated on 5 μM NF in the dark and then exposed to WL for 3 d. For WT seedlings (black bars in Fig. 3A), the majority of genes were repressed, with the strongest repression (>2 fold; −50% in Fig. 3A) shown for HEMA1, encoding the rate-limiting enzyme glutamyl-tRNA reductase, and the genes associated with the Mg-porphyrin branch of the pathway (Fig. 1, Table S1). The trunk pathway for tetrapyrrole synthesis was also downregulated (Fig. 3A). These data indicate that tetrapyrrole synthesis is regulated by plastid signals during seedling development in the light. To verify the reliability of the array data, we conducted real-time RT-PCR on 12 tetrapyrrole biosynthesis genes (Fig. 3B). In general, there was good agreement between the microarray data and the real-time PCR results with only occasional discrepancies (Fig. 3B). As the microarray experiment was conducted with seedlings grown in the presence of sucrose we also analyzed these tetrapyrrole biosynthesis genes in the absence of sucrose after 24 h. The comparison of the two growth conditions shows that with the exception of CHLI, which only responds to signals in the presence of sucrose and over the longer time period, the response to NF was strikingly similar (Fig. 3B).
Fig. 3.
The effect of NF treatment on expression of genes encoding the tetrapyrrole pathway. (A) Data from full genome microarray analysis of WT (dark bars) and gun1,5 double mutant (light bars) seedlings grown ± 5 μM NF in the presence of 1.5% (wt/vol) supplementary sucrose. Following germination in D for 3 d, seedlings were allowed to de-etiolate under WL for 3 d. Data shown are the mean and range of two independent experiments. (B) Relative expression levels of 12 tetrapyrrole synthesis genes (see label above each panel) measured in WT and gun1,5 mutant seedlings grown ± 5 μM NF. For each gene, top panels show data from seedlings grown identically to those in A, and bottom panels are for seedlings grown without sucrose for 3 d D and 1 d WL. Transcript levels were measured using real-time RT-PCR and corrected for equal cDNA template according to expression of 40S. Values shown are the mean ± SE from at least three independent experiments. A dark circle indicates the corresponding value in the microarray experiment shown in A.
To determine the effect of known plastid signaling pathways, the impact of NF treatment in the gun1,5 double mutant was examined (8, 20). Overall on NF gene expression was always greater in gun1,5 than WT, but the increase varied (Fig. 3A). Interestingly, the genes that showed the strongest response to gun1,5 are those known to be important regulatory points in the pathway. For example, the regulator GUN4 was strongly inhibited following NF treatment, but was expressed in gun1,5 on NF at a similar level to WT in the absence of NF. The HEMA1 and CHLH genes showed a similar response (Fig. 3). It should also be noted that in some cases expression of genes was greater in gun1,5 compared to WT when both genotypes were grown in the absence of NF. This has been seen before for HEMA1 (21), but is also apparent here for FC2 (encoding ferrochelatase-2), CHLH, CHLD, and GUN4 (the first two in the absence of sucrose only), further supporting a regulatory role for plastid signals in restricting tetrapyrrole synthesis during normal seedling development in the light.
Not all genes were strongly down-regulated after NF treatment. The GluTS gene showed a significant up-regulation. The product of this enzyme, glutamyl-tRNAGlu, is also used for chloroplast protein synthesis, suggesting that its response is driven by the need to synthesize new chloroplast proteins. The expression of other chloroplast tRNA synthetase genes was also maintained following NF treatment (data not shown). Three other major tetrapyrrole biosynthesis genes failed to show downregulation after NF treatment. HEMA2 and FC1 were both moderately up-regulated, as has been observed previously (22, 23). These two genes have been shown to be important for non-photosynthetic heme synthesis. Similarly expression of PPO2, encoding the mitochondrial isoform of protoporphyrinogen IX oxidase, was also increased by NF. It is possible that the strong expression of HEMA2, PPO2, and FC1 is to ensure supply of cofactor for hemoproteins that may be required in response to the severe oxidative stress induced by NF treatment (23).
In summary, although steady-state transcript levels may not be a direct representation of the levels of tetrapyrrole enzyme activity, these data are consistent with the observation that NF-treated seedlings have a severely reduced capacity to synthesize intermediates such as Mg-proto in the light.
Is There a Relationship Between Tetrapyrrole Intermediates and Nuclear Gene Expression?
To investigate further the relationship between tetrapyrroles and nuclear gene expression we used ALA feeding in the dark to increase all of the chlorophyll biosynthesis intermediates including Mg-proto (Table 1). Analysis by RT-PCR of the expression of Lhcb1.1 and FC2 (Fig. 3) (22), showed that in both WT and gun5, there is an increase in steady state transcript levels in ALA-fed tissue rather than Mg-proto repressing gene expression (Fig. S5).
We extended this analysis to a total of 16 different conditions with seedlings grown in either complete darkness, or light/dark cycles, and treated with NF, ALA, or both, and investigated if there was any correlation, either negative or positive, between these intermediates and the repression of nuclear gene expression. The expression of Lhcb1.1 was determined by RT-PCR (Fig. S6), and levels of all seven chlorophyll biosynthetic intermediates were determined by LC/MS (Fig. S7). Fig. 4 shows a graphical representation of relative levels of Lhcb1.1 transcript under the different conditions compared with levels of each of the intermediates, expressed as a percentage of the values for seedlings grown in the dark without treatments. There is clearly no relationship between Mg-proto and Lhcb1.1 transcript abundance. Although in condition 16 there is no measurable Mg-proto and the Lhcb1.1 transcript is highest, under conditions 1–3 both transcript and metabolite levels are low. Similarly, in condition 10, when there is an extremely high level of Mg-proto, there is still easily detectable Lhcb1.1 transcript. Moreover, none of the other intermediates showed a convincing correlation with Lhcb1.1 expression.
Fig. 4.
Lack of correlation between tetrapyrrole intermediates and Lhcb expression. WT Arabidopsis seedlings were grown in a total of 16 different conditions, chosen to vary the level of tetrapyrrole intermediates and/or expression of Lhcb1.1. In stage 1 (I), seedlings were grown for 4 d in complete darkness (D) or in 3 d in the dark followed by 1 d in white (100 μmol·m−2·s−1) light (L) in the absence (−) or presence (+) of 5 μM NF. In stage 2 (II), seedlings were subsequently treated for a further 16 h in complete darkness (D) or in white light (L) in the absence (−) or presence (+) of 1 mM ALA. Lhcb1.1 expression was monitored by RT-PCR and quantified relative to ACTIN (Fig. S6) with the values of the dark-grown untreated sample (condition 7) set as 100%. Tetrapyrrole intermediates were measured by LC/MS (Fig. S7). The values are shown with increasing relative expression of Lhcb1.1. Data shown are mean ±SE (n ≥ 2).
Discussion
Mg-Proto Does Not Accumulate after NF Treatment.
Several studies have identified Mg-proto as having the potential to be involved in chloroplast-to-nucleus communication, and this has become the dominant hypothesis (24). A central feature of this premise was measurements in NF-treated seedlings, which demonstrated that Mg-proto levels increased to 6,380 pmol/gFW after NF treatment compared to 414 pmol/gFW in green WT seedlings, whereas other intermediates did not accumulate (11). Mg-proto has been reported to be visualized in vivo in NF-treated seedlings, but only after subsequence ALA feeding (16).
Reliable measurement of Mg-proto levels in plant cells is critical to the verification of this hypothesis and to this end, we developed an LC-MS approach that is equally sensitive, but more reliable compared to conventional methods, in that compounds are identified unambiguously. Using this method the estimation of Mg-proto levels in dark-grown WT seedlings fed ALA (43 pmol/gFW) is in general agreement with data for light-grown tobacco leaves kept in darkness (40–50 pmol/gFW Mg-proto) (25), and etiolated barley leaves fed ALA (250 pmol/gFW for Mg-proto and Mg-proto ME combined) (26). In contrast, we found that Mg-proto failed to accumulate after NF treatment, and was well below the 20 pmol/gFW threshold for unambiguous identification and quantitation. The most likely explanation for the discrepancy between our results and those of Strand, et al. (11) is that the HPLC conditions used by the latter are not optimal for resolution of pigment molecules, so that the fluorescence they detected was due to contaminating substances. Significantly, Mochizuki and colleagues have carried out a simultaneous re-evaluation of the Mg-proto hypothesis using a molecular genetic approach, and have similarly concluded that Mg-proto cannot itself be acting as a signaling intermediate (27).
The Chlorophyll Synthesis Pathway Is Strongly Downregulated after NF Treatment.
To understand why no accumulation of tetrapyrroles was observed following NF treatment, we examined tetrapyrrole biosynthesis gene expression under these conditions. All genes of the chlorophyll biosynthesis pathway were strongly inhibited by NF treatment, although the most severe effects were seen for those encoding the enzymes of the Mg-porphyrin branch. This is consistent with the need to eliminate the synthesis of potentially dangerous Mg-porphyrins while retaining some capacity for heme synthesis to respond to the stress conditions imposed. There has always been a close link between the response to light and plastid signals (28). It is therefore interesting that nearly all of the tetrapyrrole genes responded strongly to NF while a previous study using a miniarray system showed that only a small cohort of genes (HEMA1, CHLH, CRD1, and CAO) responded strongly to light with most genes showing a more moderate response (29). It is known that the HEMA1 gene is under direct photoreceptor control while a more weakly light-regulated gene, GSA1, is not, and it was hypothesized previously (30) that the moderate light regulation seen for many tetrapyrrole genes may be mediated through plastid signaling from the developing chloroplasts. The observation here that most genes respond quite strongly to the loss of chloroplast integrity would be consistent with this hypothesis.
One significant observation from the microarray data was that GluTS, HEMA2, PPO2, and FC1, genes encoding isoforms that have previously been implicated in having a major role in heme synthesis (22, 31) under stress conditions (23), were all induced following NF treatment. The UPM1 gene also showed strong induction under these conditions. This suggests a strong requirement for siroheme in responding to stress. However, as the substrate for the enzyme encoded by UPM1, uroporphyrinogen III, is the first photosensitive tetrapyrrole, it is also possible that strong induction of UPM1 may have an immediate role in mitigating further photo-oxidative damage. One final possibility is that induction of siroheme may be part of the signaling cascade leading to sustained down regulation of nuclear gene expression. These hypotheses clearly require further testing.
Do Tetrapyrroles Have a Role in Plastid Signaling?
The conclusion drawn from our data is that NF-induced downregulation of nuclear gene expression can occur without any accumulation of Mg-proto, and there is no correlation between levels of chlorophyll biosynthetic intermediates and Lhcb expression. A simple signaling mechanism in which the accumulation of a tetrapyrrole intermediate inhibits nuclear gene expression is thus highly unlikely. Indeed, time course studies showed that any effect of Mg-proto would be extremely short-lived and, from the data presented here, light-dependent. This is not consistent with the proposal of a sustained accumulation of Mg-proto, whether in the chloroplast or following transport to the cytosol (16). Although the data presented here do not suggest an alternative model, we do note that one short-lived, light-dependent process might be the generation of ROS signals (32). It is conceivable that signaling is via a specific Mg-proto-derived ROS, as under optimal conditions no Mg-proto appears to accumulate. However, Mg-proto would be expected to generate singlet oxygen in the presence of light (32), and analysis of specific ROS marker genes suggests that NF treatment generates a much more complex ROS signal during chloroplast destruction (A.C.M. and M.J.T., unpublished results). The effects of NF treatment on nuclear gene expression would therefore be unlikely to correlate with an individual tetrapyrrole intermediate, but such a scenario may explain why signaling has been suggested to coincide with Mg-proto under some experimental conditions. Strand, et al. (11) reported that superoxide generation was not reduced in gun mutants compared to WT. Although details of the conditions were not provided, this would suggest that this ROS species at least is not a major component of any plastid signal profile. There is evidence for regulation of nuclear gene expression by both H2O2 (33) and 1O2 (34) and a role for these ROS, perhaps in combination, needs to be explored further. Alternatively, regulation of nuclear gene expression in response to the redox state of plastids is well established (35), and NF treatment and photo-oxidative destruction of tetrapyrroles may impact on this pathway.
Experimental Procedures
Plant Material and Growth Conditions.
The Arabidopsis thaliana L. gun5 mutant and the gun1,5 double mutant both in the Columbia (Col-0) WT background have been described previously (8, 20). For pigment analysis seeds were surface-sterilized and plated onto medium containing 0.8% agar and 1/2 × MS salts ± 5 μM NF. Plates were placed at 4 °C in darkness for 2 d followed by a 30 min light irradiation to synchronize germination. Seeds were germinated in darkness at 22 °C for 3 d, followed by 24 h illumination in 100 μmol.m−2.s−1 WL. Alternatively, to reproduce the conditions of Strand, et al. (11), seedlings were placed in continuous WL for 6 d. For ALA treatment, after 3 d in complete darkness 1 mM ALA was added and seedlings were incubated in darkness for 16 h before harvesting. For microarray and real-time RT-PCR experiments, growth conditions (including growth medium, light sources and treatments) were exactly as described previously (21).
Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis.
For the extraction of pigments, seedlings were ground in liquid N2, 20 μl (1 μM) deuteroporphyrin internal standard was added and the powder homogenized in methanol:0.1M NH4OH (90:10, vol/vol). The extract was centrifuged at 10,000 × g for 10 min, the supernatant kept, and the pellet re-homogenized with acetone:0.1M NH4OH (80:20, vol/vol). The supernatants were combined and the procedure repeated twice. The pooled supernatants were dried under N2 flow, resuspended in 100 μl acetone: 0.1M NH4OH (80:20, vol/vol) and then centrifuged at 10,000 × g for 10 min. The supernatant was collected and kept in darkness at 4 °C until analysis.
LC/MS analysis was carried out essentially as described (17). The extract was separated on a Finnigan Surveyor HPLC (conditions detailed in SI Text). Elution was followed by an online formic acid treatment (4 μl/min) and then MS with a Finnigan LCQDECA XP mass spectrometer with an ESI source (Thermo Fisher Scientific). Detection was in positive ion mode using SIM-MS (Single Ion Monitoring-MS) of [M+H+] ions with the m/z values given in Fig. S1 (MS-MS parameters: capillary temperature, 300 °C; capillary voltage, 3.0 kV; collision energy level, 70%). Data were analyzed using Xcalibur (Thermo Fisher Scientific). Quantification was carried out using the linear range of a standard curve constructed with known amounts of porphyrins (Fig. S2).
Gene Expression Analysis.
For microarray analysis total RNA extraction was carried out as previously described (35) but with the addition of a further purification step using the Qiagen RNeasy kit (Qiagen) according to manufacturer's instructions. Microarrays were carried out using the Affymetrix ATH1 Arabidopsis Genome Array (22K) by the GARNet/NASC facility. Further details of the conditions used are available at http://affymetrix.arabidopsis.info/narrays/experimentpage.pl?experimentid=51. Analysis of the normalized data were conducted using Microsoft Excel and Silicon Genetics GeneSpring®7 (Agilent Technologies) software packages. For real-time RT-PCR analysis, total RNA extraction was also carried out as previously described (36) but with an additional step for removal of polysaccharides as given in SI Text. The gene-specific primer pairs used are shown in Table S2.
Supplementary Material
Acknowledgments.
The gun mutants were gifts from Enrique López-Juez (Royal Holloway College, London, United Kingdom) and Joanne Chory (Salk Institute for Biological Studies, La Jolla, CA). We thank Nobuyoshi Mochizuki (Kyoto University, Kyota, Japan) for providing information before publication. We also thank John Gray (University of Cambridge, Cambridge, U.K.) for the gift of the Norflurazon, and GARNet (NASC, Nottingham, U.K.) for providing the microarray service. This work was supported by the Biotechnology and Biological Sciences Research Council [Grant Number 51/P17214], Gatsby Charitable Foundation, and EU Framework Program 5.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803054105/DCSupplemental.
References
- 1.Beale SI. Enzymes of chlorophyll biosynthesis. Photosyn Res. 1999;60:43–73. [Google Scholar]
- 2.Eckhardt U, Grimm B, Hortensteiner S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol Biol. 2004;56:1–14. doi: 10.1007/s11103-004-2331-3. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- 4.Cornah JE, Terry MJ, Smith AG. Green or red: What stops the traffic in the tetrapyrrole pathway? Trends Plants Sci. 2003;8:224–230. doi: 10.1016/S1360-1385(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 5.Oelmüller R, Mohr H. Photooxidative destruction of chloroplasts and its consequences for expression of nuclear genes. Planta. 1986;167:106–113. doi: 10.1007/BF00446376. [DOI] [PubMed] [Google Scholar]
- 6.Gray JC, Sullivan JA, Wang JH, Jerome CA, MacLean D. Coordination of plastid and nuclear gene expression. Phil Trans Roy Soc Lond B. 2003;358:135–144. doi: 10.1098/rstb.2002.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koussevitzky S, et al. Multiple signals from damaged chloroplasts converge on a common pathway to regulate nuclear gene expression. Science. 2007;316:715–719. [PubMed] [Google Scholar]
- 8.Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. Arabidopsis genomes uncoupled 5 (gun5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA. 2001;98:2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin RM, Alonso JM, Ecker JR, Chory J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science. 2003;299:902–906. doi: 10.1126/science.1079978. [DOI] [PubMed] [Google Scholar]
- 10.McCormac AC, Terry MJ. Loss of nuclear gene expression during the phytochrome A-mediated far-red block of greening response. Plant Physiol. 2002;130:402–414. doi: 10.1104/pp.003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strand A, Asami T, Alonso J, Ecker JR, Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 12.La Rocca NL, Rascio N, Oster U, Rüdiger W. Amitrole treatment of etiolated barley seedlings lead to a deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta. 2001;213:101–108. doi: 10.1007/s004250000477. [DOI] [PubMed] [Google Scholar]
- 13.Oster U, Brunner H, Rüdiger W. The greening process in cress seedlings. V. Possible interference of chlorophyll precursors accumulated after thujaplicin treatment with light-regulated expression of Lhc genes. J Photochem Photobiol. 1996;36:255–261. [Google Scholar]
- 14.Gadjieva R, Axelsson E, Olsson U, Hansson M. Analysis of gun phenotype in barley magnesium chelatase and Mg-protoporphyrin IX monomethyl ester cyclase mutants. Plant Physiol Biochem. 2005;43:901–908. doi: 10.1016/j.plaphy.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Pontier D, Albrieux C, Joyard J, Lagrange T, Block MA. Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis—Effects on chloroplast development and on chloroplast-to-nucleus signaling. J Biol Chem. 2007;282:2297–2304. doi: 10.1074/jbc.M610286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ankele E, Kindgren P, Pesquet E, Strand A. In vivo visualization of Mg-ProtoporphyrinIX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell. 2007;19:1964–1979. doi: 10.1105/tpc.106.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulin M, Smith AG. A robust method for determination of chlorophyll intermediates by tandem mass spectrometry. In: Allen J, Gantt E, Golbeck J, Osmond B, editors. Photosynthesis: Energy from the Sun; 14th International Congress on Photosynthesis; Heidelberg: Springer; 2008. pp. 1221–1228. [Google Scholar]
- 18.Kolossov VL, Rebeiz CA. Chloroplast biogenesis 88—Protochlorophyllide b occurs in green but not in etiolated plants. J Biol Chem. 2003;278:49675–49678. doi: 10.1074/jbc.C300449200. [DOI] [PubMed] [Google Scholar]
- 19.Huq E, et al. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 20.Vinti G, et al. Interactions between hy1 and gun mutants of Arabidopsis, and their implications for plastid/nuclear signaling. Plant J. 2000;24:883–894. doi: 10.1046/j.1365-313x.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 21.McCormac AC, Terry MJ. The nuclear genes Lhcb and HEMA1 are differentially sensitive to plastid signals and suggest distinct roles for the GUN1 and GUN5 plastid-signalling pathways during de-etiolation. Plant J. 2004;40:672–685. doi: 10.1111/j.1365-313X.2004.02243.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh DP, Cornah JE, Hadingham S, Smith AG. Expression analysis of the two ferrochelatase genes in Arabidopsis in different tissues and under stress conditions reveals their different roles in haem biosynthesis. Plant Mol Biol. 2002;50:773–788. doi: 10.1023/a:1019959224271. [DOI] [PubMed] [Google Scholar]
- 23.Nagai S, et al. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol. 2007;144:1039–1051. doi: 10.1104/pp.107.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nott A, Jung H-S, Koussevitzky S, Chory J. Plastid-to-nucleus retrograde signalling. Annu Rev Plant Biol. 2006;57:739–759. doi: 10.1146/annurev.arplant.57.032905.105310. [DOI] [PubMed] [Google Scholar]
- 25.Papenbrock J, Mock H-P, Kruse E, Grimm B. Expression studies in tetrapyrrole biosynthesis: Inverse maxima of magnesium chelatase and ferrochelatase activity during cyclic photoperiods. Planta. 1999;208:264–273. [Google Scholar]
- 26.Yaronskaya E, et al. Metabolic control of the tetrapyrrole biosynthetic pathway for porphyrin distribution in the barley mutant Albostrians. Plant J. 2003;35:512–522. doi: 10.1046/j.1365-313x.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A. The steady state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:15184–15189. doi: 10.1073/pnas.0803245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinti G, Fourrier N, Bowyer JR, López-Juez E. Arabidopsis cue mutants with defective plastids are impaired primarily in the photocontrol of expression of photosynthesis-associated nuclear genes. Plant Mol Biol. 2005;57:343–357. doi: 10.1007/s11103-004-7867-8. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto F, et al. Gene expression profiling of the tetrapyrrole metabolic pathway in Arabidopsis with a mini-array system. Plant Physiol. 2004;135:2379–2391. doi: 10.1104/pp.104.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormac AC, Terry MJ. Light-signalling pathways leading to the co-ordinated expression of HEMA1 and Lhcb during chloroplast development in Arabidopsis thaliana. Plant J. 2002;32:549–559. doi: 10.1046/j.1365-313x.2002.01443.x. [DOI] [PubMed] [Google Scholar]
- 31.Ujwal ML, et al. Divergent regulation of the HEMA gene family encoding glutamyl-tRNA reductase in Arabidopsis thaliana: Expression of HEMA2 is regulated by sugars, but is independent of light and plastid signaling. Plant Mol Biol. 2002;50:81–89. doi: 10.1023/a:1016081114758. [DOI] [PubMed] [Google Scholar]
- 32.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M, et al. Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol. 2003;77:226–233. doi: 10.1562/0031-8655(2003)077<0226:ioagrb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.op den Camp RGL, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfannschmidt T. Chloroplast redox signals: How photosynthesis controls its own genes. Trends Plants Sci. 2003;8:33–41. doi: 10.1016/s1360-1385(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 36.McCormac AC, Fischer A, Kumar AM, Söll D, Terry MJ. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J. 2001;25:549–561. doi: 10.1046/j.1365-313x.2001.00986.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.