Abstract
Visual object recognition is subserved by ventral temporal and occipital regions of the brain. Regions comprising the dorsal visual pathway have not been considered relevant for object recognition, despite strong categorical biases for tool-related information in those regions. Here, we show that dorsal stream processes influence object categorization. We used two techniques to render prime pictures invisible: continuous flash suppression (CFS), which obliterates input into ventral temporal regions, but leaves dorsal stream processes largely unaffected, and backward masking (BM), which allows suppressed information to reach both ventral and dorsal stream structures. Categorically congruent primes suppressed under CFS facilitate categorization of tools but have no effect on nonmanipulable objects; in contrast, primes rendered invisible through BM facilitate target categorization for both tools and nonmanipulable things. Our findings demonstrate that information computed by the dorsal stream is used in object categorization, but only for a category of manipulable objects.
Keywords: binocular rivalry, continuous flash suppression, dorsal stream, object categorization, tools
Visual object recognition is subserved by the ventral visual pathway, which projects from V1 through ventral temporal and occipital structures to anterior temporal cortex (1–4). The spatial and visuomotor analyses necessary for grasping and manipulating objects are subserved by the dorsal visual pathway, which projects from V1 through dorsal occipital to posterior parietal structures (1, 5–12). The respective autonomy of the computations mediated by the ventral and dorsal streams is well established. For instance, patients with lesions to ventral stream structures may present with visual object agnosia but normal object grasping; in contrast, patients with lesions to dorsal stream structures may present with impaired object grasping and/or manipulation, but intact visual object recognition (1, 9, 12–14). It is also known that regions within the dorsal stream that are involved in object directed action show neural specificity for manipulable objects (7, 15, 16). However, regions comprising the dorsal visual pathway have not been considered relevant for object recognition, despite those strong categorical biases. Here, we show that dorsal stream computations influence object categorization processes, albeit in a highly categorical fashion (i.e., only for objects, like tools, that are manipulable).
CFS (17, 18), an interocular suppression technique, provides a direct means for testing whether computations mediated by the dorsal stream influence object recognition. It is known that posterior parietal/dorsal occipital regions show greater activation for tool stimuli compared with face stimuli when those stimuli are rendered invisible with CFS, whereas category-specific neural responses within the ventral stream to the same stimuli are obliterated (18; see also, 19–22). In experiments 1–5, we used this property of the CFS paradigm to demonstrate that information processed by the dorsal stream influences, online, the overt retrieval of semantic knowledge about tools but not nonmanipulable things. In contrast to CFS, stimuli rendered invisible through backward masking (BM) continue to activate regions within the ventral object processing stream (23), and induce priming effects for a range of different semantic categories (24, 25). In experiment 6, we used this property of BM to show that the same categorically congruent primes used in the CFS experiments facilitate categorization responses for both tool and animal targets.
Results
Category Specific Priming Effects under CFS.
In experiments 1 and 2, participants indicated whether a visible target picture was a tool or an animal by means of a manual button response. Each target stimulus (tool or animal) was preceded by a prime stimulus (duration, 200 ms), that could be either congruent (same category as the target) or incongruent (different category as the target stimulus). Prime stimuli were rendered invisible using CFS by presenting the prime to only one eye, and a dynamic (10hz) random noise pattern to the other eye (Figs. 1 and 2). To avoid low-level visual priming effects, prime and target stimuli (throughout all experiments) were never the same basic level items (see Methods for details). Participants were unaware of both the presence and identity of the primes, as demonstrated by the percentage correct performance of participants in detection (experiment 1) and discrimination tasks (experiment 2) carried out over the prime stimuli [See Table 1, supporting information (SI) Fig. S1 a and b, and Methods for details].
Fig. 1.
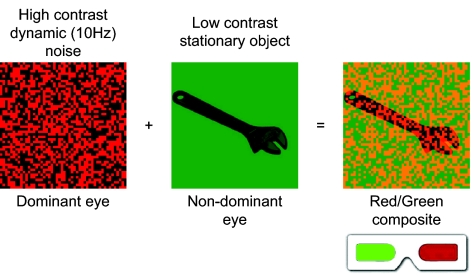
Stimuli rendered invisible with CFS. For experiments 1–5, we used CFS to render the prime stimuli invisible. In CFS a static image competes with a dynamic image, with the latter reliably suppressing the former for a prolonged time (17, 18). Low-luminance, variable low-contrast versions of the prime stimuli were created. Two different high-contrast random noise patterns were created per prime. To ensure that prime stimuli and high-contrast patterns were presented to separate eyes, prime stimuli were restricted to the green RGB channel, whereas high-contrast random patterns were restricted to the red RGB channel. Red/green anaglyph glasses were worn by participants throughout the experiments; it was ensured that the red lens (and hence the high-contrast noise pattern) corresponded to the dominant eye of each participant (the Miles test was used to determine eye dominance). Following previous studies on CFS (17, 18), we presented each random pattern for 100 ms (10 hz dynamic noise patterns), for a total prime/random noise (composite) presentation of 200 ms.
Fig. 2.
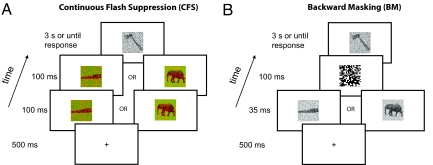
Experimental design. The experiments consisted of two independent stages, the experiment proper, and a detection (experiment 1) or discrimination (experiments 2–6) task performed immediately after the experiment proper. The procedure for the detection and discrimination tasks was the same as in the experiment proper except that, first, the target picture was not presented; second, participants were fully informed of the presence of a prime stimulus; and third, participants were instructed to perform the task (detection or discrimination) over the prime stimulus. The trial structure for the experiments was the following: (A) for experiments 1–5 a fixation cross appeared on the screen (500 ms), followed immediately by the prime picture accompanied by the first random noise pattern (100 ms), followed immediately by the prime picture accompanied by the second random noise pattern (100 ms), followed by the target picture (3,000 ms or response, whichever came first); (B) in experiment 6, the trial sequence was the following: a fixation cross appeared on the screen (500 ms), immediately followed by the prime picture (35 ms), immediately followed by a black and white random noise mask (100 ms), immediately followed by the target picture (3,000 ms or until the response of participants, whichever came first).
Table 1.
Experimental measures of prime awareness
| Experiments |
||||||
|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | |
| Mean, % | 52 | 51 | 50 | 48 | 53 | 47 |
| SD | 3.25 | 6.34 | 5.98 | 6.55 | 6.83 | 5.22 |
| SEM | 0.57 | 1.76 | 1.8 | 1.2 | 1.53 | 1.84 |
| Maximum score | 58 | 60 | 59 | 61 | 61 | 56 |
| Minimum score | 44 | 41 | 40 | 40 | 40 | 40 |
Average percentage correct performance, SD, SEM, and the range of scores (maximum and minimum individual scores) for each experiment. For experiments 1–5, these scores correspond to the performance for the contrast-levels of the primes that were included in the analysis of the data. The same contrasts were selected when d-prime measures (instead of percentage correct) was used. For data from individual participants see Fig. S1.
Analyses of response times to the target pictures in experiment 1 showed that the categorization responses of participants were facilitated by categorically congruent suppressed primes [F(1,30) = 5.90; P < 0.02; and η2 = 0.164; Fig. 3]. Planned comparisons showed that this priming effect was modulated by the category of the target. Participants were faster to categorize a tool when tool primes were presented than when animal primes were presented [t(31) = 3.44 and P < 0.002; priming effects ranged from −29 to 104 ms; mean, 18 ms; SEM, 5 ms) but there was no effect for animal targets (t < 1; mean priming effect, 3 ms; SEM, 5 ms).
Fig. 3.
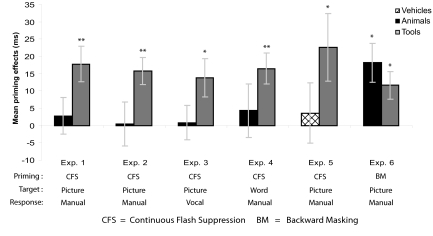
Behavioral priming effects. Average priming effects (incongruent trials minus congruent trials) plotted as a function of the experimental conditions. *, P < 0.05; **, P < 0.001. Error bars represent SEM for priming effects across subjects.
Experiment 2 followed the same protocol as experiment 1, except that a discrimination task over the primes was used as an index of successful suppression of the prime stimuli (i.e., participants had to decide whether a prime was a tool or an animal; see Table 1). The reason for using a discrimination task in experiment 2 (as opposed to a detection task in experiment 1) was to obtain a more stringent measurement of the information that is available from a suppressed stimulus for making a categorization decision. In addition, a different set of animal and tool stimuli was used as primes and targets (see Methods for details). As in experiment 1, the same pattern of semantic priming modulated by the category of the target pictures was observed. Participants were faster to categorize targets in the presence of congruent primes than in the presence of incongruent primes [F(1,11) = 9.42; P < 0.011; and η2 = 0.461; Fig. 3]. Planned comparisons showed reliable semantic priming for tool targets [t(12) = 4.08 and P < 0.002; priming effects ranged from 1 to 48 ms; mean, 16 ms; SEM, 4 ms) but not for animal targets (t < 1; mean priming effect, 0 ms; SEM, 6 ms). The data from experiment 2 demonstrate that the category-specific priming effect is obtained for prime stimuli that participants are not able to discriminate as belonging to one or another category.
The findings from experiments 1 and 2 are consistent with the view that information computed by dorsal stream structures affects object categorization and object recognition. In experiments 3–5, we further explored this effect by having participants make verbal responses to target pictures (experiment 3), manual responses to target words (experiment 4), and categorization decisions over tools and vehicles (another nonliving, but nonmanipulable object category; experiment 5).
In experiment 3, we tested whether the effect observed in experiments 1 and 2 could be explained at the level of motor-relevant information, that is, whether the category-specific priming effect is related to motor facilitation at the level of the effectors. Experiment 3 followed the same procedure and used the same materials as in experiment 2, except that instead of responding with a button response, participants pronounced the words “tool” or “animal” to indicate their categorization decision. Analyses of naming latencies again demonstrated that participants were faster to categorize an object when it was preceded by a congruent prime than by an incongruent prime [F(1, 10) = 4.97; P < 0.05; and η2 = 0.332; Fig. 3; for measures of prime awareness see Table 1, and Fig. S1c). Planned comparisons demonstrated reliable semantic priming for tool targets [t(10) = 2.48 and P < 0.032; priming effects ranged from −14 to 57 ms; mean, 14 ms; SEM, 6 ms) but not for animal targets (t < 1; mean priming effect, 1 ms; SEM, 5 ms). These results demonstrate that the scope of category-specific semantic priming induced by CFS is not limited to manual responses, and that, therefore, this priming effect is not reducible to simple motor facilitation.
In experiment 4, we studied whether the results obtained in the previous experiments were due to visual form, or visuo-motor facilitation between the prime and target pictures. Experiment 4 followed the same procedure and used the same materials as in experiment 2, except that participants categorized word targets instead of picture targets. Analyses of button responses once again demonstrated that participants were faster to categorize a target word preceded by a congruent prime than by an incongruent prime [F(1,28) = 4.155; P < 0.05; and η2 = 0.129; Fig. 3; for measures of prime awareness see Table 1, and Fig. S1d]. Planned comparisons demonstrated reliable semantic priming for tool targets [t(29) = 3.66 and P < 0.001; priming effects ranged from −46 to 75 ms; mean, 16 ms; SEM, 4.5 ms) but not for animal targets (t < 1; mean priming effect, 4 ms; SEM, 7.7 ms). These results demonstrate that the priming effect is obtained for primes and targets that share minimal visual characteristics, but nevertheless maintain a semantic relationship.
In experiment 5, we tested whether the observed category-specificity of the priming effect depends on contrasting two categories that differ in manipulability (i.e., tools vs. animals), or, alternatively, whether it is due to the contrast of artifacts (i.e., tools) with natural entities (animals). The same experimental procedure that was used in experiment 2 was used in experiment 5. We also used the same tool stimuli as in experiment 2, but the animal primes and targets were replaced with images of vehicles. As in the previous experiments, participants were faster to categorize a target in the context of a congruent prime, than in the context of an incongruent prime [F(1,18) = 4.12; P = 0.057; and η2 = 0.186; Fig. 3; for measures of prime awareness see Table 1, and Fig. S1e]. Planned comparisons demonstrated reliable semantic priming for tool targets [t(19) = 2.306 and P < 0.033; priming effects ranged from −41 to 152 ms; mean, 23 ms; SEM, 9.8 ms] but not for vehicle targets (t < 1; mean priming effect, 4 ms; SEM, 8.7 ms). These data suggest that manipulability is the critical dimension underlying the specificity of the observed priming effect.
Finally, in experiment 6 we studied two questions that were left unanswered in the previous experiments. First, are the results obtained in experiments 1–4 because animals and tools, or at least the particular prime pictures that were used, differ in terms of their general ability to lead to priming? Second, can the specificity of the priming effect we have reported be traced to the over-representation of tool knowledge in dorsal structures, and the fact that such structures receive information about CFS suppressed stimuli? To address these questions, we used BM, a technique that is known to elicit priming for a range of categories, including those that are not over-represented in dorsal stream structures, and is known to result in direct activation of ventral stream structures by the prime stimuli.
Priming Effects under BM.
Previous research demonstrates that primes rendered invisible through BM lead to reliable semantic priming effects (24, 25), as well as reduced but significant neural activity in ventral temporal areas (23). Experiment 6 followed the same protocol and used the same materials as in experiment 2; the only difference was that primes were rendered invisible by using a backward mask. Primes were presented for 35 ms, immediately followed by a high-contrast noise-pattern mask that stayed on the screen for ≈100 ms (See Fig. 2 B and Methods for details). The analysis of the response times to target pictures showed once again that congruent primes facilitated object categorization [F(1,6) = 46; P < 0.001; and η2 = 0.885; Fig. 3; for measures of prime awareness see Table 1 and Fig. S1f). In contrast to experiments 1–4, planned comparisons demonstrated reliable priming for both tool and animal targets [for tool targets: t(7) = 2.94 and P < 0.022; priming effects ranging from −1 to 29 ms; mean, 12 ms; SEM, 4 ms; and for animal targets: t(7) = 3.24 and P < 0.014; priming effects ranging from 1 to 43 ms; mean, 18 ms; SEM, 6 ms]. The results from experiment 6 indicate that the prime pictures used in experiments 1–4 do not differ in their general ability to elicit priming. Also, they suggest that the category-specific nature of the priming effects obtained under CFS is related to the over-representation of tool properties in the dorsal stream, and to the fact that such dorsal stream structures receive information about CFS suppressed stimuli (18–22).
Discussion
The results presented in this report constitute a previously undescribed demonstration of high-level priming induced by CFS, or interocular suppression techniques more generally (26–28). Previous attempts to obtain high-level priming effects with interocular suppression techniques may have failed because they did not distinguished between stimuli that do (i.e., tools) and do not (i.e., animals, vehicles) have strong representations in the dorsal object processing stream. In Fig. 3, we summarize the results of experiments 1–6: categorically congruent primes rendered invisible through CFS facilitated categorization responses for tool but not animal or vehicle targets, when compared with categorically incongruent primes. These results are robust across different stimuli, measures of prime awareness (detection vs. discrimination), modality of response (manual vs. vocal), target format (picture vs. written word), and semantic category contrasts (tools vs. animals and tools vs. vehicles). In contrast, the same categorically congruent primes rendered invisible through BM facilitated categorization responses for both animal and tool targets.
The overall pattern of results indicates that semantic priming effects are modulated by interactions between the content of the stimulus and the computations that it engenders. Specifically, the dimension of “being a manipulable object” seems to be critical for priming effects to be induced by CFS suppressed stimuli. The pattern of results obtained suggests that dorsal stream computations mediating object directed action influence object recognition processes for manipulable objects.
An important issue that is raised by the findings that we have reported concerns the nature of the information that is processed by dorsal stream structures, and which ultimately affects object recognition processes. The tool stimuli that were used in these experiments all had an elongated principal axis. Thus, one issue that arises is whether similar effects would be observed for manipulable/graspable objects that do not share this visuo-motor characteristic (see ref. 15 for discussion). More generally, our findings raise questions about whether dorsal stream structures represent detailed and “abstract” knowledge about visually presented objects. For instance, it could be argued that the information computed by the dorsal stream that is relevant for observing priming from CFS stimuli is relatively abstract and concerns the category membership of the stimulus. However, as discussed above, neuropsychological evidence indicates that patients with lesions to ventral occipital-temporal regions can have profound difficulties naming objects, but unimpaired visuo-motor abilities with the same objects (e.g., patient DF; 1). Those data place an important upper boundary on what the dorsal stream can be assumed to represent about an object, at least as that information is explicitly available to individuals/patients. Nevertheless, our findings, and the experimental paradigm we have presented, offer a previously undescribed way of studying these issues in the normally functioning and intact brain.
Whereas there is a range of evidence (both behavorial and physiological) with human and nonhuman primates demonstrating that binocularly suppressed stimuli have different effects on ventral and dorsal stream structures (18, 19–22), much remains unknown about how information reaches dorsal stream structures. One possibility is that information reaches the dorsal processing stream through subcortical routes (18, 20). An important possibility opened up by our findings is that information arriving through subcortical structures is filtered along lines that map onto conceptual categories. Consistent with this hypothesis, Pasley et al. (20) found that suppressed emotional faces activated the amygdala, and that the provenance of this activation could be traced to the superior colliculus. Of particular relevance to the present study is the fact that regions within the posterior parietal cortex are the target of projections from the superior colliculus (29). Another possibility is that stimuli are not filtered along categorical lines within subcortical structures, but are rather sorted based on the response preferences of the cortical regions that receive subcortical input.
Our findings also indicate that there is more than one way in which an object may be invisible. By rendering stimuli invisible with CFS and BM, we took advantage of the different kinds of information that became available to cognitive systems in each technique. We believe that these differences in the availability of information are responsible for the dramatic disparity in subsequent behavior, including high-level decisions, observed in our experiments. Along the lines of the distinction advanced by Dehaene et al. (30) between unconscious and preconscious processes, it is possible to distinguish different types of unconscious processes according to the pathways that information takes from the eye to the cortex.
Methods
Participants and Apparatus.
For this study, 114 Harvard University undergraduate students participated in the experiments in exchange for course credit or payment (32 participated in experiment 1, 13 in experiment 2, 11 in experiment 3, 30 in experiment 4, 20 in experiment 5, and 8 in experiment 6). All participants had normal, or corrected to normal vision and gave written informed consent. The project was approved by the Committee for the Use of Human Subjects at Harvard University. All participants were right handed (Edinburgh Handedness questionnaire), and were naïve as to the experimental hypotheses.
All experiments were run on a Dell PC, with a ViewSonic ultrabrite A90+f monitor. The monitor refresh rate was 100 Hz for experiments 1–5 and 85 Hz for experiment 6. Stimulus presentation was controlled by DMDX (31). The tool and animal pictures that were used in the experiments have been described elsewhere (15). The vehicle pictures were obtained from the internet.
Continuous Flash Suppression.
For experiment 1, 10 pictures were selected as experimental stimuli, five animals and five tools (as defined in ref. 15). For each category, one of the pictures was selected as a prime, whereas the others were used as targets. For experiments 2–3, 16 pictures were selected as experimental stimuli (eight animals and eight tools). In experiment 5, we used the same tool pictures, and we selected eight pictures of vehicles as experimental stimuli, replacing the animal pictures. For each category, half of the items were selected to be targets, whereas the other half was selected as primes. Care was taken so that the selected primes for experiments 2, 3, and 5 were not used as primes in experiment 1, and the targets were not used as targets in experiment 1. The stimuli were presented centrally, and subtended ≈7° of visual angle; 70% additive noise was added to the target stimuli by using Photoshop, to avoid ceiling performance. For experiment 4, the words corresponding to the picture targets used in experiment 2 were used as targets, whereas the same prime pictures were used as in experiments 2–3. Participants were seated comfortably, and at a distance of ≈50 cm from the screen.
Experiment 1 was run by using four levels of contrast for the primes, whereas experiments 2–5 were run by using three levels of contrast for the primes. For experiments 1–5, there were four targets per category that were presented with either one (experiment 1) or four categorically congruent primes (experiments 2–5), and one or four categorically incongruent primes. These stimuli assignments were repeated 10 times in experiment 1, for a total of 160 trials per contrast level (640 total trials), and three times in experiments 2–5, for a total of 192 trials per contrast level (576 total trials).
For experiments 1–5, the contrast of the prime pictures was adjusted for each participant so that prime invisibility was successfully achieved. Percentage correct performance of participants on the prime detection or discrimination task was used to select, offline, the particular contrast level for the main analysis of the experiment proper. For all experiments, the highest level of contrast for which the performance of participants was not above chance, as defined by a z test for one proportion (exp. 1–5), and for which discrimination was not different between the two categories, as determined by a z test for two proportions (experiments 2–5), was selected for the main analysis. The data for participants whose performance in the detection or discrimination tasks did not meet specified criteria for inclusion for any of the contrast-levels of the prime stimuli were discarded.
For experiments 1–5, after completing the experiment, participants performed a prime detection (experiment 1) or discrimination task (experiments 2–5) by using the same contrast levels that were used during the experiment (Table 1). In the detection task after experiment 1, the two primes (one animal, one tool) were repeated 12 times; 24 random patterns without prime pictures were used as noise alone trials. This trial set was repeated for each contrast level. Participants were asked to indicate if they detected something other than the noise patterns. In experiments 2–5, each prime was repeated 10 times, for a total of 80 trials per contrast level. Participants were asked to categorize, to the best of their ability, the primes as animals or tools.
BM.
Experiment 6 used the same stimuli as experiment 2. We added 70% additive noise to the prime stimuli by using Photoshop to facilitate masking. A black and white backward mask was generated, by using the same algorithm that was used to generate the high-contrast random noise patterns for CFS. Experiment 6 followed the same design as experiments 2. The discrimination task was the same as that used in experiments 2–5, as well as the criteria for prime invisibility.
Analyses.
For all six experiments, a 2 (Target Category, animals vs. tools) X 2 (Prime Category, animals vs. tools) ANOVA was performed. The F values for the interaction between these two factors are reported. Planned comparisons were performed over the two-way interaction between target category and prime category, for each target category.
Supplementary Material
Acknowledgments.
We thank Petra Pajtas and Lukas Strnad for their help collecting data; Fang Fang and Bruno Breitmeyer for their advice on the experimental design; and M. Clara Barata and Petra Pajtas for their comments on earlier versions of this manuscript. A.C. was supported by National Institute on Deafness and other Communication Disorders Grant R01 DC006842 and by the Fondazione Cassa di Risparmio di Trento e Rovereto. J.A. was supported by Fundação para a Ciência e a Tecnologia, Portugal Grant SFRH/BD/28994/2006. B.Z.M. was supported by a National Science Foundation Graduate Research Fellowship. K.N. was supported by National Institutes of Health Grant HSD-DHB-MOD 0433136/0433226.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805867105/DCSupplemental.
References
- 1.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 2.Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 3.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cerebral Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- 5.Ungerleider LG, Mishkin M. In: Analysis of Visual Behavior. Ingle DJ, Goodale MA, Mansfield RJW, editors. Cambridge, Mass: The MIT Press; 1982. pp. 549–586. [Google Scholar]
- 6.Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- 7.Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cerebral Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Jeannerod M, Decety J, Michel F. Impairment of grasping movements following a bilateral posterior parietal lesion. Neuropsychologia. 1994;32:369–380. doi: 10.1016/0028-3932(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 10.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: The cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- 11.Culham J, et al. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- 12.Carey DP, Harvey M, Milner AD. Visuomotor sensitivity for shape and orientation in a patient with visual form agnosia. Neuropsychologia. 1996;34:329–337. doi: 10.1016/0028-3932(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 13.Ochipa C, Rothi LJ, Heilman KM. Ideational apraxia: A deficit in tool selection and use. Ann Neurol. 1989;25:190–193. doi: 10.1002/ana.410250214. [DOI] [PubMed] [Google Scholar]
- 14.Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cognitive Neuropsych. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- 15.Mahon BZ, et al. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nat Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- 19.Kreiman G, Fried I, Koch C. Single-neuron correlates of subjective vision in the human medial temporal lobe. Proc Natl Acad Sci USA. 2002;99:8378–8383. doi: 10.1073/pnas.072194099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- 21.Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene S, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 24.Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: A comparison, review, and update. Percept Psychophys. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- 25.Finkbeiner M, Caramazza A. Modulating the masked congruence priming effect with the hands and the mouth. J Exp Psychol Hum Percept Perform. 2008;34:894–918. doi: 10.1037/0096-1523.34.4.894. [DOI] [PubMed] [Google Scholar]
- 26.Zimba LD, Blake R. Binocular rivalry and semantic processing: Out of sight, out of mind. J Exp Psychol Hum Percept Perform. 1983;9:807–815. doi: 10.1037//0096-1523.9.5.807. [DOI] [PubMed] [Google Scholar]
- 27.Cave CB, Blake R, McNamara TP. Binocular rivalry disrupts visual priming. Psychol Sci. 1998;9:299–302. [Google Scholar]
- 28.Blake R, Logothetis NK. Visual competition. Nat Rev Neuroscis. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 29.Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehaene S, Changeux J-P, Naccache L, Sackur Jrm, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Forster KI, Forster JC. DMDX: A Windows display program with millisecond accuracy. Behav Res Meth Ins C. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.