Abstract
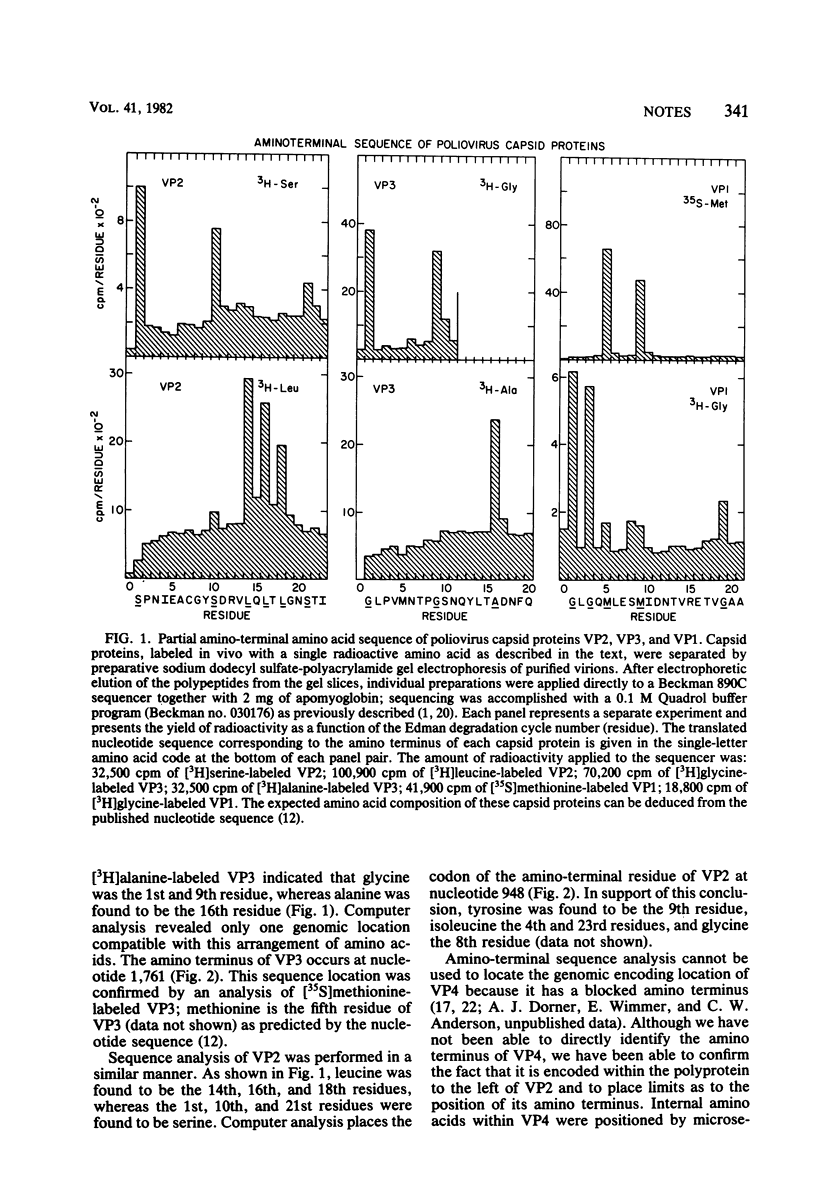
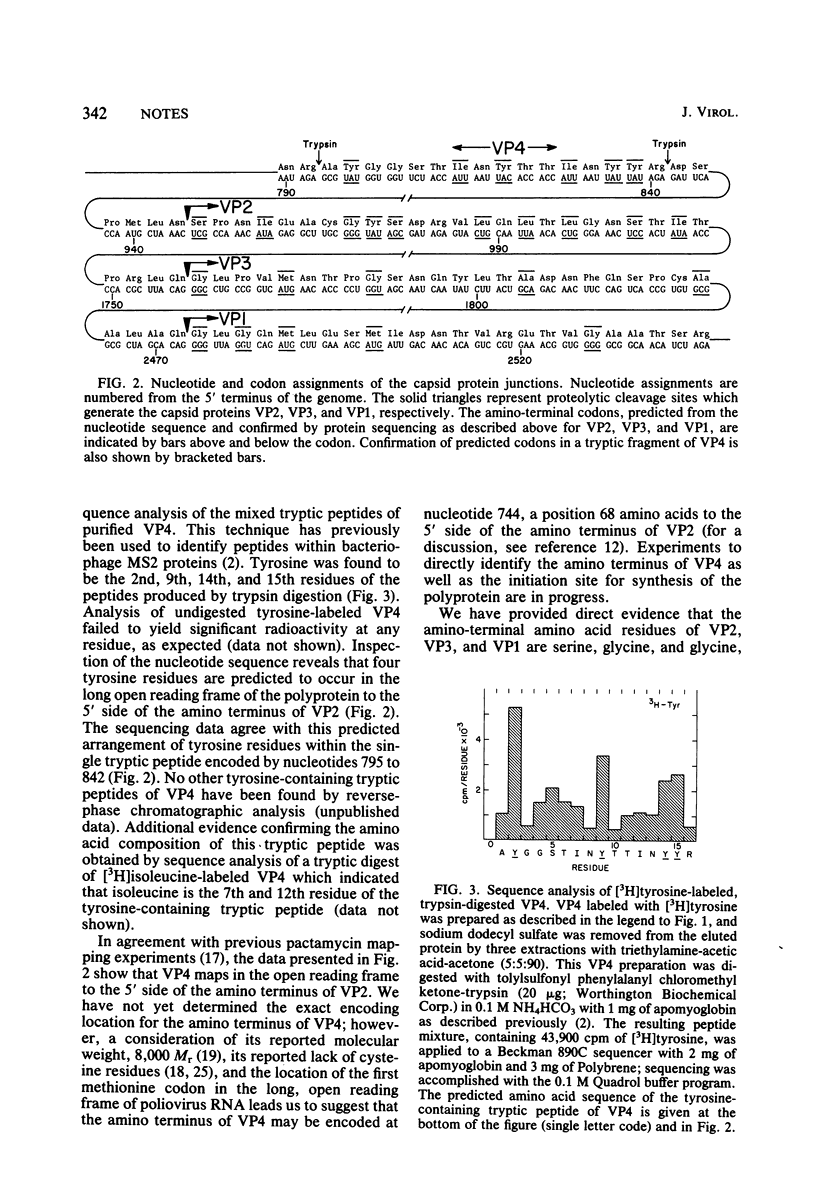
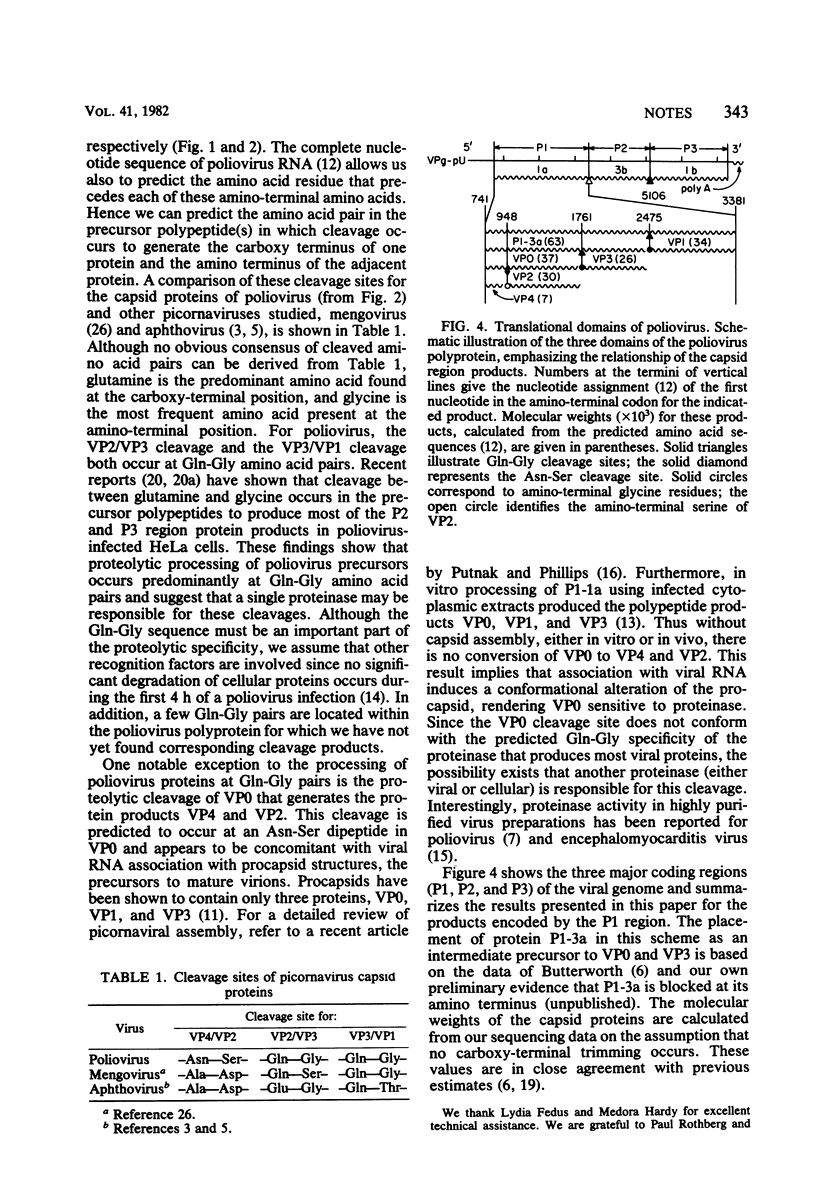
Partial amino-terminal sequence analysis was performed on radiolabeled polio-virus capsid proteins VP1, VP2, and VP3. A computer-assisted comparison of the amino acid sequences obtained with that predicted by the nucleotide sequence of the poliovirus genome allows assignment of the amino terminus of each capsid protein to a unique position within the virus polyprotein. Sequence analysis of trypsin-digested VP4, which has a blocked amino terminus, demonstrates that VP4 is encoded at or very near to the amino terminus of the polyprotein. The gene order of the capsid proteins is VP4-VP2-VP3-VP1. Cleavage of VP0 to VP4 and VP2 is shown to occur between asparagine and serine, whereas the cleavages that separate VP2/VP3 and VP3/VP1 occur between glutamine and glycine residues. This finding supports the hypothesis that the cleavage of VP0, which occurs during virion morphogenesis, is distinct from the cleavages that separate functional regions of the polyprotein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B. Amino-terminal sequence of adenovirus type 2 proteins: hexon, fiber, component IX, and early protein 1B-15K. Virology. 1980 Jul 15;104(1):27–41. doi: 10.1016/0042-6822(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Gesteland R. F., Reid B. R., Anderson C. W. Normal tRNAs promote ribosomal frameshifting. Cell. 1979 Dec;18(4):1119–1131. doi: 10.1016/0092-8674(79)90225-3. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L., Swaney J. B., Vande Woude G. F. Isolation of the structural polypeptides of foot-and-mouth disease virus and analysis of their C-terminal sequences. Virology. 1973 Apr;52(2):520–528. doi: 10.1016/0042-6822(73)90347-4. [DOI] [PubMed] [Google Scholar]
- Bhown A. S., Mole J. E., Hunter F., Bennett J. C. High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem. 1980 Mar 15;103(1):184–190. doi: 10.1016/0003-2697(80)90254-7. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Highfield P. E., Cross G. A., Rowlands D. J., Lowe P. A., Brown F., Harris T. J. Molecular cloning of foot and mouth disease virus genome and nucleotide sequences in the structural protein genes. Nature. 1981 Apr 30;290(5809):800–802. doi: 10.1038/290800a0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Doyle M., Perrault J., Kingsbury D. T., Etchison J. Proteinase activity in purified animal viruses. Biochem Biophys Res Commun. 1972 Jan 31;46(2):634–639. doi: 10.1016/s0006-291x(72)80187-6. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Chow N. L., Lively M. O., Powers J. C. Proteolytic events in replication of animal viruses. Ann N Y Acad Sci. 1980;343:304–318. doi: 10.1111/j.1749-6632.1980.tb47260.x. [DOI] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of poliovirus-specific polypeptide aggregates. J Virol. 1973 Sep;12(3):556–563. doi: 10.1128/jvi.12.3.556-563.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Identification of a viral protein involved in post-translational maturation of the encephalomyocarditis virus capsid precursor. J Virol. 1975 Apr;15(4):918–928. doi: 10.1128/jvi.15.4.918-928.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Determination of the gene sequence of poliovirus with pactamycin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2852–2856. doi: 10.1073/pnas.68.11.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen R., Boeyé A., Strosberg A. D. Amino terminal sequence ambiguity in three capsid polypeptides of poliovirus. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1596–1601. doi: 10.1016/0006-291x(78)91185-3. [DOI] [PubMed] [Google Scholar]
- Wouters M., Vandekerckhove J. Amino acid composition of the poliovirus capsid polypeptides isolated as fluorescamine conjugates. J Gen Virol. 1976 Dec;33(3):529–533. doi: 10.1099/0022-1317-33-3-529. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. IV. Amino- and carboxyl-terminal analyses of the major capsid polypeptides. Virology. 1976 May;71(1):111–121. doi: 10.1016/0042-6822(76)90098-2. [DOI] [PubMed] [Google Scholar]



