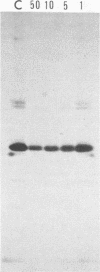
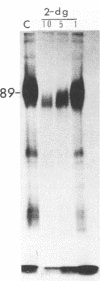
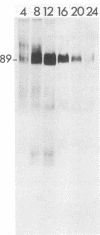
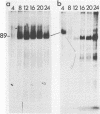
Abstract
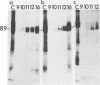
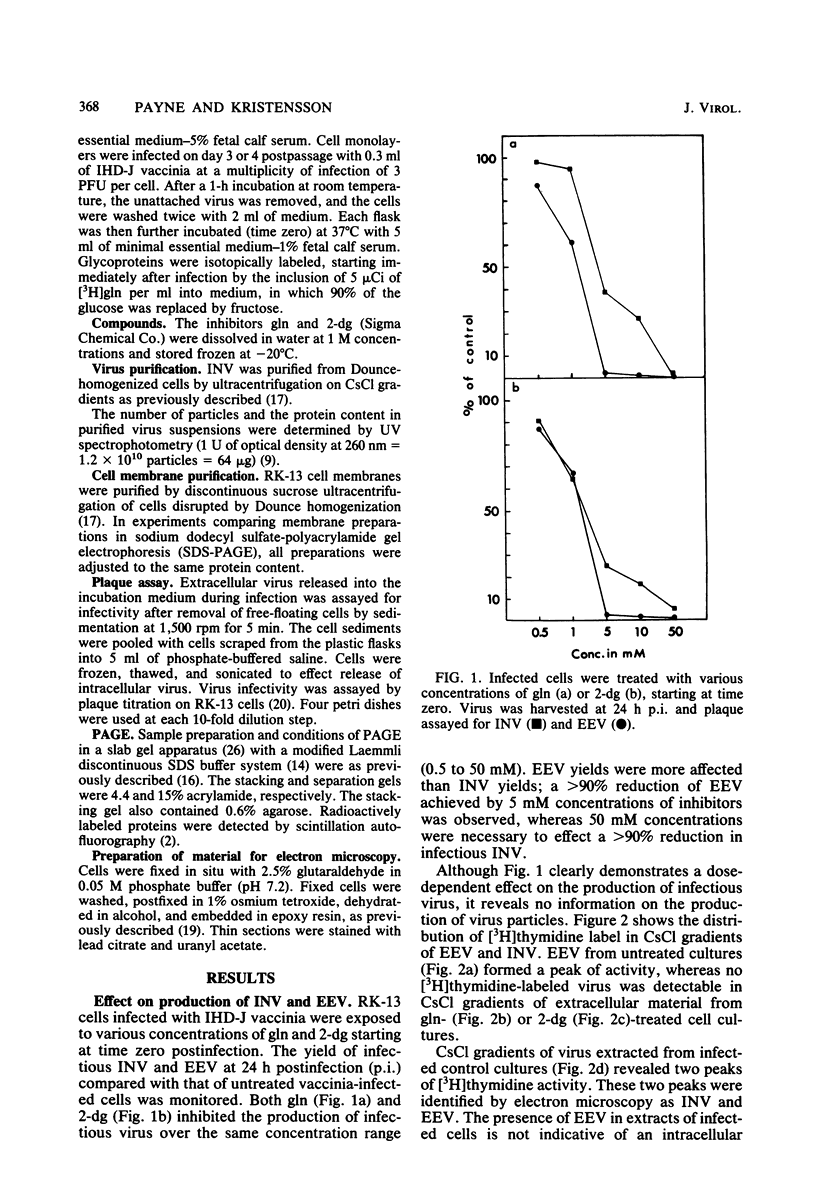
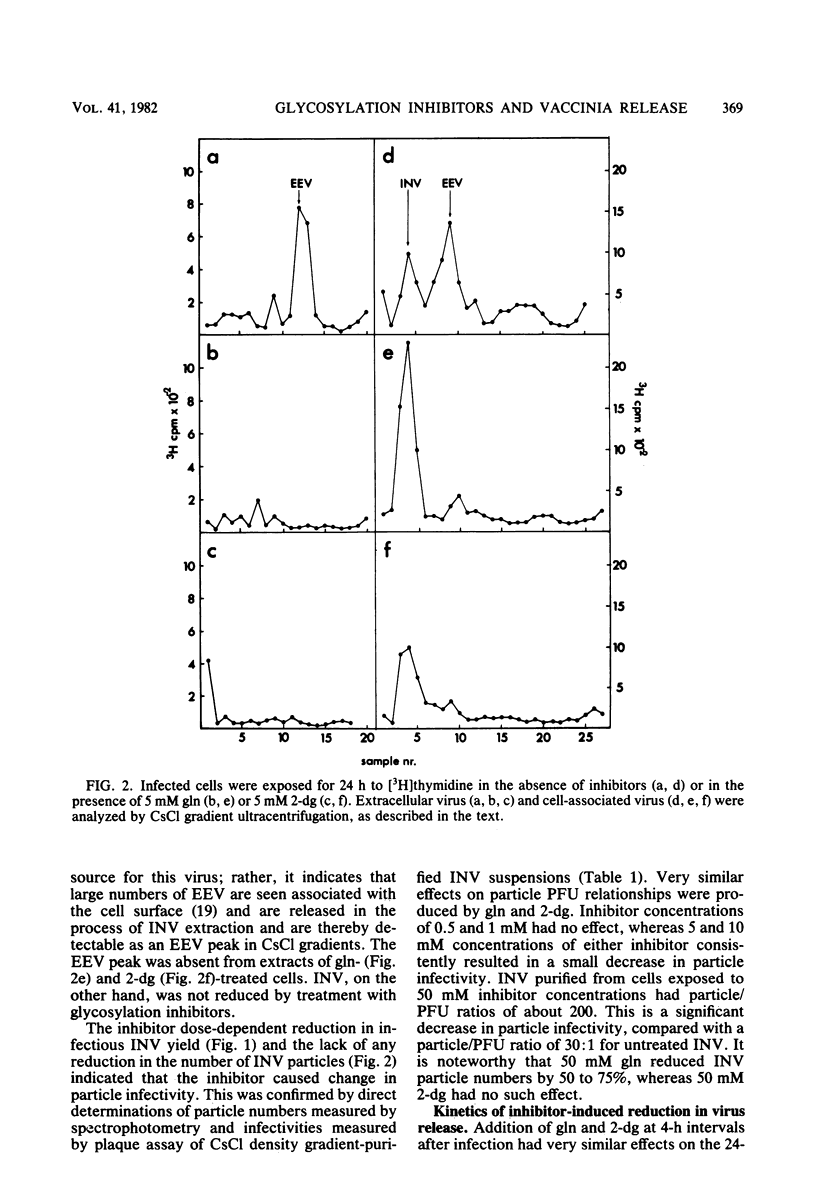
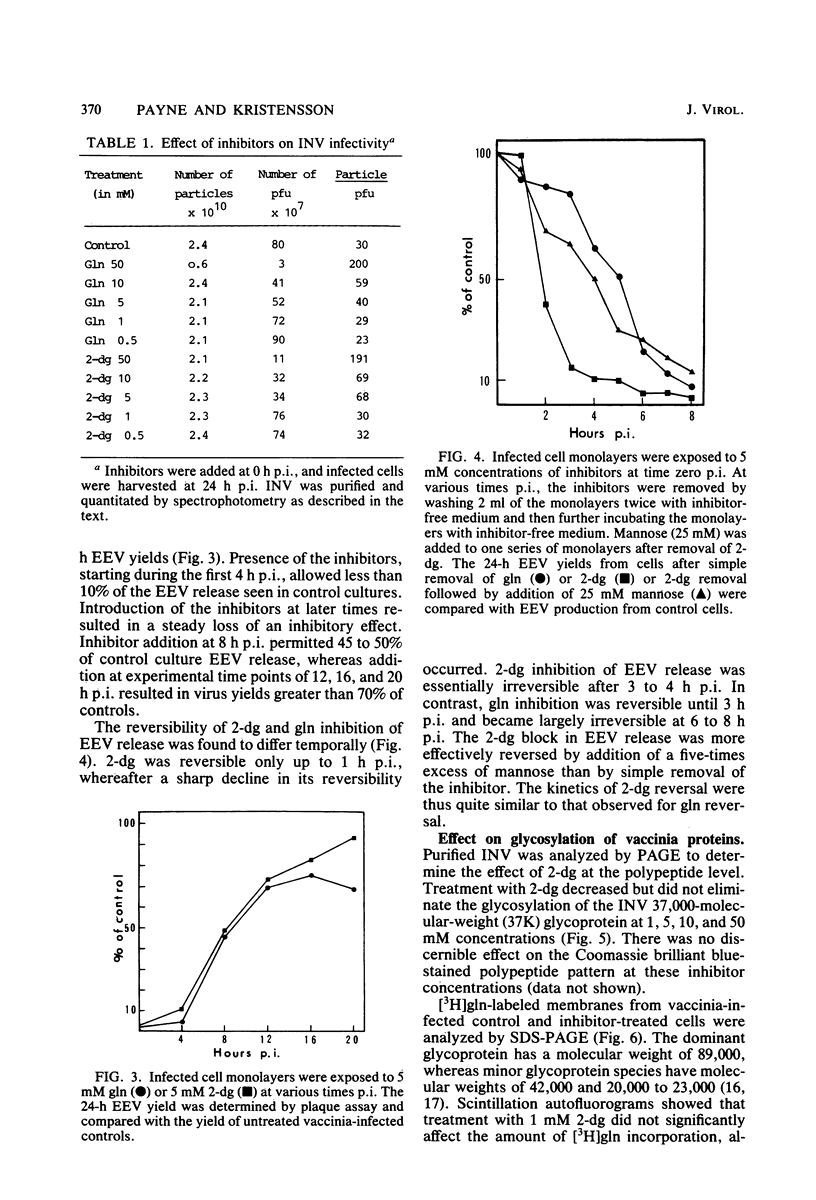
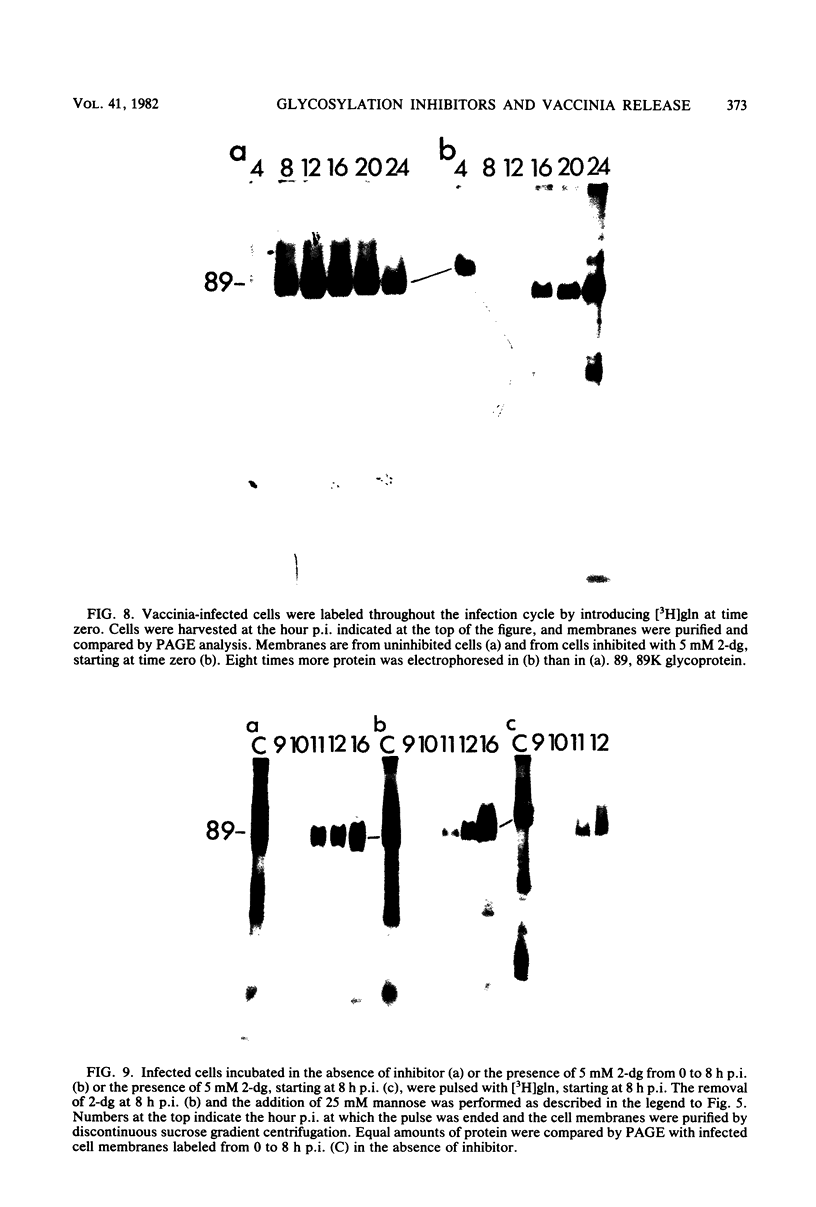
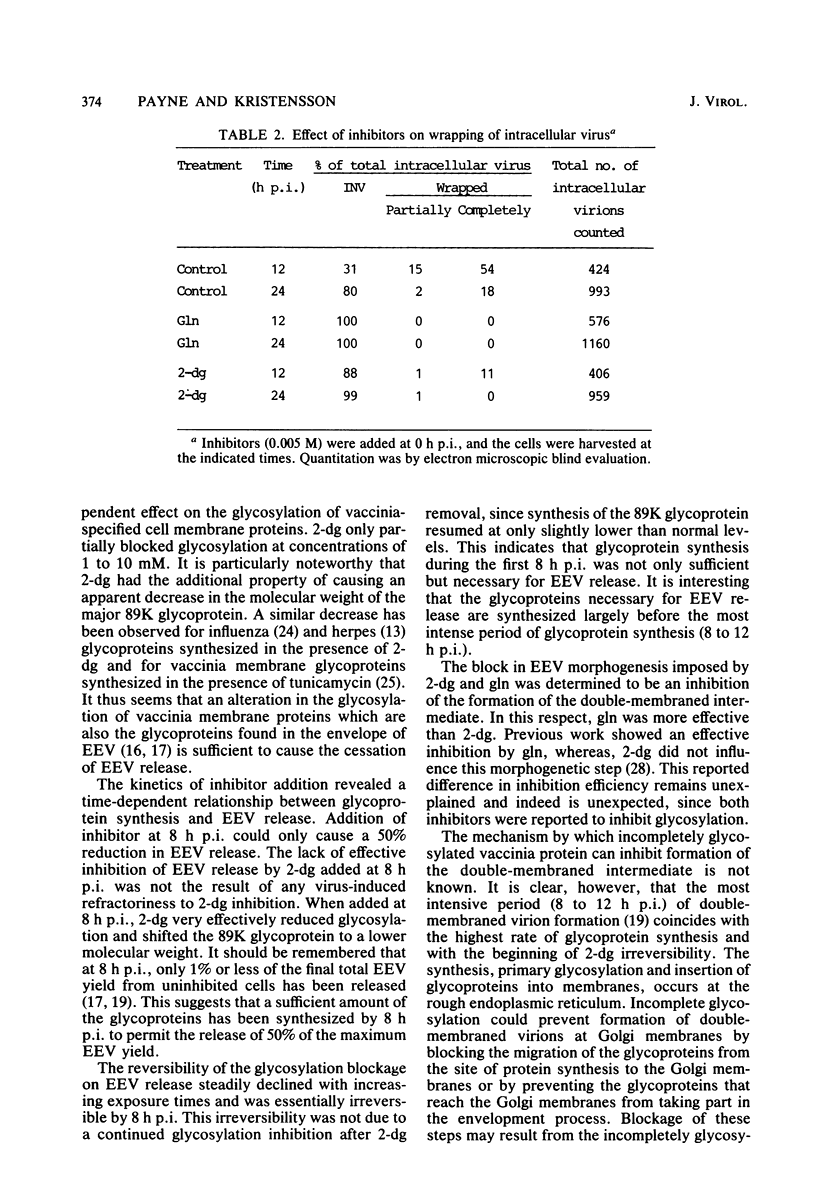
Addition of 1 to 10 mM 2-deoxy-D-glucose (2-dg) or glucosamine (gln) to the growth medium of vaccinia virus-infected cells inhibited the release of extracellular enveloped vaccinia virus (EEV) without affecting the production of intracellular naked vaccinia virus (INV) particles. In contrast, INV infectivity (particles per PFU) was decreased sevenfold by 50 mM 2-dg. Treatment with 2-dg reduced but did not eliminate glycosylation of the INV 37,000-molecular-weight glycoprotein. The kinetics of sensitivity to inhibitor addition experiments and inhibitor reversal experiments indicated that EEV release was dependent on glycosylation before 8 h postinfection. This was supported by polyacrylamide gel electrophoretic analysis of the synthesis kinetics for cell membrane-associated vaccinia glycoproteins in 2-dg-inhibited infected cells. The dependence of vaccinia protein glycosylation before 8 h postinfection for efficient EEV release was observed in spite of the fact that the period of greatest glycoprotein synthesis was 8 to 12 h postinfection. The presence of 2-dg resulted in an incompletely glycosylated 89,000-molecular-weight glycoprotein, as indicated by a reduction in the apparent glycoprotein molecular weight. The morphological event affected by the inhibitors was the acquisition by INV of a double-membrane structure from the Golgi apparatus. This morphological intermediate is necessary for release of EEV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Hapel A. J., Boulter E. A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971 Oct;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. doi: 10.1177/003591576906200349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Moss B. Glycoprotein synthesis in cells infected with vaccinia virus. II. A glycoprotein component of the virion. Virology. 1971 Nov;46(2):233–246. doi: 10.1016/0042-6822(71)90026-2. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Glycopeptides of vaccinia virus. I. Preliminary characterization and hexosamine content. Virology. 1970 Sep;42(1):87–99. doi: 10.1016/0042-6822(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Oie M. Adsorption and penetration of the trypsinized vaccinia virion. Virology. 1980 Feb;101(1):50–60. doi: 10.1016/0042-6822(80)90482-1. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Rott R., Schwarz R. T. Carbohydrate-induced conformational changes of Semliki forest virus glycoproteins determine antigenicity. Virology. 1980 Apr 30;102(2):286–299. doi: 10.1016/0042-6822(80)90096-3. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kato N., Eggers H. J., Rolly H. Inhibition of release of vaccinia virus by N1-isonicotinoly-N2-3-methyl-4-chlorobenzoylhydrazine. J Exp Med. 1969 Apr 1;129(4):795–808. doi: 10.1084/jem.129.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J Virol. 1979 Jul;31(1):147–155. doi: 10.1128/jvi.31.1.147-155.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash V. J., Norrby E., Payne L. Single radial immunodiffusion test for detecting antibodies against surface antigens of intracellular and extracellular vaccinia virus. J Gen Virol. 1977 Jun;35(3):465–472. doi: 10.1099/0022-1317-35-3-463. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Inhibition of glycosylation of the influenza virus hemagglutinin. J Virol. 1974 Nov;14(5):1023–1034. doi: 10.1128/jvi.14.5.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Squires E. J. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J Gen Virol. 1971 Oct;13(1):19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Stern W., Dales S. Biogenesis of vaccinia. Effects of inhibitors of glycosylation on virus-mediated activities. Virology. 1977 May 1;78(1):315–322. doi: 10.1016/0042-6822(77)90102-7. [DOI] [PubMed] [Google Scholar]