Abstract
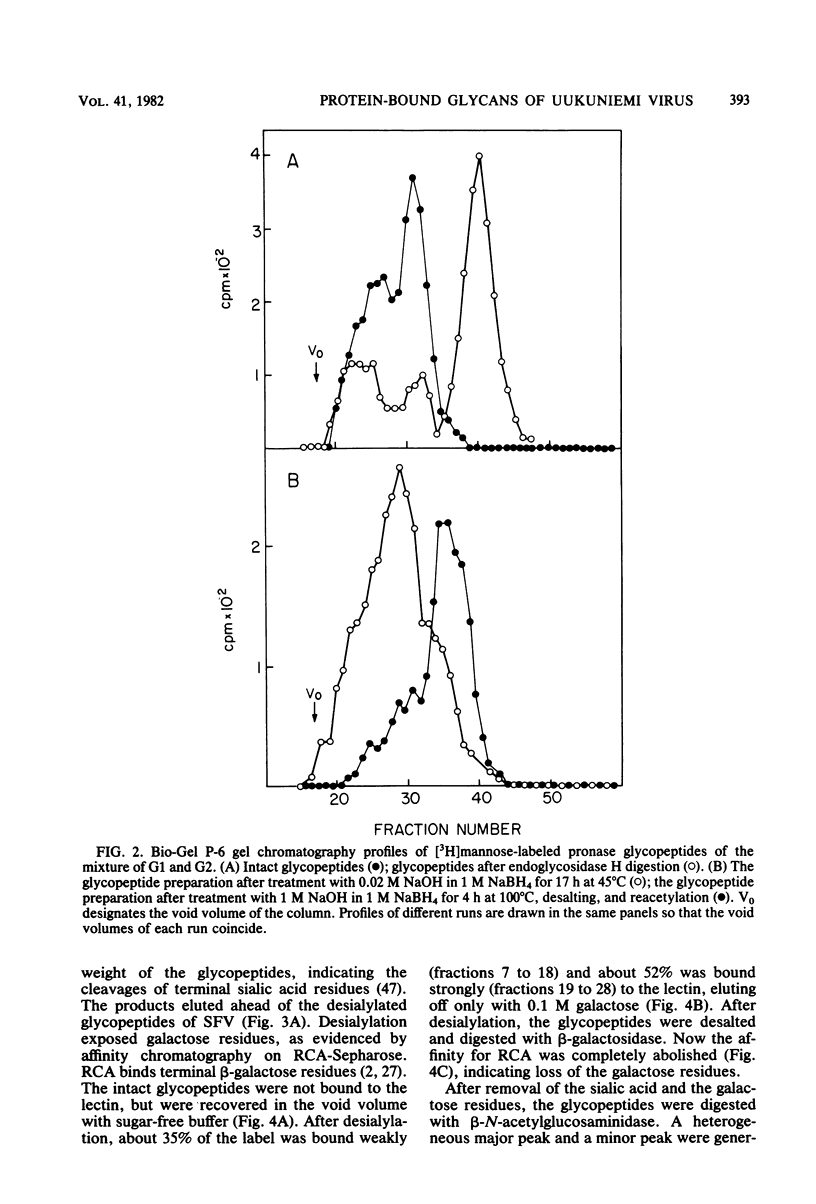
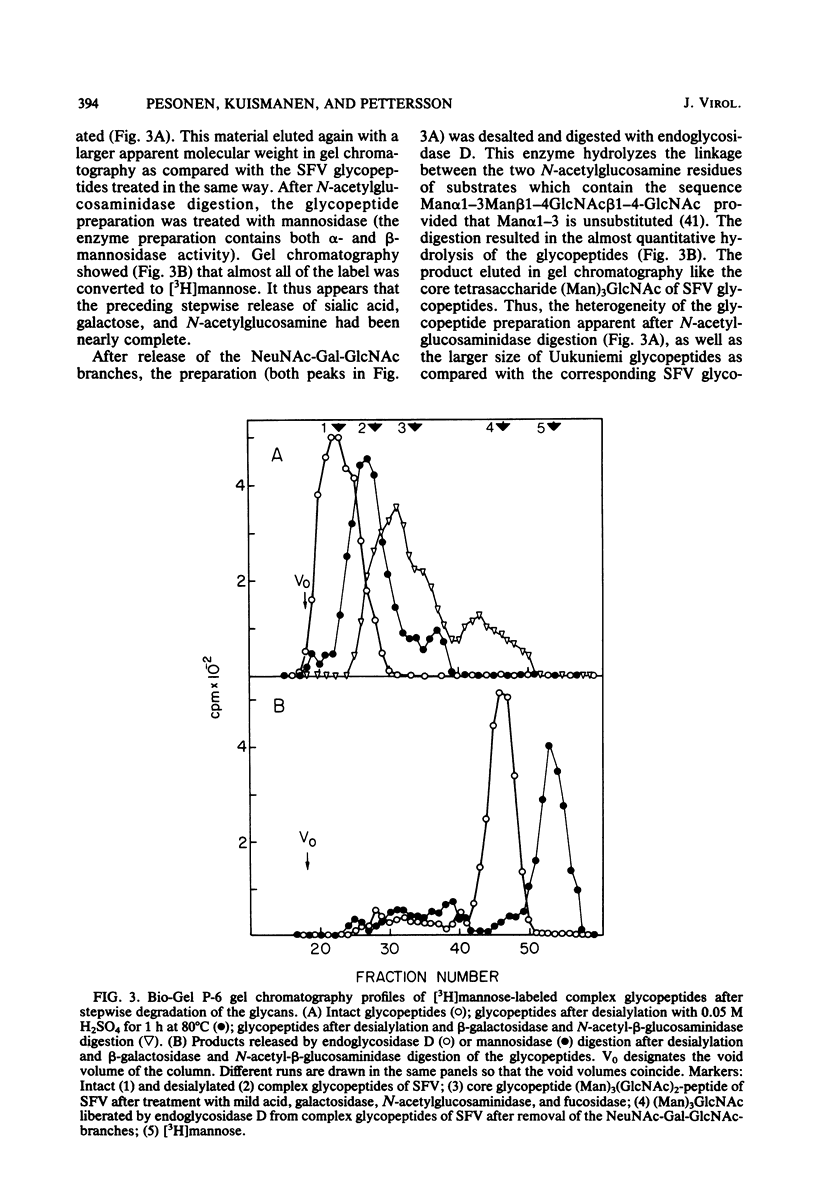
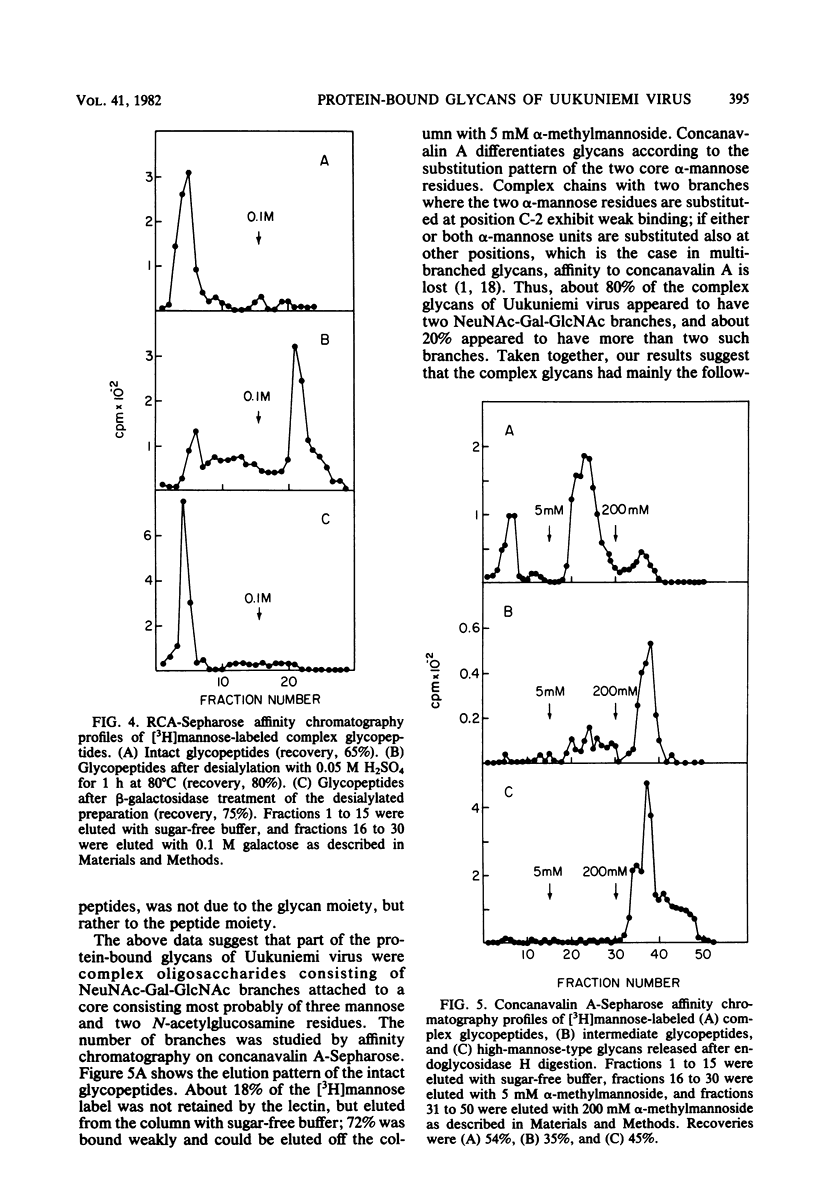
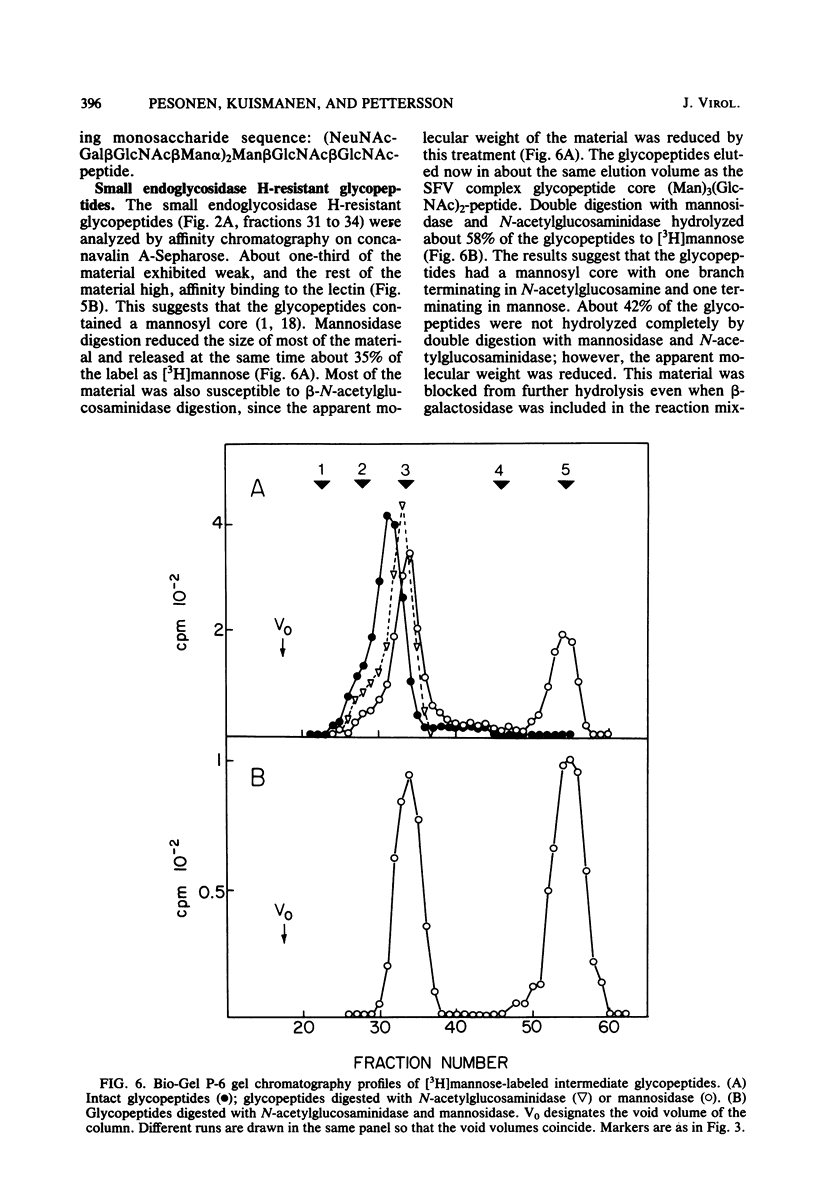
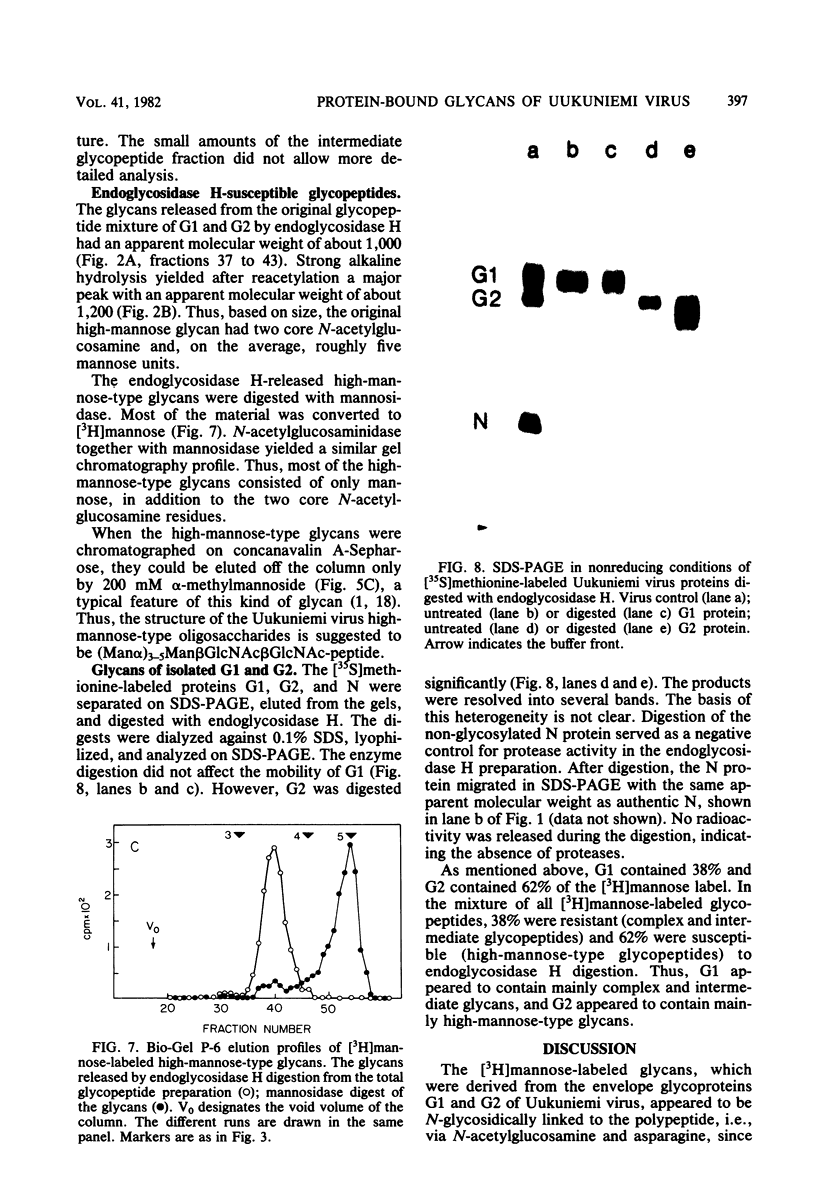
Uukuniemi virus, a member of the Bunyaviridae family, was grown in BHK-21 cells in the presence of [3H]mannose. The purified virions were disrupted with sodium dodecyl sulfate and digested with pronase. The [3H]mannose-labeled glycopeptides of the mixture of the two envelope glycoproteins G1 and G2 were characterized by degrading the glycans with specific exo-and endoglycosidases, by chemical methods, and by analyzing the products with lectin affinity and gel chromatography. The glycopeptides of Uukuniemi virus fell into three categories: complex, high-mannose type, and intermediate. The complex glycopeptides probably contained mainly two NeuNAc-Gal-GlcNAc branches attached to a core (Man)3(GlcNAc)2 peptide. The high-mannose-type glycans were estimated to contain at least five mannose units attached to two N-acetylglucosamine residues. Both glycan species appeared to be similar to the asparagine-linked oligosaccharides found in many soluble and membrane glycoproteins. The results suggested that the intermediate glycopeptides contained a mannosyl core. In about half of the molecules, one branch appeared to be terminated in mannose, and one appeared to be terminated in N-acetylglucosamine. Such glycans are a novel finding in viral membrane proteins. They may represent intermediate species in the biosynthetic pathway from high-mannose-type to complex glycans. Their accumulation could be connected with the site of maturation of the members of the Bunyaviridae family. Electron microscopic data suggest that the virions bud into smooth-surfaced cisternae in the Golgi region. The relative amounts of [3H]mannose in the complex, high-mannose-type, and intermediate glycans were 25, 62, and 13%, respectively, which corresponded to the approximate relative number of oligosaccharide chains of 2:2.8:1, respectively, in the roughly equimolar mixture of G1 and G2. Endoglycosidase H digestion of isolated [35S]methionine-labeled G1 and G2 proteins suggested that most of the complex and intermediate chains were attached to G1 and that most of the high-mannose-type chains were attached to G2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979 Oct 10;254(19):9795–9799. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Keegstra K. Carbohydrate structure of Sindbis virus glycoprotein E2 from virus grown in hamster and chicken cells. J Virol. 1979 Feb;29(2):546–554. doi: 10.1128/jvi.29.2.546-554.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Cash P., Hendershot L., Bishop D. H. The effect of glycosylation inhibitors on the maturation and intracellular polypeptide synthesis induced by snowshoe hare bunyavirus. Virology. 1980 May;103(1):235–240. doi: 10.1016/0042-6822(80)90142-7. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Bovine colostrum UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. Separation from UDP-N-acetylglucosamine:alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase II, partial purification, and substrate specificity. J Biol Chem. 1980 May 25;255(10):4885–4893. [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980 May 25;255(10):4894–4902. [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- Li E., Kornfeld S. Structural studies of the major high mannose oligosaccharide units from Chinese hamster ovary cell glycoproteins. J Biol Chem. 1979 Mar 10;254(5):1600–1605. [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Lyons M. J., Heyduk J. Aspects of the developmental morphology of California encephalitis virus in cultured vertebrae and arthropod cells and in mouse brain. Virology. 1973 Jul;54(1):37–52. doi: 10.1016/0042-6822(73)90112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila K., Renkonen O. Separation of A- and B-type glycopeptides of Semliki Forest virus by concanavalin A affinity chromatography and preliminary characterization of the B-type glycopeptides. Virology. 1978 Dec;91(2):508–510. doi: 10.1016/0042-6822(78)90401-4. [DOI] [PubMed] [Google Scholar]
- Munro J. R., Narasimhan S., Wetmore S., Riordan J. R., Schachter H. Intracellular localization of GDP-L-fucose:glycoprotein and CMP-sialic acid: apolipoprotein glycosyltransferases in rat and pork livers. Arch Biochem Biophys. 1975 Jul;169(1):269–277. doi: 10.1016/0003-9861(75)90341-0. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology. 1973;1(4):297–316. doi: 10.1159/000148858. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Pesonen M., Haahtela K., Renkonen O. Core tetrasaccharide liberated by endo-beta-D-N-acetylglucosaminidase D from lactosamine-type oligosaccharides of Semliki Forest virus membrane proteins. Biochim Biophys Acta. 1979 Nov 15;588(1):102–112. doi: 10.1016/0304-4165(79)90375-1. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Renkonen O. Serum glycoprotein-type sequence of monosaccharides in membrane glycoproteins of Semliki Forest virus. Biochim Biophys Acta. 1976 Dec 2;455(2):510–525. doi: 10.1016/0005-2736(76)90321-7. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Saraste J., Hashimoto K., Käriäinen L. Reversible defect in the glycosylation of the membrane proteins of Semliki Forest virus ts-1 mutant. Virology. 1981 Feb;109(1):165–173. doi: 10.1016/0042-6822(81)90481-5. [DOI] [PubMed] [Google Scholar]
- Pesonen M. Sequence analysis of lactosamine type glycans of individual membrane proteins of Semliki Forest virus. J Gen Virol. 1979 Nov;45(2):479–487. doi: 10.1099/0022-1317-45-2-479. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., von Bonsdorff C. H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975 Feb;15(2):386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973 Dec;56(2):608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Rasilo M. L., Renkonen O. The molecular size of glycans liberated by hydrazinolysis from Semliki Forest virus proteins. Biochim Biophys Acta. 1979 Jan 18;582(2):307–321. doi: 10.1016/0304-4165(79)90393-3. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Saikku P., Von Bonsdorff C. H., Oker-Blom N. The structure of Uukuniemi virus. Acta Virol. 1970 Mar;14(2):103–107. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorndam A. V., Trent D. W. Oligosaccharides of the California encephalitis viruses. Virology. 1979 May;95(1):1–7. doi: 10.1016/0042-6822(79)90396-9. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- von Bonsdorff C. H., Pettersson R. Surface structure of Uukuniemi virus. J Virol. 1975 Nov;16(5):1296–1307. doi: 10.1128/jvi.16.5.1296-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Saikku P., Oker-Blom N. The inner structure of Uukuniemi and two Bunyamwera supergroup arboviruses. Virology. 1969 Oct;39(2):342–344. doi: 10.1016/0042-6822(69)90057-9. [DOI] [PubMed] [Google Scholar]