Abstract
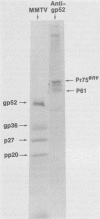
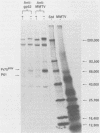
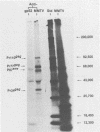
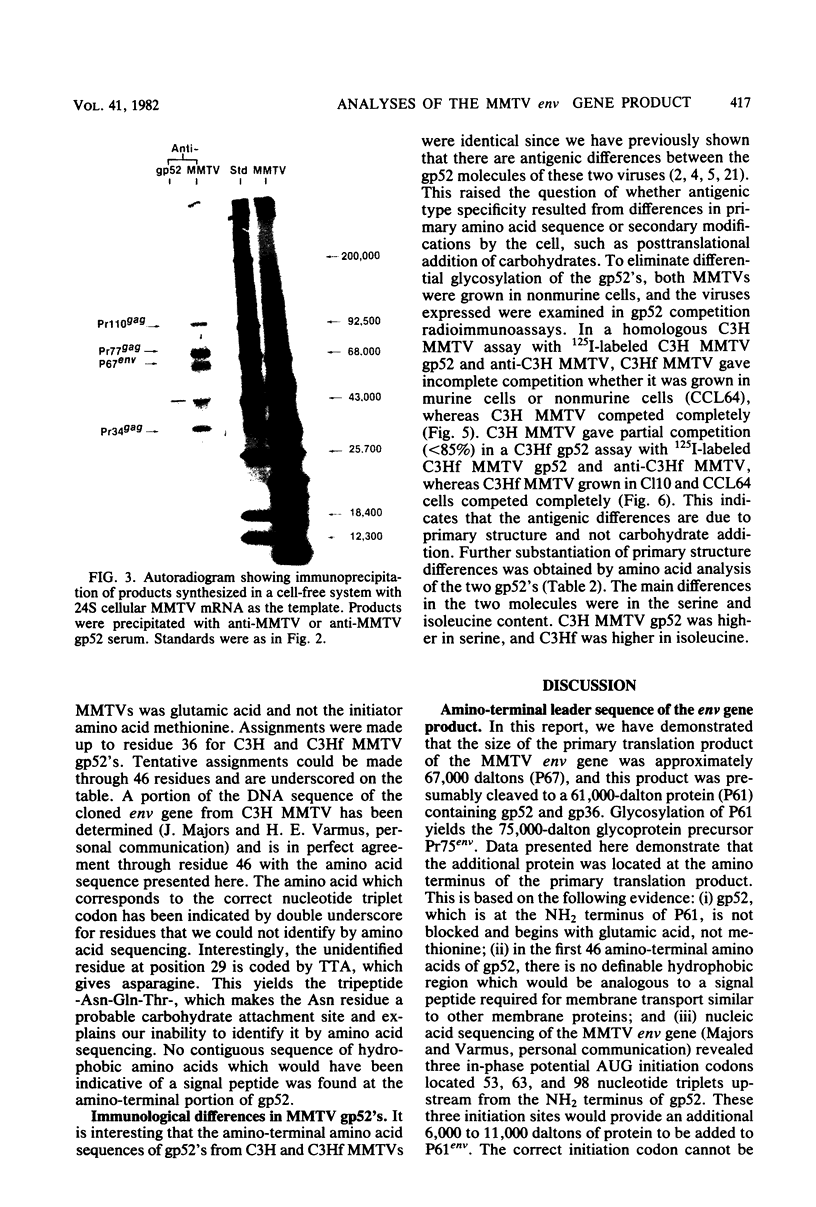
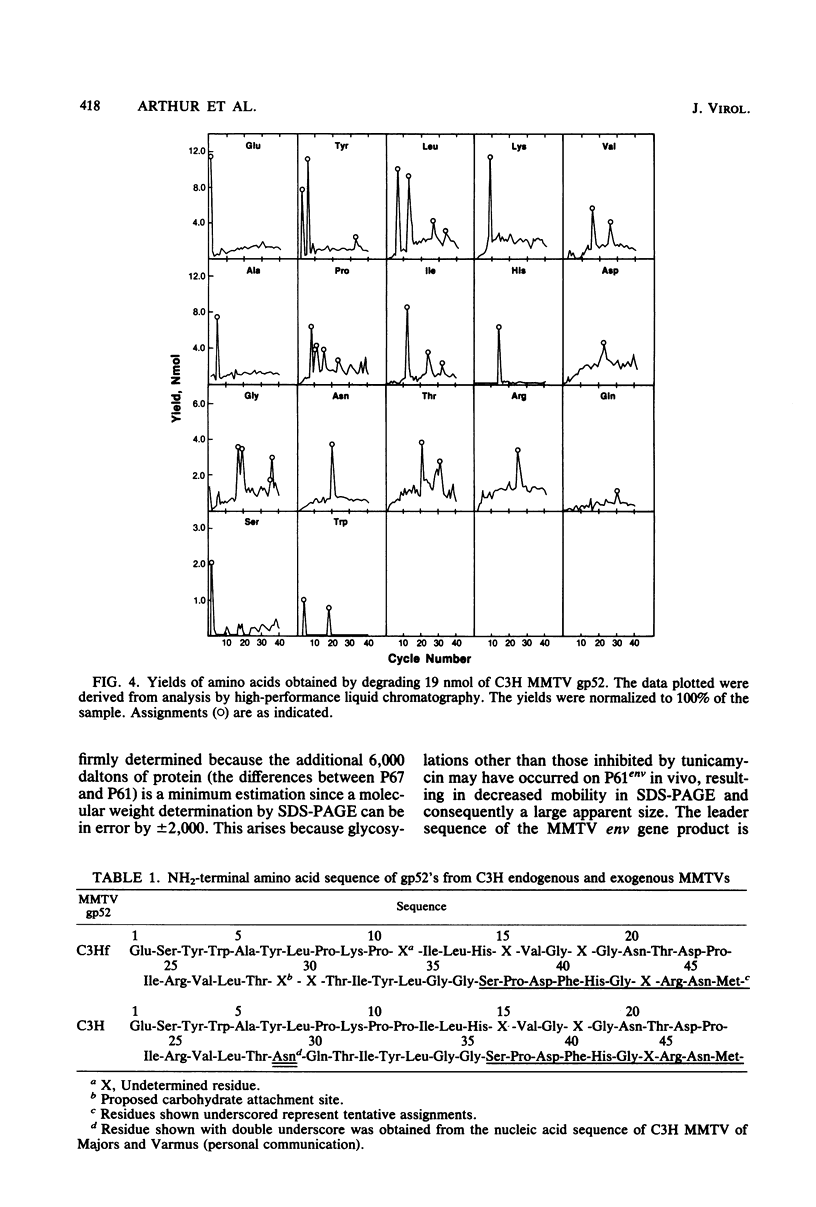
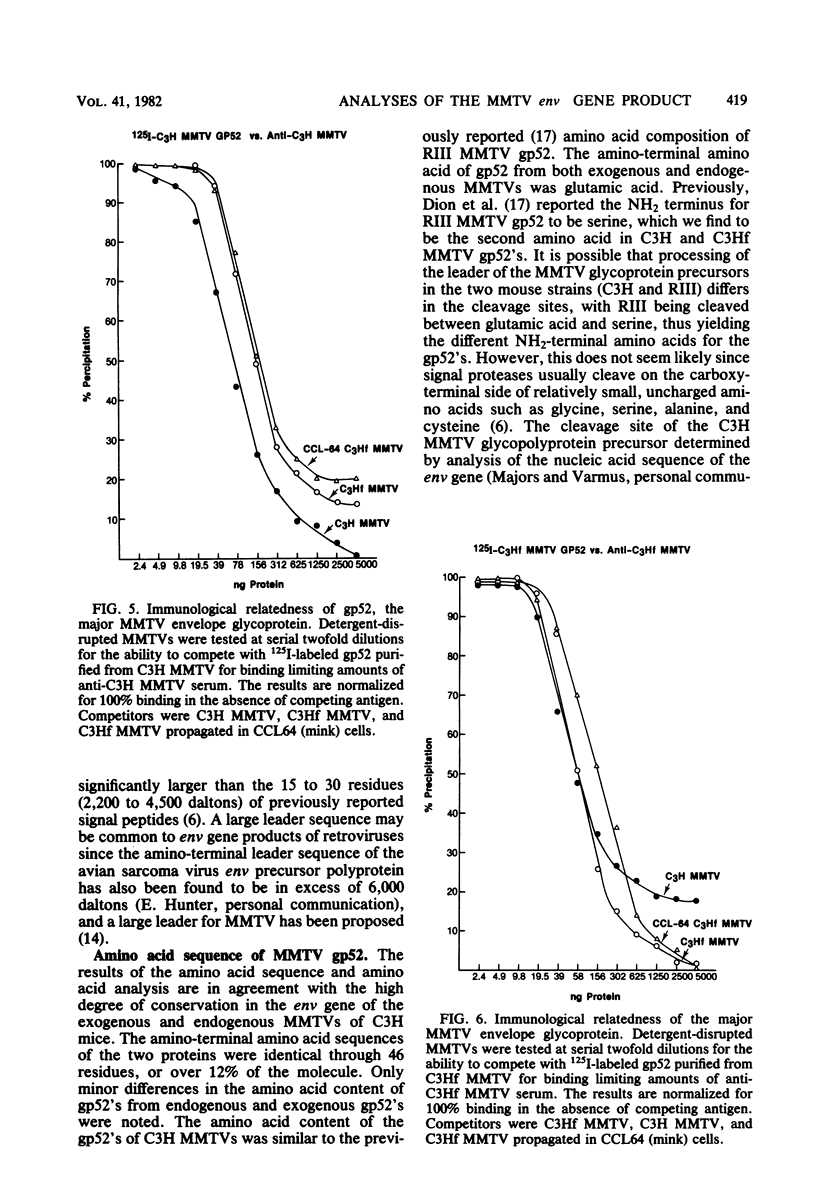
The envelope proteins of mouse mammary tumor virus (MMTV) are synthesized from a subgenomic 24S mRNA as a 75,000-dalton glycosylated precursor polyprotein which is eventually processed to the mature glycoproteins gp52 and gp36. In vivo synthesis of this env precursor in the presence of the core glycosylation inhibitor tunicamycin yielded a precursor of approximately 61,000 daltons (P61env). However, a 67,000-dalton protein (P67env) was obtained from cell-free translation with the MMTV 24S mRNA as the template. To determine whether the portion of the protein cleaved from P67env to give P61env was removed from the NH2-terminal end of P67env and as such would represent a leader sequence, the NH2-terminal amino acid sequence of the terminal peptide gp52 was determined. Glutamic acid, and not methionine, was found to be the amino-terminal residue of gp52, indicating that the cleaved portion was derived from the NH2-terminal end of P67env. The NH2-terminal amino acid sequences of gp52's from endogenous and exogenous C3H MMTVs were determined though 46 residues and found to be identical. However, amino acid composition and type-specific gp52 radioimmunoassays from MMTVs grown in heterologous cells indicated primary structure differences between gp52's of the two viruses. The nucleic acid sequence of cloned MMTV DNA fragments (J. Majors and H. E. Varmus, personal communication) in conjunction with the NH2-terminal sequence of gp52 allowed localization of the env gene in the MMTV genome. Nucleotides coding for the NH2 terminus of gp52 begin approximately 0.8 kilobase to the 3' side of the single EcoRI cleavage site. Localization of the env gene at that point agrees with the proposed gene order -gag-pol-env- and also allows sufficient coding potential for the glycoprotein precursor without extending into the long terminal repeat.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altrock B. W., Arthur L. O., Massey R. J., Schochetman G. Common surface receptors on both mouse and rat cells distinguish different classes of mouse mammary tumor viruses. Virology. 1981 Mar;109(2):257–266. doi: 10.1016/0042-6822(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Altrock B. W., Schochetman G. Type-specific determinants on proteins of an endogenous C3H mouse mammary tumor virus (MMTV) distinguish this virus from highly oncogenic exogenous MMTVs. Virology. 1981 Apr 30;110(2):270–280. doi: 10.1016/0042-6822(81)90059-3. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Fine D. L. Immunological characterization of mouse mammary tumor virus p10 and its presence in mammary tumors and sera of tumor-bearing mice. J Virol. 1979 Apr;30(1):148–156. doi: 10.1128/jvi.30.1.148-156.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur L. O., Fine D. L. Naturally occurring humoral immunity to murine mammary tumor virus (MuMTV) and MuMTV GP52 in mice with low mammary tumor incidence. Int J Cancer. 1978 Dec;22(6):734–740. doi: 10.1002/ijc.2910220616. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Lovinger G. G., Schochetman G. Establishment of a C3Hf mammary tumor cell line expressing endogenous mouse mammary tumor virus: antigenic and genetic relationships of this virus with highly oncogenic mouse mammary tumor viruses. J Virol. 1979 Dec;32(3):852–859. doi: 10.1128/jvi.32.3.852-859.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H., Noyes A. N., Boyer M. L., Marr K. Hemoglobin 3 chains in apes. Primary structures and the presumptive nature of back mutation in a normally silent gene. J Biol Chem. 1973 Feb 10;248(3):992–1003. [PubMed] [Google Scholar]
- Copeland T. D., Grandgenett D. P., Oroszlan S. Amino acid sequence analysis of reverse transcriptase subunits from avian myeloblastosis virus. J Virol. 1980 Oct;36(1):115–119. doi: 10.1128/jvi.36.1.115-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Composition, arrangement and cleavage of the mouse mammary tumor virus polyprotein precursor Pr77gag and p110gag. Cell. 1979 Aug;17(4):1003–1012. doi: 10.1016/0092-8674(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Structure and processing of the mouse mammary tumor virus glycoprotein precursor pr73env. J Virol. 1980 Aug;35(2):349–361. doi: 10.1128/jvi.35.2.349-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Intracellular synthesis of mouse mammary tumor virus polypeptides: indication of a precursor glycoprotein. J Virol. 1975 Aug;16(2):250–258. doi: 10.1128/jvi.16.2.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Farwell D. C., Pomenti A. A., Williams C. J. Characterization of purified structural proteins of murine mammary tumor virus. Virology. 1979 Jul 15;96(1):319–322. doi: 10.1016/0042-6822(79)90203-4. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Smythers G. W., Marquardt H., Oroszlan S. Amino-terminal amino acid sequence and carboxyl-terminal analysis of Rauscher murine leukemia virus glycoproteins. Virology. 1978 Mar;85(1):319–322. doi: 10.1016/0042-6822(78)90437-3. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Massey R. J., Arthur L. O., Nowinski R. C., Schochetman G. Monoclonal antibodies identify individual determinants on mouse mammary tumor virus glycoprotein gp52 with group, class, or type specificity. J Virol. 1980 Jun;34(3):635–643. doi: 10.1128/jvi.34.3.635-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Gene order of mouse mammary tumor virus precusor polyproteins and their interaction leading to the formation of a virus. Virology. 1979 Dec;99(2):358–371. doi: 10.1016/0042-6822(79)90015-1. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Nusse R., van der Ploeg L., van Duijn L., Michalides R., Hilgers J. Impaired maturation of mouse mammary tumor virus precursor polypeptides in lymphoid leukemia cells, producing intracytoplasmic A particles and no extracellular B-type virions. J Virol. 1979 Oct;32(1):251–258. doi: 10.1128/jvi.32.1.251-258.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D., Henderson L. E., Stephenson J. R., Gilden R. V. Amino-terminal sequence of bovine leukemia virus major internal protein: homology with mammalian type C virus p30 structural proteins. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2996–3000. doi: 10.1073/pnas.76.6.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T., Summers M. R., Smythers G., Gilden R. V. Amino acid sequence homology of mammalian type C RNA virus major internal proteins. J Biol Chem. 1975 Aug 25;250(16):6232–6239. [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. Enzymatic conversion of proteins to glycoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):134–138. doi: 10.1073/pnas.74.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Schlom J. Independent polypeptide chain initiation sites for the synthesis of different classes of proteins for an RNA tumor virus: mouse mammary tumor virus. Virology. 1976 Sep;73(2):431–441. doi: 10.1016/0042-6822(76)90404-9. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]