Abstract
We have studied initiation and regulation of early transcription of simian virus (SV40) DNA in vitro by eucaryotic RNA polymerase II, using both a crude HeLa cell extract and a partially purified calf thymus polymerase supplemented with a HeLa cell S100 fraction. Analysis of initiation sites by primer-directed cDNA synthesis and sequencing of cDNA's has revealed that early transcription is initiated at a multiplicity of sites corresponding to the 5' termini of early viral mRNA's. The pattern of in vitro initiation closely resembles the pattern of 5' termini of early mRNA's late in the lytic cycle, with principal initiations between residues 5184 to 5194, upstream from the early Hogness-Goldberg (TATA) sequence, and at residue 5123, well downstream from this sequence. In vitro transcription is initiated to a lesser extent at sites between residues 5150 and 5155, the principal 5' termini of early mRNA's in transformed cells and early in lytic infection, located 21 to 26 nucleotides downstream from the TATA sequence. Initiation occurs at identical sites and with similar efficiencies on form I and linearized DNA templates. There are minor differences in the efficiency of initiation at specific sites by the two transcriptional systems. Studies using a DNA template cleaved just downstream from the TATA sequence and a second template cleaved through a pair of 72-base-pair tandem repeats starting 87 nucleotides upstream from the TATA sequence have revealed that neither the TATA sequence nor the repeats are essential for early transcription in vitro. However, removal of the TATA and upstream sequences shifts initiation of transcription principally to the residue 5123 site. Comparison of the relative efficiencies of transcription on intact wild-type DNA, the two cleaved DNAs, and DNA from a deletion mutant suggests that all or most of the sequences constituting an early promoter lie within the genomic region 60-70 to 140 nucleotides upstream from the principal 5' termini of the early mRNA's.
Full text
PDF

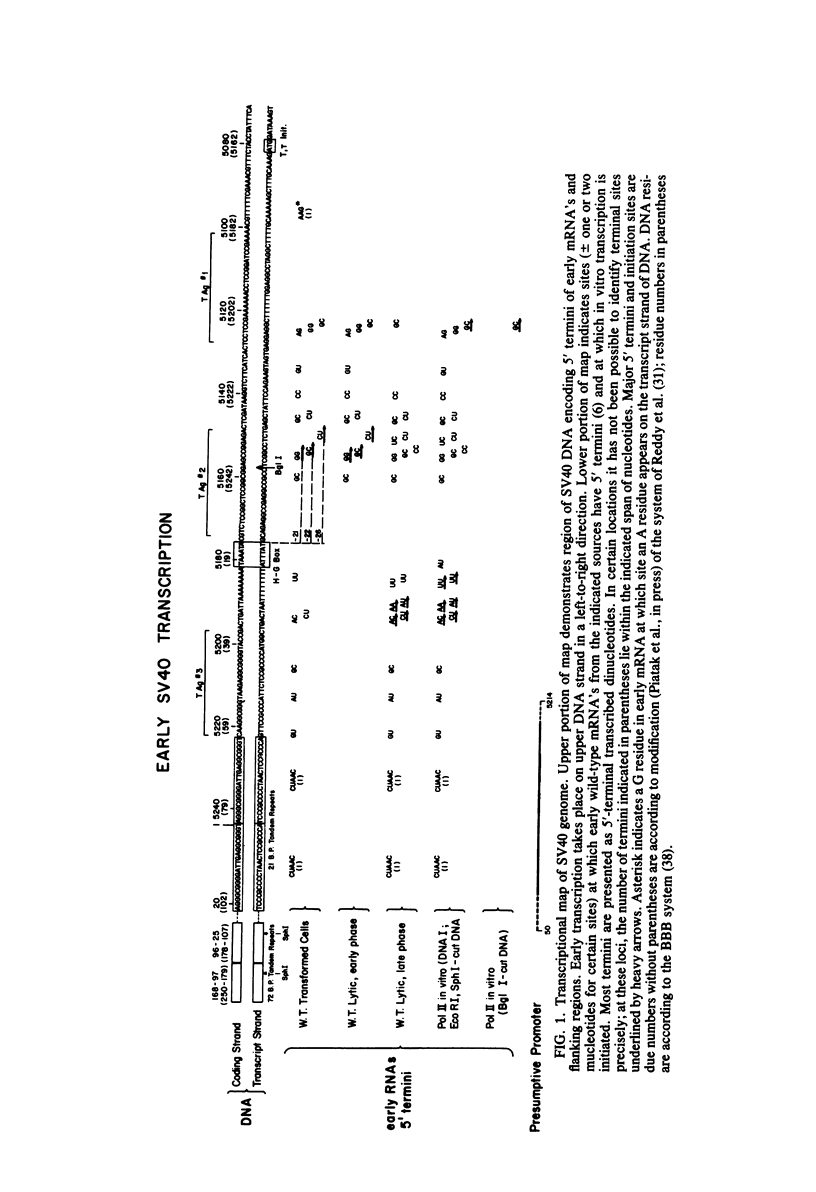

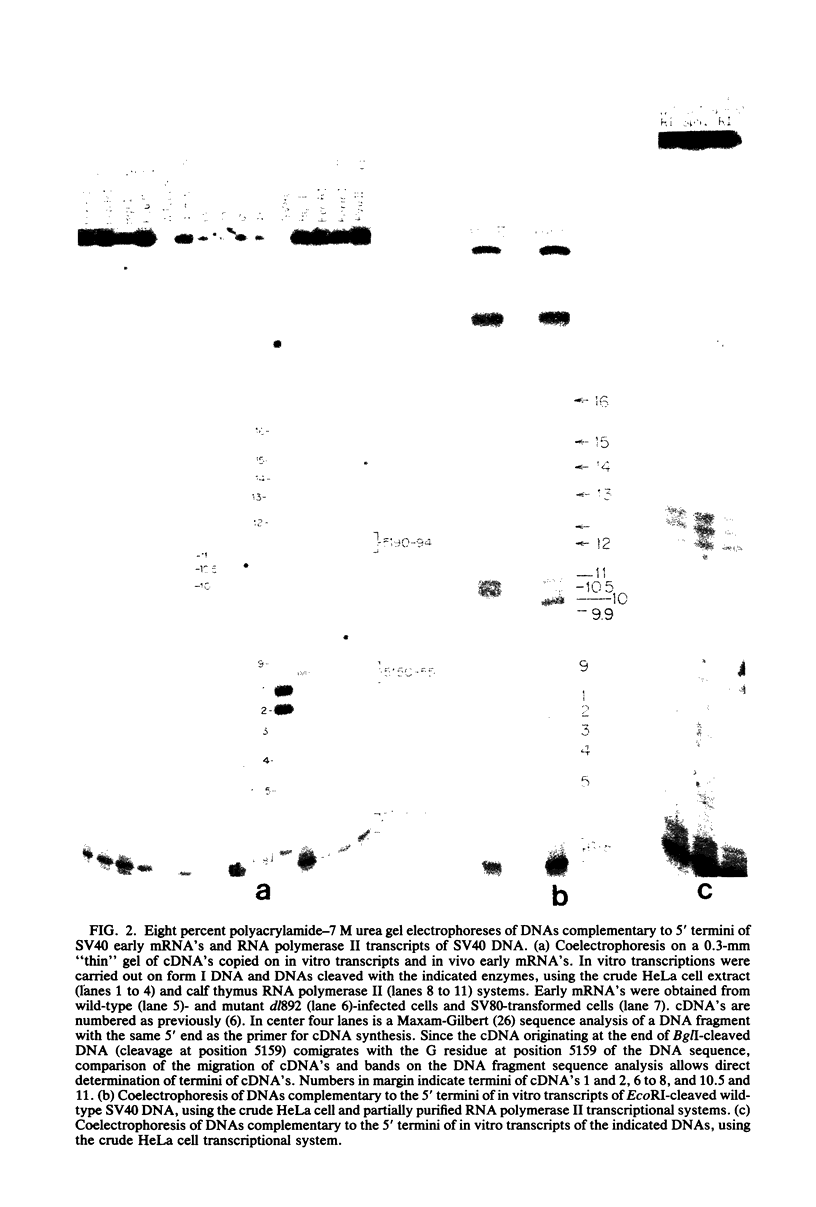








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Mukamel A., Groner Y. Sequence heterogeneity at the 5' termini of late simian virus 40 19S and 16S mRNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3078–3082. doi: 10.1073/pnas.76.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P. Simian virus 40 early mRNA's contain multiple 5' termini upstream and downstream from a Hogness-Goldberg sequence; a shift in 5' termini during the lytic cycle is mediated by large T antigen. J Virol. 1981 Oct;40(1):224–240. doi: 10.1128/jvi.40.1.224-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Piatak M., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Transcription of the SV40 genome in virus-transformed cells and early lytic infection. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):31–39. doi: 10.1101/sqb.1980.044.01.006. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Kahana C., Canaani D., Groner Y. Specific in vitro initiation of transcription of simian virus 40 early and late genes occurs at the various cap nucleotides including cytidine. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2174–2178. doi: 10.1073/pnas.78.4.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Characterization of the 5'-terminal cap structures of early simian virus 40 mRNA. J Virol. 1980 Sep;35(3):955–961. doi: 10.1128/jvi.35.3.955-961.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Characterization of the 5'-terminal capped structures of late simian virus 40-specific mRNA. J Virol. 1978 Mar;25(3):824–830. doi: 10.1128/jvi.25.3.824-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kaufman R. J., Manley J., Gefter M., Sharp P. A. Transcription of Simian virus 40 DNA in a HeLa whole cell extract. J Biol Chem. 1981 Jan 10;256(1):478–482. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hodo H. G., 3rd, Blatti S. P. Purification using polyethylenimine precipitation and low molecular weight subunit analyses of calf thymus and wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1977 May 31;16(11):2334–2343. doi: 10.1021/bi00630a005. [DOI] [PubMed] [Google Scholar]
- Kahana C., Gidoni D., Canaani D., Groner Y. Simian virus 40 early mRNA's in lytically infected and transformed cells contain six 5'-terminal caps. J Virol. 1981 Jan;37(1):7–16. doi: 10.1128/jvi.37.1.7-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M. Organization and transcription of the simian virus 40 genome. Curr Top Microbiol Immunol. 1979;87:43–172. doi: 10.1007/978-3-642-67344-3_3. [DOI] [PubMed] [Google Scholar]
- Luse D. S., Roeder R. G. Accurate transcription initiation on a purified mouse beta-globin DNA fragment in a cell-free system. Cell. 1980 Jul;20(3):691–699. doi: 10.1016/0092-8674(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Chambon P. The SV40 early region TATA box is required for accurate in vitro initiation of transcription. Nature. 1981 Mar 26;290(5804):310–315. doi: 10.1038/290310a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Weissman S. M. Gaps and duplicated sequences in the leaders of SV40 16S RNA. Nucleic Acids Res. 1978 Nov;5(11):4195–4213. doi: 10.1093/nar/5.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Kobayashi N., Mizuno D., Natori S. Purification of a factor from Ehrlich ascites tumor cells specifically stimulating RNA polymerase II. Biochemistry. 1976 Nov 16;15(23):5064–5070. doi: 10.1021/bi00668a018. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Dhar R., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. III. Construction of the total sequence of EcoRII-G fragment of SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):355–367. [PubMed] [Google Scholar]
- Subramanian K. N., Shenk T. Definition of the boundaries of the origin of DNA replication in simian virus 40. Nucleic Acids Res. 1978 Oct;5(10):3635–3642. doi: 10.1093/nar/5.10.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wu G. J. Adenovirus DNA-directed transcription of 5.5S RNA in vitro. Proc Natl Acad Sci U S A. 1978 May;75(5):2175–2179. doi: 10.1073/pnas.75.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]





