Abstract
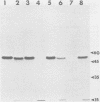
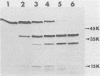
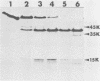
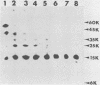
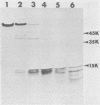
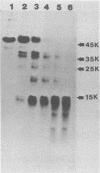
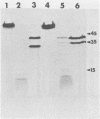
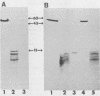
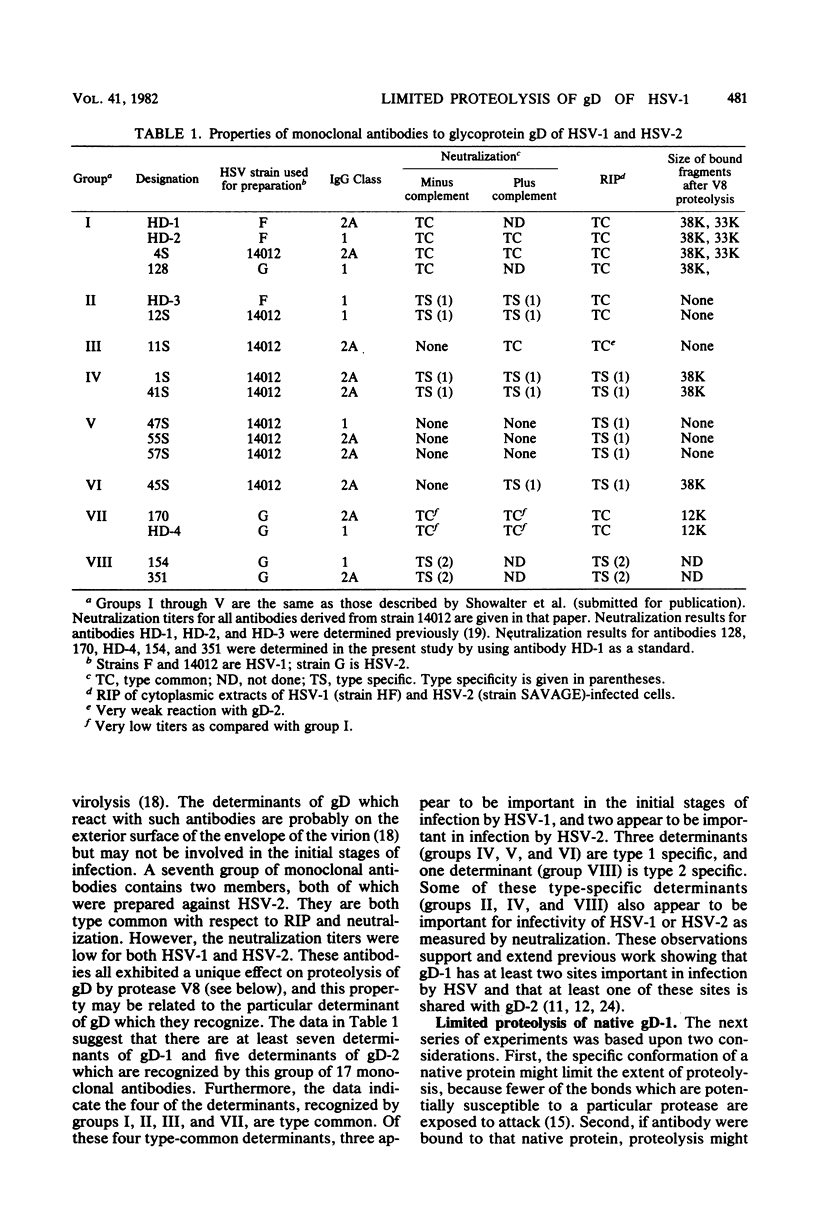
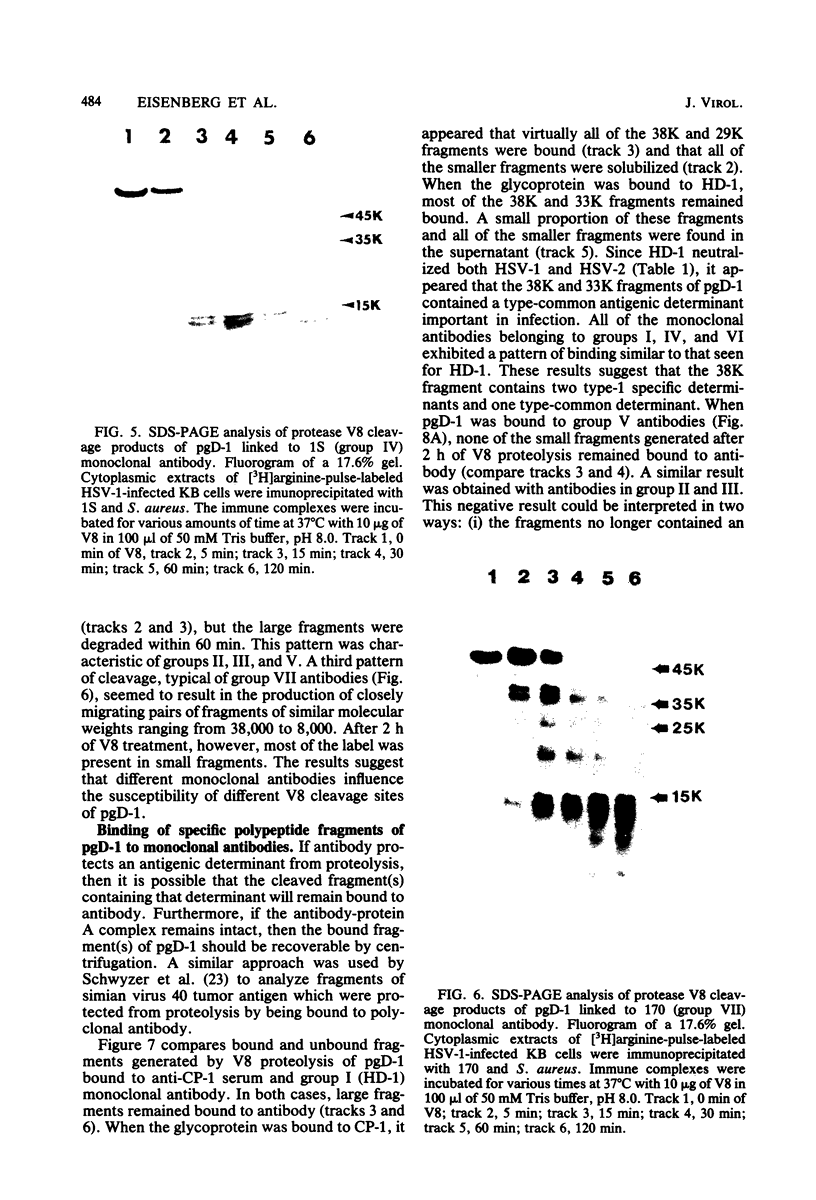
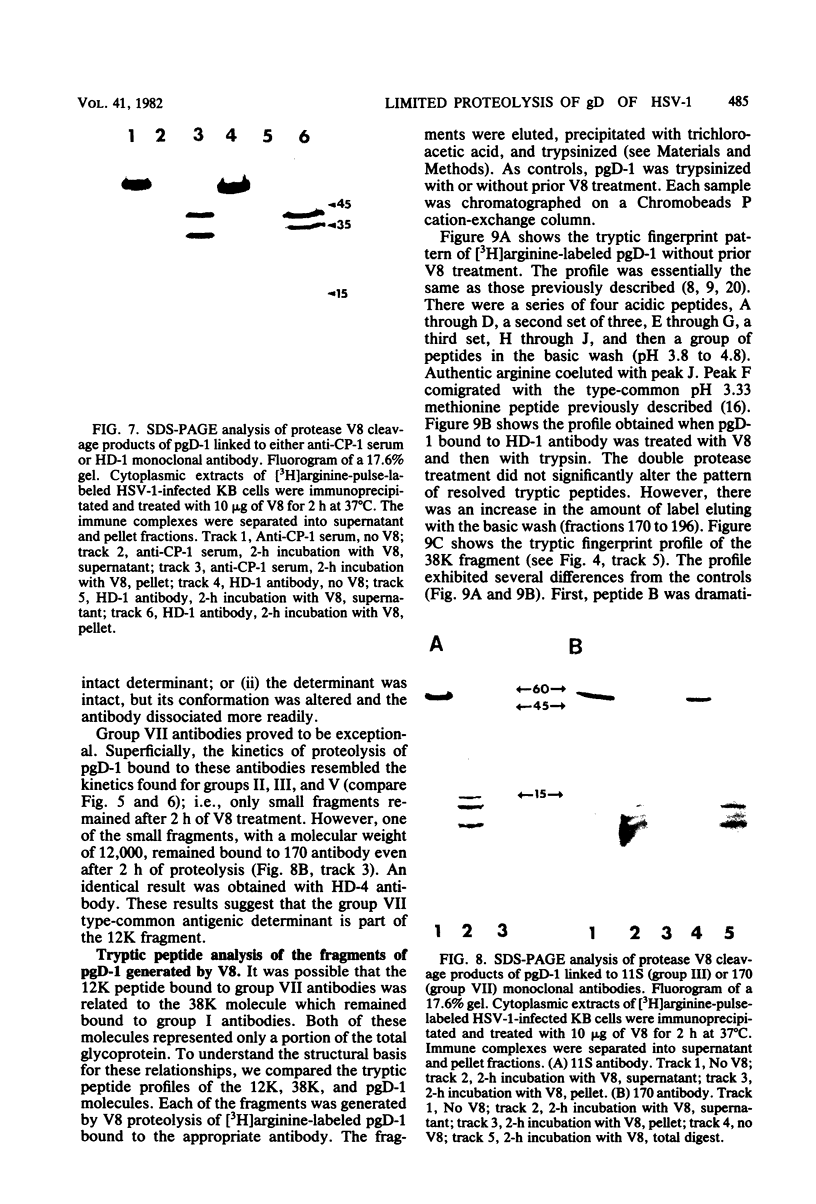
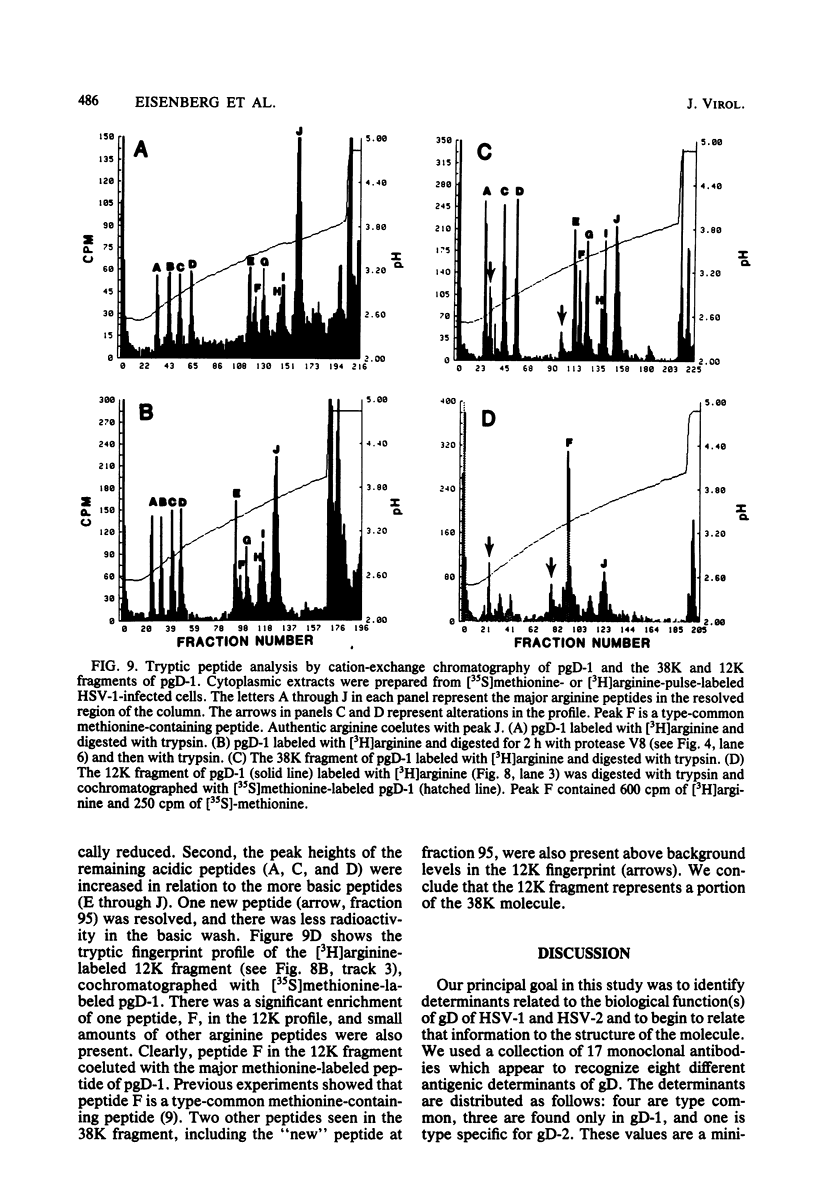
We examined the properties of 17 monoclonal antibodies to glycoprotein gD of herpes simplex type 1 (HSV-1) (gD-1) and HSV-2 (gD-2). The antibodies recognized eight separate determinants of gD, based on differences in radioimmuno-precipitation and neutralization assays. The determinants were distributed as follows: three were gD-1 specific, one was gD-2 specific, and four were type common. Several type-specific and type-common determinants appeared to be involved in neutralization. We developed a procedure for examining the effect that binding of monoclonal antibody has on proteolysis of native gD-1 by Staphylococcus aureus protease V8. We showed that several different patterns of protease V8 cleavage were obtained, depending on the monoclonal antibody used. The proteolysis patterns were generally consistent with the immunological groupings. With four groups of antibodies, we found that fragments of gD-1 remained bound to antibody after V8 treatment. A 38,000-dalton fragment remained bound to antibodies in three different groups of monoclonal antibodies. This fragment appeared to contain one type-common and two type-specific determinants. A 12,000-dalton fragment remained bound to antibodies belonging to one type-common group of monoclonal antibodies. Tryptic peptide analysis revealed that the 12,000-dalton fragment represented a portion of the 38,000-dalton fragment and was enriched in a type-common arginine tryptic peptide.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R. Chemically defined antiviral vaccines. Annu Rev Microbiol. 1980;34:593–618. doi: 10.1146/annurev.mi.34.100180.003113. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen G. H., Factor M. N., Ponce de Leon M. Inhibition of herpes simplex virus type 2 replication by thymidine. J Virol. 1974 Jul;14(1):20–25. doi: 10.1128/jvi.14.1.20-25.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972 Nov;10(5):1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W. Intertypic recombinants of herpes simplex viruses. J Gen Virol. 1980 May;48(1):1–23. doi: 10.1099/0022-1317-48-1-1. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Nowinski R. C. Lysis of retroviruses with monoclonal antibodies against viral envelope proteins. Virology. 1980 Feb;101(1):296–299. doi: 10.1016/0042-6822(80)90507-3. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M., Hessle H., Cohen G. H. Separation of Herpes simplex virus-induced antigens by Concanavalin A affinity chromatography. J Virol. 1973 Oct;12(4):766–774. doi: 10.1128/jvi.12.4.766-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Glycoproteins specified by herpes simplex virus type 1: their synthesis, processing and antigenic relatedness to HSV -2 glycoproteins. IARC Sci Publ. 1975;(11 Pt 1):49–61. [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Sarmiento M., Manservigi R. The structural proteins and glycoproteins of herpesviruses: a review. IARC Sci Publ. 1978;(24 Pt 1):157–167. [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]