Abstract
Several membrane-associating signals, including covalently linked fatty acids, are found in various combinations at the N termini of signaling proteins. The function of these combinations was investigated by appending fatty acylated N-terminal sequences to green fluorescent protein (GFP). Myristoylated plus mono/dipalmitoylated GFP chimeras and a GFP chimera containing a myristoylated plus a polybasic domain were localized similarly to the plasma membrane and endosomal vesicles, but not to the nucleus. Myristoylated, nonpalmitoylated mutant chimeric GFPs were localized to intracellular membranes, including endosomes and the endoplasmic reticulum, and were absent from the plasma membrane, the Golgi, and the nucleus. Dually palmitoylated GFP was localized to the plasma membrane and the Golgi region, but it was not detected in endosomes. Nonacylated GFP chimeras, as well as GFP, showed cytosolic and nuclear distribution. Our results demonstrate that myristoylation is sufficient to exclude GFP from the nucleus and associate with intracellular membranes, but plasma membrane localization requires a second signal, namely palmitoylation or a polybasic domain. The similarity in localization conferred by the various myristoylated and palmitoylated/polybasic sequences suggests that biophysical properties of acylated sequences and biological membranes are key determinants in proper membrane selection. However, dual palmitoylation in the absence of myristoylation conferred significant differences in localization, suggesting that multiple palmitoylation sites and/or enzymes may exist.
INTRODUCTION
Inside the living cell, covalent lipid modifications of proteins are used to alter the physical and functional properties of proteins. This process, called protein lipidation, is subdivided into four major categories based on the identity of the lipid attached to the protein. Prenylation, glypiation, cholesteroylation, and fatty acylation represent covalent modifications of proteins by isoprenoids (farnesyl and geranylgeranyl), glycosylphosphatidylinositol structures, cholesterol, and fatty acids, respectively (Casey, 1995; Porter et al., 1996; Bhatnagar and Gordon, 1997). Recently, protein lipidation has been shown to be an important new type of protein modification involved in several aspects of cellular signaling (Casey, 1995; Milligan et al., 1995; Mumby, 1997; Dunphy and Linder, 1998). However, the reason that various and multiple lipid moieties are attached to proteins is still not well understood.
Protein fatty acylation involves two main types of fatty acids, myristate and palmitate (Bhatnagar and Gordon, 1997). Myristoylation is the permanent cotranslational linkage of the 14-carbon fatty acid myristate to an N-terminal glycine of a protein via an amide bond catalyzed by the enzyme N-myristoyl transferase. Palmitoylation is the reversible posttranslational linkage of the 16-carbon fatty acid palmitate to variably located cysteine residues via a thioester bond. Membrane-associated protein S-acyltransferase (PAT) activities have been partially purified (Berthiaume and Resh, 1995; Dunphy et al., 1996; Veit et al., 1996, 1998), and a cytosolic acyl protein thioesterase (APT1) was recently cloned (Duncan and Gilman, 1998), but their relevance to fatty acylation regulation remains to be elucidated.
Examples of proteins having both myristate and palmitate at their N termini include several Src-related protein tyrosine kinases (PTKs) (Alland et al., 1994; Resh, 1994), α subunits of heterotrimeric G proteins (Wedegaertner et al., 1995; Morales et al., 1998; Galbiati et al., 1999), and the A-kinase anchoring protein AKAP18 (Fraser et al., 1998). In addition, several proteins contain myristate and a polybasic domain, including Src and myristoylated alanine-rich C-kinase substrate (Kaplan et al., 1992; McLaughlin and Aderem, 1995), or two or more covalently linked palmitates at their N termini, as in GAP-43/neuromodulin or L-type voltage-dependent calcium channel subunit β2a (Zuber et al., 1989; Chien et al., 1998).
N-Myristoylation, like farnesylation, has been shown to be insufficient by itself to stably anchor proteins to membranes (Shahinian and Silvius, 1995). Typically, myristoylation and prenylation signals are linked to a second signal that assists in membrane anchoring. One secondary signal is a series of positively charged residues adjacent or distal to the lipidation site, a combination used by proteins such as c-Src, K-Ras, and myristoylated alanine-rich C-kinase substrate (Hancock et al., 1990; Resh, 1993; McLaughlin and Aderem, 1995). Another has been shown to be palmitoylation, used by Yes, Fyn, and Lck PTKs and H- and N-Ras GTPases. Furthermore, in those PTKs and GTPases, myristoylation and farnesylation have been shown to be prerequisites for palmitoylation to occur (Koegl et al., 1994). The presence of a polybasic domain second signal in the case of multiply palmitoylated proteins such as GAP-43 has been proposed but remains controversial, because conflicting reports of its significance exist (Liu et al., 1993, 1994).
The heterogeneous nature of N-terminal fatty acylated sequences includes differences in the number of palmitates and the position(s) that they occupy, in the presence or absence of myristate or positive charges, and in amino acid composition (Casey, 1995; Milligan et al., 1995). In an attempt to explain or rationalize this observed heterogeneity, we postulated that these sequences might contain specific subcellular localization information. To test this hypothesis, various N-terminal sequences corresponding to the first 11–16 amino acids of PTKs, Gα proteins, and GAP-43 were fused to the cytosolic reporter protein green fluorescent protein (GFP), and the fate of the resulting chimeric proteins was studied with the use of confocal microscopy. GFP was selected as the reporter protein in our studies because of its intrinsic fluorescence properties and because it has been demonstrated to be an excellent reporter protein for subcellular localization studies (Pines, 1995; Gerdes and Kaether, 1996; Girotti and Banting, 1996; Liu et al., 1997). Our data support the possibility of more than one role for the various fatty acylated N-terminal domains in subcellular localization.
MATERIALS AND METHODS
Plasmid Design and Construction
Appropriate oligonucleotides containing BglII and BamHI restriction endonuclease recognition sites at the 5′ and 3′ ends, respectively, S65T GFP cDNA, and Vent DNA polymerase (New England Biolabs, Beverly, MA) were used in the PCR reactions. Amplified fragments were digested with corresponding restriction enzymes, gel purified, and ligated into the appropriately digested pCMV5 mammalian expression vector (Andersson et al., 1989). pCMV5 possesses the SV40 replication origin and uses the strong cytomegalovirus promoter to drive efficient heterologous gene expression in SV40 large T antigen–transformed cells, such as COS-7 cells. The plasmid also contains the human growth hormone fragment containing transcription termination and polyadenylation signals for correct eukaryotic mRNA processing. Large-scale plasmid preparations were done for each construct with the use of the Monster 4G Maxiprep Kit (Bio-101), and each construct was sequenced with the use of automated DNA sequencing on an Applied Biosystems (Foster City, CA) sequencer in the Biochemistry DNA Core Facility at the University of Alberta and shown to conform to the original design. Standard molecular biology protocols were from Sambrook et al. (1989).
Cell Lines, Antibodies, and Reagents
COS-7 cells were from the American Type Culture Collection (Rockville, MD) and were maintained in 10% FBS in DMEM (Life Technologies, Grand Island, NY) with 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 2 mM l-glutamine (Sigma Chemical, St. Louis, MO) and were passed twice per week with a 0.25% trypsin/1 mM EDTA wash (Life Technologies). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Rabbit polyclonal anti-GFP antibody (Ab) was developed in our laboratory with the use of highly purified recombinant GFP made in Escherichia coli as antigen; goat polyclonal anti-calreticulin Ab was a kind gift from Dr. M. Michalak (University of Alberta); mouse anti-GFP was from Chemicon; rabbit anti-giantin was from Dr. E.K. Chan (Scripps Institute, La Jolla, CA); and mouse monoclonal anti-CD63 was from Dr. K. Jimbo (University of Alberta). Mouse monoclonal anti-Yes PTK was from Transduction Laboratories (Lexington, KY). Donkey anti-rabbit immunoglobulin G (IgG)-Texas Red (TR) and IgG-FITC, donkey anti-mouse IgG-TR and IgG-FITC, and donkey anti-goat IgG-TR and IgG-FITC secondary Abs, as well as normal donkey serum, were obtained from Jackson Immunoresearch (West Grove, PA). Protein A–Sepharose CL-4B was from Amersham-Pharmacia. BODIPY TR-ceramide (stored as a DMSO stock) and DiI were obtained from Molecular Probes (Eugene, OR). Paraformaldehyde, nocodazole (NZ), and Triton X-100 were from Sigma Chemical. DiI-LDL was prepared and stored in 0.9% NaCl and 0.01% EDTA as described by Pitas et al. (1981). NZ was stored as a 4 mM stock in DMSO at −20°C.
Transfection, Live Cell Fluorescence, and Immunofluorescence Microscopy
COS-7 cells were seeded at 2 × 105 cells/well onto six-well tissue culture plates containing flame-sterilized 22- × 22-mm glass coverslips (no. 1 thickness, Fisher Scientific, Pittsburgh, PA). Before seeding, coverslips were coated with 5 μg/ml poly-l-lysine (Sigma Chemical) to promote cell adhesion. Twenty-four hours after seeding, cells grown on coverslips were transfected for 2.5 h with the DEAE-dextran/DMSO shock method (Cullen, 1987). Cells were analyzed 16–24 h after transfection to allow for GFP chromophore development (Olson et al., 1995). For live cell fluorescence analysis, coverslips were removed from media, washed with prewarmed PBS, and mounted on glass slides in PBS with the use of vacuum grease or nail polish as a sealant. For immunocytochemistry, cells were washed in PBS, fixed in 4% paraformaldehyde in PBS, pH 7.4, for 20 min, and permeabilized with 0.1% Triton X-100 in PBS for 1 min at room temperature, followed by a 1-h block with 4% normal donkey serum in PBS. All Abs used were diluted in 4% normal donkey serum in PBS to prevent nonspecific binding. For colocalization of GFP chimeras and various organelles, the intrinsic GFP fluorescence, mouse anti-GFP (1:200), or rabbit anti-GFP (1:2000) was used, and anti-calreticulin (1:50), anti-giantin (1:2000), or anti-CD63 (1:100) was used to detect the endoplasmic reticulum (ER), Golgi, and lysosomes, respectively. BODIPY TR-ceramide (1.5 μM) was added for 0.5–2 h before viewing of living transfected cells to detect the Golgi apparatus, as reported by Ralston (1993). DiI-LDL (1 μg/ml) was added to living transfected cells 1 h before fixation to allow for incorporation of the fluorescent lipoprotein particle into endosomes. The red DiI-LDL fluorescence was detected with a Texas Red filter set. To disperse intracellular organelles, transfected cells were treated with 20 μM NZ for 1 h before fixation to allow for effective depolymerization of microtubules (Kalcheva et al., 1998). The subcellular localization of proteins was assessed by generating images acquired on two confocal laser scanning systems. The first system consisted of a Leitz Aristoplan fluorescence microscope illuminated by a 100-W mercury burner for direct observation and an Ar/Kr laser with major emissions at 488, 568, and 647 nm for scanning, with ×100 1.32 numerical aperture or ×63 1.40 numerical aperture oil immersion objectives. Images were also collected with a Zeiss (Thornwood, NY) laser scanning confocal microscope (model LSM 510) mounted on a Zeiss Axiovert M100 inverted microscope with a ×63 Apochromatic lens (1.40 numerical aperture) (Cross Cancer Institute, University of Alberta). Each image is collected within the linear range of fluorescence intensity based on the imaging software, with FITC or Texas Red filters. Image overlays represent samples acquired with the use of the sequential mode for double-label collection to avoid cross-talk between the fluorophores. Scans were optimized for chromophore detection. Final image manipulations were done in Adobe (Mountain View, CA) Photoshop 5.0. To ensure optimal comparisons, images of cells of similar sizes (15–30 μm diameter) were captured with the use of similar pinhole and laser intensities.
Metabolic Labeling
Transiently transfected COS-7 cells were grown in 100-mm-diameter dishes for 24 h. The next day, cells were split into two 100-mm dishes, and they were metabolically labeled 24 h later. For [3H]myristate labeling, cells were starved for 1 h with 3 ml of DMEM, 2 mM l-glutamine, 10 μg/ml BSA, to which 100 μCi per plate [3H-9,10(n)]myristic acid (52.0 Ci/mmol) (Amersham-Pharmacia) was added for 4 h at 37°C. 16-[125I]Iodohexadecanoic acid (125I-IC16; specific activity of 2–3 Ci/mmol) was prepared as described by Berthiaume et al. (1995), without the HPLC purification step, and was used to assess palmitoylation. For 125I-IC16 labeling, cells were starved for 1 h in DMEM containing 2 mM l-glutamine and 10 μg/ml BSA and labeled with 90 μCi of 125I-IC16 per plate for 4 h at 37°C (Alland et al., 1994).
Immunoprecipitation
Metabolically labeled cells were rinsed twice with cold STE buffer (100 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA). Cells were scraped off the plate, collected by centrifugation, and lysed in 2 ml of cold lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 2 mM EDTA, 2 mM MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.1 mM PMSF) for 30 min on a Nutator rotating device at 4°C. Lysates were clarified at 14,000 rpm for 15 min in an Eppendorf microfuge to remove the nuclei and cellular debris. One milliliter of clarified supernatant was incubated with either 2 μl of polyclonal anti-GFP or 2 μl of preimmune serum for 1 h at 4°C. The immune complexes were precipitated with 20 μl of a 50% (wt/vol) slurry of protein A–Sepharose CL-4B in cold lysis buffer for 1 h at 4°C. Samples were pelleted and washed three times with cold lysis buffer, resuspended in sample buffer containing 20 mM DTT, boiled for 2 min, and analyzed by SDS-PAGE (12.5%). Gels containing samples labeled with [3H]myristate and 125I-IC16 fatty acid were transferred onto Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Western blot analyses were performed on these membranes with 1× Blotto as a blocking and diluting solution. Anti-GFP Ab and protein A–HRP (Pierce, Rockford, IL) were both incubated for 1 h at 22°C at 1:5000 dilutions. ECL detection was performed with the ECL Plus kit (Amersham-Pharmacia). Three 5-min washes with PBS were done between steps. After this, PVDF membranes were put into LE Transcreen cassettes with Biomax MS film and exposed for 14 d for [3H]myristate samples or into a phosphorimager screen cassette (Molecular Dynamics, Sunnyvale, CA) for 125I-labeled samples for 7 d.
Subcellular Fractionation
COS-7 cells expressing chimeric GFP constructs were fractionated into soluble (S100) and membrane (P100) fractions as described by Alland et al. (1994). The 1.0-ml supernatant (S100) was transferred to an Eppendorf tube, and 250 μl of 5× cold lysis buffer was added. The pellet (P100) was resuspended in 1.0 ml of hypotonic lysis buffer/sucrose/EDTA solution and homogenized for 5–10 strokes in a 1-ml Dounce homogenizer, and the suspension was adjusted to 1× cold lysis buffer. Fractions were immunoprecipitated as described above, and chimeras were detected by Western blotting with the use of the rabbit polyclonal anti-GFP Ab. To estimate relative amounts of GFP chimera in either the membrane pellets or the supernatant and correct for potential losses resulting from processing, we calculated the percentages of GFP in the pellet and supernatant fractions as follows: %S = [S/(S + P)] × 100 and %P = [P/(S + P)] × 100 instead of %S = (S/T) × 100 and %P = (P/T) × 100, where S, P, and T represent the amount of GFP present in the supernatant, pellet, and total fractions, and %S and %P represent the percentage of GFP protein found in the supernatant and pellet fractions, respectively.
RESULTS
Plasmid Design and Construction
To determine whether various short N-terminal fatty acylated sequences contain different subcellular targeting information, PCR-mediated mutagenesis was used (Horton et al., 1989) to append various 11- to 16-amino acid N-terminal sequences known to be acylated from the Src PTK and Gα protein families and GAP-43 onto the cDNA of the S65T mutant of GFP (Clontech, Palo Alto, CA) (Heim et al., 1996) (Table 1). The rationale was to allow the study of features specific to the N-terminal fatty acylation domain that are important for membrane localization independent of other protein-protein interaction modules found within these signaling proteins (e.g., SH2 and SH3 domains of Src-related PTKs). To further investigate the specific contributions of myristate and palmitate in FynGFP and YesGFP, and of palmitoylation in GAP-43GFP, two series of mutations were created. One series abolished palmitoylation by mutating palmitoylated cysteine residues to serine residues [Fyn(C3,6S), Yes(C3S), and GAP-43(C3,4S)]. The other series [Fyn(G2A) and Yes(G2A)] abolished both myristoylation and palmitoylation by substituting the glycine residue essential for myristoylation for an alanine (Gordon et al., 1991). Because N-myristoylation is a prerequisite for the palmitoylation of adjacent cysteine residues, the prevention of myristoylation abolishes palmitoylation of those cysteines (Alland et al., 1994; Resh, 1994).
Table 1.
Fatty acylated sequences appended to the amino terminus of GFP
| Protein | Amino acid sequencea | Chargeb | First signalc | Second signald | Localizatione |
|---|---|---|---|---|---|
| Fyn | (M) GCVQCKDKEA | 0 | myr | pal (Cys3,6) | pm + endo |
| Fyn(C3,6S) | (M) GSVQSKDKEA | 0 | myr | None | endo + ER |
| Fyn(G2A) | (M) ACVQCKDKEA | 0 | None | None | cyto |
| Yes | (M) GCIKSKENKS | +2 | myr | pal (Cys3) | pm + endo |
| Yes(C3S) | (M) GSIKSKENKS | +2 | myr | None | endo + ER |
| Yes(G2A) | (M) ACIKSKENKS | +2 | None | None | cyto |
| Gαo | (M) GCTLSAEERA | −1 | myr | pal (Cys3) | pm + endo |
| Lck | (M) GCGCSSHPED | −2 | myr | pal (Cys3,5) | pm + endo |
| Src14 | (M) GSSKSKPKDPSQR | +3 | myr | Polybasic | pm, endo, ER |
| Src16 | (M) GSSKSKPKDPSQRRR | +5 | myr | Polybasic | pm + endo |
| GAP-43 | MLCCMRRTKQV | +3 | pal | (Polybasic)f | pm + Golgi |
| GAP-43(C3,4S) | MLSSMRRTKQV | +3 | None | (Polybasic) | cyto |
A neutrally charged seven-amino acid linker corresponding to amino acids 12–18 of Fyn (TKLTEER) was appended between the N-terminal amino acid sequences and GFP.
M, methionine removed by methionyl aminopeptidase (cotranslational). G, myristoylated glycine residue (cotranslational); C, palmitoylated cysteine residue (posttranslational).
Net charge of the sequence at physiological pH.
First membrane-targeting signal. myr, myristoylation; pal, palmitoylation.
Second membrane-targeting signal. Polybasic, a positively charged amino acid sequence.
Subcellular localization of chimeric proteins as displayed in Figures 3–7; cyto, cytoplasm; endo, endosomes; pm, plasma membrane.
Putative second signal.
To ensure proper access to the fatty acylation enzymes and potentially biological membranes, a seven-amino acid hydrophilic linker (TKLTEER) corresponding to residues 12–18 of Fyn PTK was introduced between GFP and the sequence of interest. Previous reports have shown that putatively acylated N-terminal sequences appended to GFP without a linker region can prevent acylation (Liu et al., 1997; Fraser et al., 1998). For the SrcGFP constructs (Src14GFP and Src16GFP), 14 and 16 amino acids, respectively, were appended to GFP to include positively charged basic residues known to be important in membrane binding (Silverman and Resh, 1992; Sigal et al., 1994).
Metabolic Labeling and Immunoprecipitation of Chimeric GFPs Expressed in COS-7 Cells
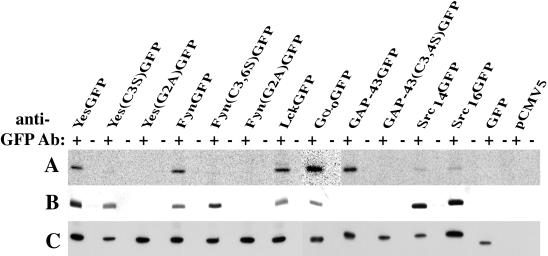
COS-7 cells were transiently transfected with various pCMV5 plasmids containing different chimeric GFP cDNAs and metabolically labeled. To assess proper chimera palmitoylation and myristoylation, 125I-IC16, a palmitate analogue, and [3H-9,10(n)]myristic acid were used, respectively. Finally, to assess protein production, Western blot analysis was used.
125I-IC16 labeling (Figure 1A) was evident in constructs bearing an N-terminal cysteine residue available for acylation (FynGFP, YesGFP, LckGFP, GαoGFP, and GAP-43GFP). Substitution of cysteine residues known to be palmitoylated for serine residues abolished the incorporation of the 125I-IC16 label into the corresponding chimeric GFPs. Mutants bearing the G2A mutation demonstrated a lack of incorporation of both 125I-IC16 and [3H]myristate. Because this mutation is known to prevent myristoylation, which is a prerequisite for palmitoylation (Koegl et al., 1994), these results conform with previous observations made with full-length signaling proteins (Zuber et al., 1989; Parenti et al., 1993; Alland et al., 1994; Koegl et al., 1994). Differences in labeling intensity in monopalmitoylated versus dipalmitoylated chimeras could not be discerned in our system. Of note, two Src constructs, Src14GFP and Src16GFP, chimeras bearing no cysteines available for palmitoylation, showed very weak 125I incorporation. This is probably due to metabolic interconversion of 125I-IC16 to 125I-IC14 by β-oxidation. This phenomenon has been observed previously (Alland et al., 1994). [3H]Myristate (Figure 1B) was incorporated into all constructs bearing a glycine residue at position 2. [3H]Myristate was not incorporated into GAP-43GFP, showing an apparent lack of conversion from [3H]myristate to palmitate or incorporation of [3H]myristate into palmitoylation sites by a nonspecific PAT, even when gels were exposed to film for >2 mo (our unpublished results). Finally, typical expression levels of the different GFP chimeras achieved under our experimental conditions are within 1 order of magnitude of each other as judged by signal intensity in our Western blot analyses (Figure 1C). Expression levels did not appear to correlate with the acylation status of GFP chimeras. Our results confirm that short (11–16 amino acids) N-terminal peptide sequences are necessary and sufficient to confer proper fatty acylation.
Figure 1.
Metabolic labeling of chimeric GFPs. COS-7 cells transiently expressing N-terminal fatty acylated GFP chimeras and mutants were metabolically labeled with 125I-IC16 to assess chimera palmitoylation (A) and with [3H-9,10(n)]myristic acid to assess chimera myristoylation (B). (C) Western blot analysis of corresponding transiently transfected COS-7 cells to assess protein production. Plus (+) and minus (−) signs refer to anti-GFP serum and preimmune serum, respectively.
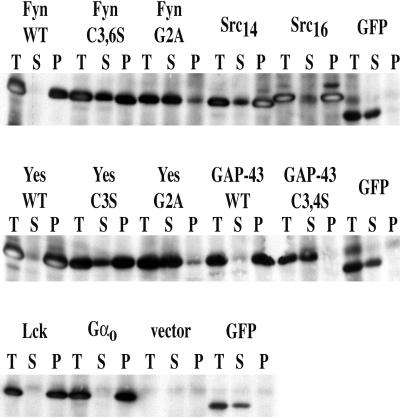
Subcellular Fractionation of Transfected COS-7 Cells
Distributions of chimeric GFPs between total, soluble, and membrane fractions can be seen in Figure 2. Cells were harvested at the same time (18–24 h after transfection) that the live cell images shown in Figure 3 were made. Myristoylated and palmitoylated or dually palmitoylated GFP constructs (FynGFP, YesGFP, LckGFP, GαoGFP, and GAP-43GFP) were associated with the membrane (P100) fraction to >90%. Nonacylated mutants [Fyn(G2A)GFP, Yes(G2A)GFP, and GAP-43(C3,4S)GFP] and GFP alone were found nearly completely in the soluble fraction (>90%). Myristoylated but not palmitoylated Fyn(C3,6S)GFP and Yes(C3S)GFP were found distributed in both fractions, but the majority (at least ∼70% on average) were found in the particulate fraction. Src14GFP (net charge of +3) was in the S100 fraction and showed an intermediate distribution between myristoylated-alone constructs and Src16GFP (net charge of +5), which was distributed similar to dually acylated chimeras (vast majority in P100). In some cases, recovery yields of GFP from the cell fractionation experiments were not always 100%. This may represent losses from nonacylated chimeras or GFP being trapped in the nucleus. Alternatively, acylated GFP chimeras could remain associated with nuclear pellet membranes. In addition, nonspecific losses may occur during processing in the Dounce homogenizer, because acylated GFPs are typically “sticky” as a result of their hydrophobic anchors. On the other hand, in several cases, recovery was apparently 100% or very close to 100% (e.g., YesGFP, Src14GFP, and Src16GFP).
Figure 2.
Subcellular fractionation of COS-7 cells expressing various GFP chimeras. WT, wild type; T, total cell lysate; S, supernatant fraction (S100); P, pellet fraction (P100).
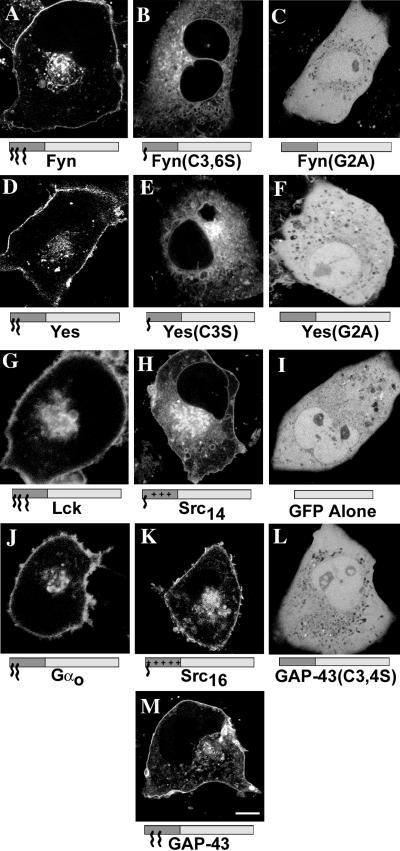
Figure 3.
Subcellular localization of various N-terminal fatty acylated GFP chimeras and mutants in living COS-7 cells. GFP fluorescence of COS-7 cells expressing FynGFP (A), Fyn(C3,6S)GFP (B), Fyn(G2A)GFP (C), YesGFP (D), Yes(C3S)GFP (E), Yes(G2A)GFP (F), LckGFP (G), Src14GFP (H), GFP alone (I), GαoGFP (J), Src16GFP (K), GAP43(C3,4S)GFP (L), and GAP-43GFP (M). Intrinsic GFP fluorescence was detected by confocal laser scanning microscopy. In the models below the images, the dark gray box indicates the appended acylation amino acid sequence and the light gray box indicates GFP. Small acyl chain (∼), myristate; large acyl chain (∧∧∧), palmitate; plus (+) signs, polybasic region. Bar, 10 μm.
The procedure we used to immunoprecipitate and detect GFP chimeras is extremely efficient; typically, 100 ng to 1 μg of apparent protein can be recovered in a single lane on the PVDF membrane. In some lanes of Figure 2, some white areas were seen within a band. We believe that these white areas are due to the high sensitivity of ECL detection used combined with the large amount of GFP present on the PVDF membrane, and not to overexposure of the autoradiographic film, which typically was <5 s. Those white areas within bands might represent ECL substrate depletion in the middle of the band area.
Overall, the relative distributions of these chimeric constructs correlate with the number of membrane-binding signals (e.g., myristate, palmitate, or polybasic domain) present in their N termini. Our subcellular fractionation data also correlate with those previously reported with the use of full-length acylated signaling proteins and chimeras (Kaplan et al., 1992; Alland et al., 1994; Wolven et al., 1997; Arni et al., 1998; Galbiati et al., 1999).
Localization of GFP Chimeras by Confocal Microscopy
To assess whether various N-terminal fatty acylated sequences contain different subcellular localization information in cells without possible artifacts from fixation and permeabilization, we transfected and viewed living COS-7 cells expressing different chimeric GFPs (shown in Table 1). COS-7 cells expressing myristoylated and dipalmitoylated FynGFP (Figure 3A) demonstrated plasma membrane and focal perinuclear localization with nuclear exclusion and low cytosolic levels at early time points in the transient transfection procedure (i.e., <24 h after transfection). Myristoylated Fyn(C3,6S)GFP (Figure 3B), bearing a single lipid modification, was localized to multiple intracellular membranes, including the nuclear envelope, and appeared enriched in perinuclear vesicles. This construct was also excluded from the nucleus and was not detected in the plasma membrane. Finally, Fyn(G2A)GFP, a nonacylated mutant, showed widespread cytoplasmic distribution in cells with negative staining of nucleoli and vesicular/vacuolar structures (Figure 3C).
To assess whether the N-terminal combination of one myristate and one palmitate would confer localization different from the combination of one myristate and two palmitates found in FynGFP, we expressed the myristoylated and monopalmitoylated YesGFP chimera in COS-7 cells and looked for cellular distribution of the green fluorescence. Like FynGFP, YesGFP was found at the plasma membrane and the perinuclear area, was excluded from the nucleus, and showed low cytosolic levels (Figure 3D). Myristoylated Yes(C3S)GFP, like Fyn(C3,6S)GFP, was localized to several intracellular membranes (and also appeared enriched in perinuclear vesicles), was excluded from the nucleus, and was typically absent from the plasma membrane (Figure 3E). Nonacylated Yes(G2A)GFP showed a widespread distribution in cells (Figure 3F). Under our experimental conditions, the fluorescence patterns of dually acylated FynGFP and YesGFP chimeras were indistinguishable, as were the fluorescence patterns of the corresponding myristoylated but not palmitoylated mutant chimeric GFPs.
To assess whether the amino acid composition found at the N terminus and the number and position of palmitates were important factors in the subcellular distribution of dually fatty acylated chimeric GFPs, the myristoylated and dipalmitoylated (at positions 3 and 5) LckGFP chimera and the myristoylated and monopalmitoylated (at position 3) GαoGFP were expressed in COS-7 cells (Figure 3, G and J). LckGFP and GαoGFP were localized in a way that was apparently indistinguishable from that of FynGFP and YesGFP. As such, it appears that the amino acid composition surrounding the fatty acylated residues and the number and position of covalently linked palmitate(s) are not essential determinants of subcellular distribution in our system. The minimal feature common to all four constructs was a myristoylated glycine (at position 2) adjacent to a palmitoylated cysteine (at position 3). This dually fatty acylated motif alone may account for the similar localization observed.
To compare the subcellular localization information encoded by the N-terminal combination of myristate and palmitate to that of myristate and a polybasic domain, two SrcGFP constructs were engineered and expressed in COS-7 cells. Src14GFP, bearing a myristate group and a positively charged domain bearing a net charge of +3, showed localization to the plasma membrane, the nuclear membrane, and several widespread intracellular membranes/structures and was excluded from the nucleus (Figure 3H). In contrast, Src16GFP (net positive charge of +5) was found primarily at the plasma membrane and on a juxtanuclear compartment analogous to that seen with dually fatty acylated YesGFP and FynGFP (Figure 3K). In those cases, the number of positively charged residues appeared to be a critical determinant of intracellular localization, and interestingly, the localization of Src16GFP was indistinguishable from that of the myristoylated and palmitoylated GFP chimeras.
To assess potential targeting differences between N-terminal combinations of myristate and palmitate and combinations of two palmitates (without myristate), the dipalmitoylated GAP-43GFP chimera was engineered and expressed in COS-7 cells. GAP-43GFP was localized to the plasma membrane and showed weak perinuclear punctate fluorescence in living cells (Figure 3M). This is in contrast to myristoylated and palmitoylated GFP constructs, which demonstrated a consistent focal perinuclear accumulation. In addition, the apparent inability of GAP-43(C3,4S)GFP to localize to membranes argues against an independent membrane-associating role for the putative second signal polybasic domain in this construct. All fatty acylation–deficient chimeric GFPs and GFP alone were found throughout the cytosol and nucleus, with the absence of fluorescence in vesicular lumenal areas (apparent negative staining) (Figure 3, C, F, I, and L).
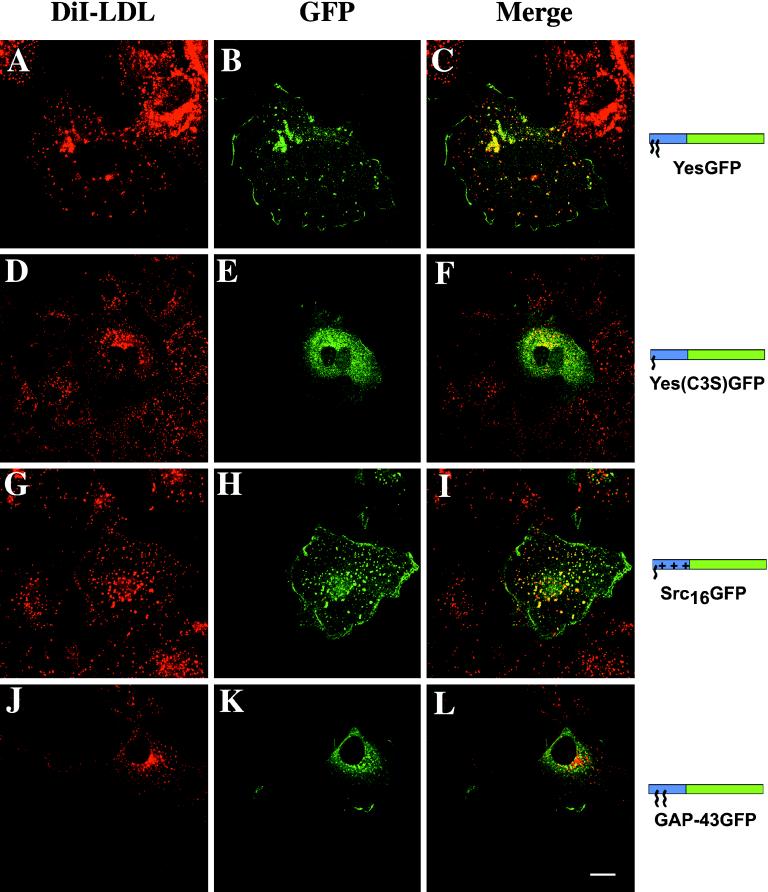
Colocalization of Chimeric GFPs with Endosomes
In COS-7 cells, ERGIC, the Golgi, trans-Golgi network (TGN) compartments, and some endosomes are tightly packed in the perinuclear area. To improve visualization of colocalization data, NZ was used to disrupt microtubules and cause dispersion of these organelles. This allowed for better refinement of the colocalization of acylated GFP chimeras with various organelle markers. To identify the intracellular membranes targeted by the various chimeric GFPs, immunocytochemistry was used to colocalize GFP chimeras with known organelle markers. To determine whether YesGFP was located on endosomes, COS-7 cells expressing YesGFP were incubated with fluorescent DiI-LDL followed by NZ treatment. Similar subcellular localization of DiI-LDL internalized via the endocytic pathway and YesGFP are shown (Figure 4, A and B). The merged image (Figure 4C) showed significant colocalization of signals (yellow) in vesicular structures containing similar levels of fluorescence. Yes(C3S)GFP fluorescence was more dispersed and localized to various intracellular membranes and was enriched in perinuclear vesicles (Figure 4E), whose distribution closely resembled that of internalized DiI-LDL (Figure 4D). When merged with the DiI-LDL signal, the resultant image shows bright perinuclear yellow vesicles, indicative of significant overlap of the vesicles with the endosomal marker (Figure 4F). Yes(G2A)GFP, a nonacylated chimera, was localized throughout the cell and did not colocalize with the endosomal marker (our unpublished results). The colocalization patterns of FynGFP and Fyn(C3,6S)GFP with the endosomal marker were indistinguishable from those obtained with YesGFP. Likewise, LckGFP and GαoGFP were also found at the plasma membrane and on endosomes (our unpublished results). The partial colocalization between the chimeric GFPs and the endosomal marker could be due to multiple types of endosomes that do not all contain endocytosed DiI-LDL or simply to differences in the signal intensities of the two constituents in different vesicles.
Figure 4.
Colocalization of chimeric GFPs with endosomes in fixed COS-7 cells. Before immunocytochemistry, cells were treated for 1 h with 20 μM NZ. The GFP chimera fluorescence was detected by rabbit anti-GFP Ab followed by FITC-conjugated anti-rabbit Ab (A, D, G, and J). The same cells were incubated with DiI-LDL (B, E, H, and K). Merged images are shown on the right (C, F, I, and L). Bar, 10 μm. Model description as in Figure 3. Blue box, acylation sequence; green box, GFP.
Src16GFP, which is myristoylated and contains a polybasic domain, also colocalized with the endosomal marker in a manner that was apparently indistinguishable from that of YesGFP or FynGFP (Figure 4, G–I). In contrast with YesGFP, FynGFP, and Src16GFP, dually palmitoylated (but not myristoylated) GAP-43GFP did not colocalize with the endosomal marker (Figure 4, J–L).
When expressed at high levels, myristoylated plus palmitoylated or polybasic domain chimeric GFPs (e.g., YesGFP or Src16GFP, respectively) showed partial colocalization with the integral membrane lysosomal marker CD63 in the perinuclear region (our unpublished results). Hence, overexpression may allow some acylated/partially acylated chimeras to reside on lysosomal membranes.
Colocalization of Chimeric GFPs with the Golgi and ER Compartments
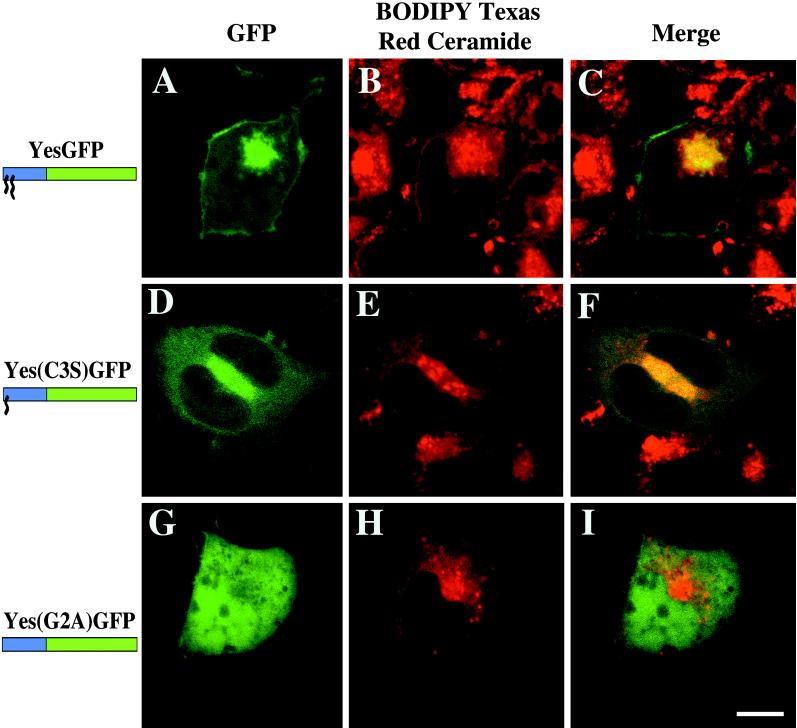
The intrinsic fluorescence of the YesGFP chimera in living cells is shown in Figure 5A, and the Golgi apparatus staining is shown by the accumulation of BODIPY TR-ceramide (Figure 5B). The merged image (Figure 5C) demonstrates colocalization of the apparent Golgi signal with the intracellular fluorescence of YesGFP. In Figure 5D, Yes(C3S)GFP expression demonstrates targeting to various intracellular membranes. When merged with the Golgi marker signal (Figure 5E), the resultant image (Figure 5F) shows significant colocalization (yellow) with part of the Yes(C3S)GFP fluorescence. Nonacylated Yes(G2A)GFP was distributed throughout the cell (Figure 5G) and did not colocalize with BODIPY TR-ceramide (Figure 5, H and I). Similar results were found with dually acylated FynGFP and myristoylated Fyn(C3,6S)GFP (our unpublished results).
Figure 5.
Colocalization of various chimeric GFPs with the Golgi marker BODIPY TR-ceramide in living COS-7 cells. GFP intrinsic fluorescence of YesGFP, Yes(C3S)GFP, and Yes(G2A)GFP is shown in A, D, and G, respectively. The distribution of BODIPY TR-ceramide is shown in B, E, and H. The merged images are shown in C, F, and I. Bar, 10 μm.
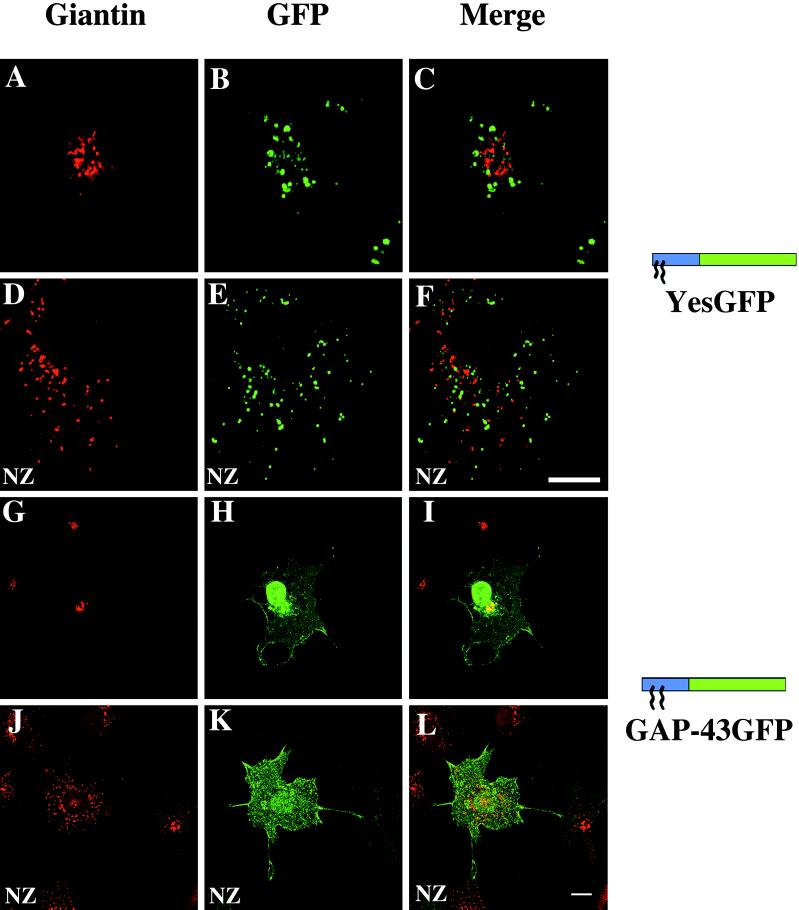
Because of the transport requirement for internalization of BODIPY TR-ceramide and its possible localization to organelles other than the Golgi apparatus, Golgi colocalization was also assessed in fixed cells with an established resident Golgi marker, giantin (Linstedt and Hauri, 1993). In contrast to the results obtained with BODIPY TR-ceramide in living cells, all constructs bearing myristate [e.g., Yes(C3S)GFP], myristate and palmitate (e.g., YesGFP), or myristate plus the polybasic domain (e.g., Src16GFP) did not colocalize with giantin. Typical results obtained with cells expressing YesGFP are shown in Figure 6. The giantin distribution is shown in red in Figure 6, A and D, in the absence or presence, respectively, of the microtubule-disrupting agent NZ. In those cells, the YesGFP signal depicted in green in Figure 6, B and E, when combined with the giantin signal, did not show any colocalization (Figure 6, C and F). In contrast, dipalmitoylated GAP-43GFP, which displayed only slight perinuclear fluorescence in living COS-7 cells, showed significant perinuclear focal fluorescence in fixed and permeabilized cells (Figure 6G). When merged with the giantin signal (Figure 6H), there was significant overlap (yellow) in distribution with giantin (Figure 6I), although the GAP-43GFP signal did extend beyond that defined by giantin. As such, GAP-43GFP localization was consistent with that of the Golgi but not restricted to this organelle. Paradoxically, upon NZ treatment, the perinuclear colocalization of GAP-43GFP (Figure 6J) with the giantin signal (Figure 6K) was abrogated (Figure 6L).
Figure 6.
Colocalization of chimeric GFPs with the Golgi apparatus in fixed COS-7 cells. The GFP chimeras were detected by incubation with mouse anti-GFP Ab followed by FITC-conjugated anti-mouse Ab (A, D, G, and J). The same cells were incubated with rabbit anti-giantin Ab followed by TR-conjugated anti-rabbit Ab (B, E, H, and K). Merged images are shown on the right (C, F, I, and L). Images A–C and G–I represent untreated cells, and images D–F and J–L represent cells treated for 1 h with 20 μM NZ before viewing. Bars, 10 μm.
Because the BODIPY TR-ceramide is internalized to the Golgi compartment via endocytosis, this ceramide analogue might also be found in endosomes and areas of the TGN. Using immunocytochemistry, we found that all lipidated GFPs colocalized significantly with the endosomal marker DiI-LDL except GAP-43, which colocalized with the Golgi marker. The data in Figure 5 show overlap between acylated GFPs (which are found on the internal membranes of endosomes, as shown in Figure 4) and the BODIPY TR-ceramide. Thus, these data support the possibility that BODIPY-TR ceramide may accumulate in endosomes as well as in the Golgi apparatus in COS-7 cells.
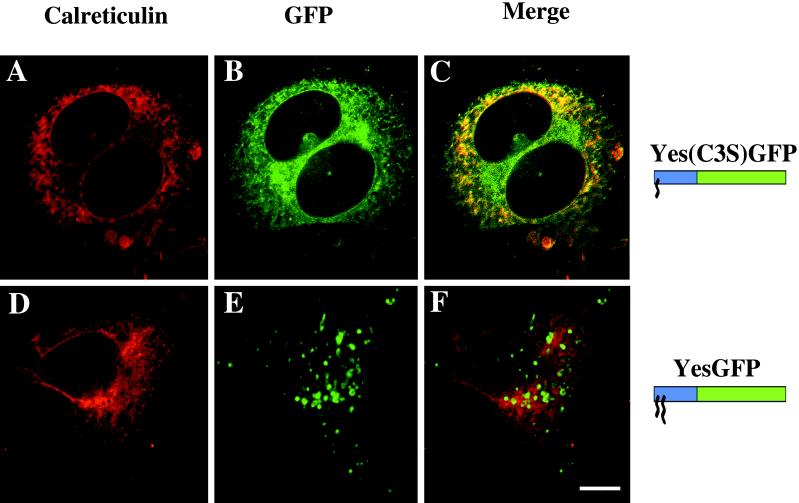
To identify the intracellular membranes targeted by the mutant chimeric GFPs, immunocytochemistry was used to colocalize GFP chimeras with a known ER marker, calreticulin. Yes(C3S)GFP was localized to various intracellular membranes (Figure 7B, green). When merged with the calreticulin staining (Figure 7A, red), the resultant image shows significant colocalization with the ER marker (Figure 7C, yellow). Identical results were seen with the Fyn(C3,6S)GFP chimera (our unpublished results). For YesGFP, the staining of intracellular vesicles identified as endosomes (Figure 7E) did not overlap with that of calreticulin (Figure 7D), as seen in the merged image (Figure 7F). Other chimeras demonstrating endosomal localization also did not colocalize with calreticulin (our unpublished results).
Figure 7.
Colocalization of chimeric GFPs with the ER marker calreticulin in fixed COS-7 cells. Yes(C3S)GFP and YesGFP were detected by mouse anti-GFP Ab followed by FITC-conjugated anti-mouse Ab for YesGFP, Yes(C3S)GFP, and Yes(G2A)GFP (A and D, respectively). The distribution of calreticulin, a lumenal protein of the ER, was detected by goat anti-calreticulin Ab followed by TR-conjugated donkey anti-goat Ab (B and E). The merged images are shown in C and F. Bar, 10 μm.
Colocalization of YesGFP and Yes PTK
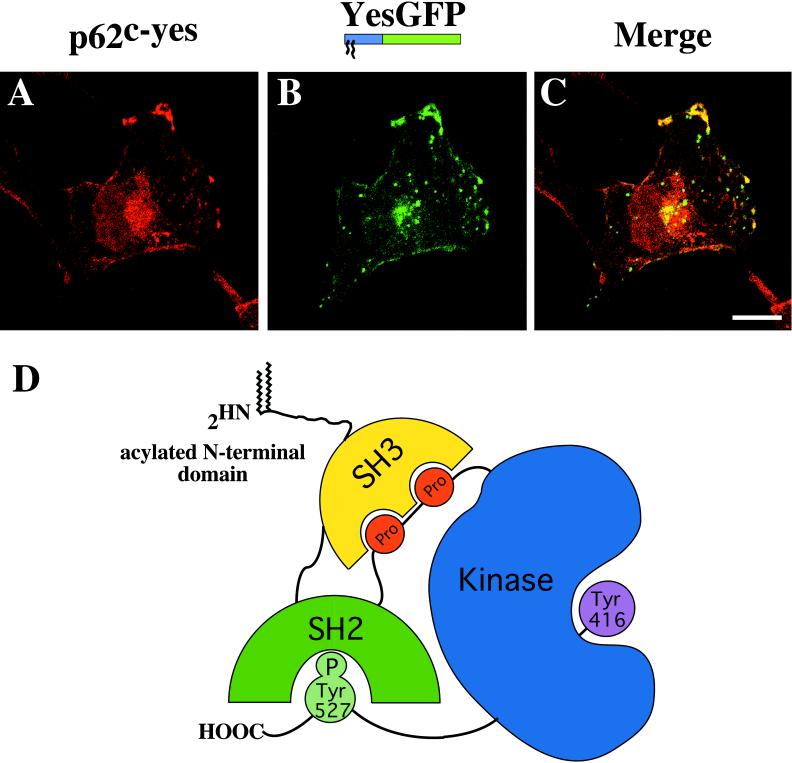
To assess whether the localization mediated by some of the fatty acylated domains appended to GFP reflected the localization of endogenous full-length proteins from which these domains were derived, a double immunofluorescence study was carried out between YesGFP and the full-length Yes PTK, which is expressed in COS-7 cells. As shown in Figure 8, the signals provided by endogenous Yes (Figure 8A, red) and YesGFP (Figure 8B, green) were distributed in a similar manner and, when merged, showed significant colocalization at the plasma membrane and in the perinuclear area (Figure 8C, yellow). Intracellular vesicular structures prominent in COS-7 cells expressing YesGFP were absent or reduced in intensity in images of endogenous Yes PTK fluorescence.
Figure 8.
N-terminal domain YesGFP chimera is localized similarly to endogenous full-length Yes PTK. (A) Endogenous Yes PTK detected with mouse anti-Yes Ab followed by TR-conjugated anti-mouse Ab. (B) transfected YesGFP chimera detected with rabbit anti-GFP Ab followed by FITC-conjugated anti-rabbit Ab. (C) Merged image showing the significant colocalization between the two proteins. (D) Model of the three-dimensional crystal structure of the Src PTK family showing the intramolecular protein domain interactions and putative exposure of the acylated N-terminal region. Bar, 10 μm.
DISCUSSION
N-terminal Fatty Acylated Sequences Mediate Differential Subcellular Localization
We dissected the molecular components of several N-terminal fatty acylated sequences required for plasma membrane localization and identified a series of intracellular membranes/vesicles in which acylated GFPs were also found. These localization patterns could be categorized into two subsets. First, a myristoylated and palmitoylated GlyCys motif or a combination of myristate and a significant polybasic domain (as in Src16GFP) led to endosomal and plasma membrane localization. A reduction in the length and net charge of the polybasic domain in SrcGFP led to a more widespread localization to a variety of intracellular membranes, including the nuclear envelope (e.g., Src14GFP). Second, a doubly palmitoylated (but not myristoylated) motif (GAP-43GFP) conferred localization to the plasma membrane and the Golgi area but not to the endosomes.
In our system, the myristoylated and palmitoylated GlyCys motif (found in Fyn, Yes, Lck, and Gαo) may be necessary and sufficient to confer localization to the plasma membrane and the endosomes. Neither the number/position of palmitates nor the amino acids surrounding the acylation sites influenced localization in our system. Because of the fact that these various acylated sequences conferred similar localization properties to GFP, our results argue in favor of common biophysical properties of these acylated sequences specifying membrane localization. In agreement with this interpretation are recent reports by Melkonian et al. (1999) and Galbiati et al. (1999), who demonstrated that the dually acylated N-terminal motif of Gαi1 was sufficient to target GFP to caveolin-enriched detergent-resistant membranes (DRMs). Also, potential differences in palmitate turnover on the various acylated GFP chimeras did not alter either the apparent subcellular localization, as judged by confocal microscopy, or the ability of chimeric GFPs to associate productively with biological membranes.
Our data clearly demonstrate that the first 11 amino acids of the Yes PTK are sufficient to localize GFP in membrane domains where endogenous full-length Yes PTK can be found. Additionally, the localization of our dually acylated chimeras in COS-7 cells agree with the findings of several previous studies on the subcellular localization of corresponding full-length signaling proteins. Src family PTKs and G protein α subunits are targeted to the plasma membrane and the intracellular membranes, including punctate structures, the TGN, endosomes, and the Golgi area, in many cell types (Krueger et al., 1991; Ley et al., 1994; Denker et al., 1996; van’t Hof and Resh, 1997; Wolven et al., 1997; Galbiati et al., 1999). Also, N-terminal Lck and Fyn fusion chimeras are targeted in a similar manner (Wolven et al., 1997; Zlatkine et al., 1997). However, in contrast to these findings, endogenous Fyn targets exclusively to the centrosomal area in hematopoietic cells (Ley et al., 1994; Campbell et al., 1998), Lck is found only at the plasma membrane in NIH 3T3 cells, and CD4 (a plasma membrane receptor that binds Lck) cotransfection can abrogate TGN/endosome-targeted Lck in HeLa cells (Bijlmakers et al., 1997).
Taken together, these results demonstrate that N-terminal acylation is a key spatial determinant in proper plasma membrane and endosome localization but is likely not the sole mediator of the subcellular localization of the corresponding full-length acylated signaling proteins within cells. As such, cell type–specific factors, protein-protein interaction modules downstream of the acylation sites, the status of palmitoylation, or even protein tertiary conformation may play essential roles in determining the net effect on protein localization. To illustrate this, when Src family PTKs were not activated, the binding sites of SH3 and SH2 domains were occupied by an intramolecular proline-rich region and a C-terminal regulatory phosphotyrosine residue, respectively, in the Hck and Src PTK crystal structures (see model, Figure 8D) (Sicheri et al., 1997; Xu et al., 1997). As such, the SH3 and SH2 domains cannot account for subcellular localization of Src-related PTKs in the inactive state. This strengthens the importance of fatty acylation in proper localization. Furthermore, controlling the presence of palmitates on dually acylated signaling PTKs, or the phosphorylation state of serine residues within the polybasic region of the c-Src N terminus, could allow shuttling between membranes of various organelles and the plasma membrane.
Myristoylated Src16GFP containing additional positively charged residues was capable of specific plasma membrane and endosome localization, with no apparent ER localization, compared with Src14GFP. Interestingly, full-length c-Src has been located at the plasma membrane and the endosomes (Kaplan et al., 1992; David-Pfeuty et al., 1993; Luttrell et al., 1999). Changes in the states of activation and/or phosphorylation of c-Src may result in regulated differential localization (Kaplan et al., 1992; Silverman and Resh, 1992; Schwartzberg, 1998). Clearly, though, the N terminus alone plays an important role in PTK localization.
The similarity in localization mediated by a combination of myristoylation and palmitoylation and myristoylation plus the polybasic domain on GFP was intriguing. It suggests that the inner leaflet of the plasma membrane and the outer leaflet of the endosome exhibit a combination of hydrophobic and electrostatic properties that can accommodate both types of acylated proteins. The concentration of negatively charged phospholipids (e.g., phosphatidylserine) in the inner leaflet of the plasma membrane is well documented (Devaux, 1991; Zwaal and Schroit, 1997). Because a significant portion of these negatively charged phospholipids are known to contain two saturated acyl chains (Holub, 1980), this population could preferentially be found in glycosphingolipid-enriched lipid raft or DRM domains (Harder and Simons, 1997). Through the formation of a liquid-ordered membrane phase, these domains are believed to selectively recruit lipids with saturated acyl chains and proteins modified by such lipids (Brown and London, 1998). In addition, endosomal membranes, which can support a liquid-ordered membrane phase (Brown and London, 1998), are known to be the most net negatively charged of all cellular membranes, as indicated by their characteristic furthest migration toward the anode in free-flow electrophoresis experiments (Cavenaugh et al., 1996). These points illustrate that acylation coupled with a polybasic region could lead to targeting to lipid raft domains in the plasma membrane or endosomes, analogous to myristoylated and palmitoylated proteins. Although not described as a DRM-associating signal by Melkonian et al. (1999), c-Src containing myristate and a polybasic domain has been shown to cofractionate and colocalize with caveolin-1 (Li et al., 1996; Song et al., 1997), which is often enriched in DRMs.
The dipalmitoylation motif, present in GAP-43GFP, appears to be equally effective at directing proteins to the plasma membrane. Immunofluorescence results with a polyclonal GFP Ab (which would detect both mature fluorescent GFP molecules and a nascently synthesized nonfluorescent population) demonstrate that there is a significant intracellular fluorescence concentration that colocalizes with the Golgi marker giantin. Golgi targeting by wild-type and N-terminal chimeras of GAP-43 has been documented previously in COS-7 cells (Liu et al., 1993, 1994; Arni et al., 1998). The association of related N-terminal palmitoylated growth cone proteins, SCG-10 and SNAP-25, with the Golgi compartment has also been reported (Di Paolo et al., 1996; Gonzalo and Linder, 1998). Upon NZ treatment, Golgi localization of GAP-43GFP was abrogated but plasma membrane localization was not. This finding indicates that GAP-43 may be palmitoylated at the plasma membrane and that an intact microtubule network may be required for accumulation of the chimera on the Golgi membranes. Potentially, NZ treatment may also impede palmitoylation of newly synthesized GAP-43GFP. As such, our localization results with the GAP-43GFP chimera are consistent with those reported previously and are consistent with a role for dual palmitoylation in Golgi area localization.
Myristoylation Is Sufficient for Intracellular Membrane Association and Nuclear Exclusion
The inability of myristoylated chimeras to associate productively with the plasma membrane, which represents 5% of total membranes (Alberts et al., 1994), is not well understood. Weak membrane-binding properties of myristate (Shahinian and Silvius, 1995) may allow the rapid dissociation of myristoylated proteins from a variety of membranes. As such, rapid dissociation may represent a means to sample different membranes. Thus, soon after cotranslational myristoylation, more abundant intracellular membrane fractions could sequester most of the myristoylated chimera away from the plasma membrane. To illustrate this possibility, we saw very significant localization of various myristoylated GFPs on ER membranes, which represent up to 60% of total cellular membranes.
Because of the detection capability of the confocal microscope and the significant chimeric protein production in our system, we believe that the presence of GFP chimeras at the plasma membrane should have been detected if myristoylation provides a random means of membrane sampling. Potentially, rapid endocytic events may remove myristoylated GFPs from the inner leaflet of the plasma membrane. This possibility is confirmed by our results, which show that myristoylated GFPs colocalize with the endocytic marker DiI-LDL. In addition, our myristoylated GFPs did not colocalize with the Golgi marker giantin (our unpublished results). Thus, in addition to random sampling of membranes, our data suggest that an exposed myristate (as found in our GFP chimeras) could confer specific localization information that allows enrichment in the ER and in endosomal membranes. This is in contrast to cytosolic myristoylated proteins in which the myristate is sequestered in a hydrophobic pocket (e.g., calcineurin and cAMP-dependent protein kinase) and unable to influence membrane association (Zheng et al., 1993; Griffith et al., 1995).
Our observations are thus consistent with myristoylation acting as an intracellular membrane-associating signal. Although the membrane association of a myristoylated fluorescent peptide in the absence of palmitoylation or polybasic residues has been demonstrated to be transient (Shahinian and Silvius, 1995), myristoylated GFPs in our study were found associated primarily with membranes, as judged by confocal microscopy of living cells and subcellular fractionation. As such, our data are not necessarily consistent with previous data obtained in vitro. Inside the living cell, myristate might contribute a better association with membranes than in vitro, possibly via association with DRMs or lipid rafts. Consistent with this possibility, Utsumi et al. (1996) showed that an N-myristoylated tumor necrosis factor fusion protein was completely bound to dipalmitoylphosphatidylcholine (DPPC) liposomes, whereas the G2A mutant chimera was not. These DPPC liposomes would promote a liquid-ordered membrane phase reminiscent of DRMs or rafts (Brown and London, 1998). Also, a glycosylphosphatidylinositol-linked protein, placental alkaline phosphatase, was Triton X-100 insoluble in dioleoylphosphatidylcholine/DPPC (1:1) liposomes, showing DRM association even when a disordered fluid membrane phase was present (Schroeder et al., 1998).
Intracellular membrane association may explain the nuclear exclusion of acylated proteins, because, other than the nuclear envelope, the nucleus is devoid of membranes. Sequestration of multiple substrate signaling proteins (such as c-Src) outside of the nucleus may be an important factor in preventing interference with nuclear signaling, as proposed by David-Pfeuty et al. (1993).
Evidence for Multiple Palmitoylation Mechanisms
The plasma membrane/endosome localization observed with myristoylated/palmitoylated chimeras versus the plasma membrane/Golgi region localization observed with dually palmitoylated chimeras suggests that two PAT activities may exist. In cases in which the covalent addition of palmitate would act as the retention signal, the localization of putative PAT(s) could play an active role in plasma membrane, endosome, and Golgi localization. Consistent with this possibility, Dunphy et al. (1996) showed that a PAT activity that palmitoylates G protein α subunits is concentrated in the plasma membrane/endosome fraction. In addition, we have characterized a PAT activity that palmitoylates myristoylated PTKs that was enriched in both crude plasma membrane and Golgi membrane fractions and absent in the ER fraction isolated from rat liver (our unpublished results). A second PAT activity presumed to be in the ERGIC/cis-Golgi has been shown to palmitoylate viral glycoproteins and presumably endogenous cellular proteins such as GAP-43, SCG-10, and SNAP-25 (Veit et al., 1996). These proteins are known to use the secretory pathway to reach the plasma membrane and are known to associate with the Golgi/TGN (Di Paolo et al., 1996; Gonzalo and Linder, 1998). It is likely that our GAP-43GFP chimera may be acylated by this PAT activity.
Mechanisms of Fatty Acylation–dependent Subcellular Localization
In terms of area and mass, the plasma membrane and the endosomes represent only a minor membrane component in most eukaryotic cells. Thus, the ∼20- to 50-fold enrichment of dual signal–containing GFPs in the plasma membrane/endosomes compared with other intracellular membranes demonstrates the specificity generated by combining fatty acids and polybasic domains at the N termini of proteins. Overall, our subcellular localization data are consistent with the kinetic bilayer trapping mechanism proposed by Shahinian and Silvius (1995), with palmitoylation acting as a plasma membrane retention signal and with an enhanced function for myristoylation acting as a possible retention signal for endosomes or ER membranes. In the kinetic bilayer trapping model, singly acylated proteins or peptides are postulated to randomly and rapidly diffuse on and off various intracellular membranes until they are retained at their final destination via covalent addition of a second lipid (often palmitate) (Schroeder et al., 1996). In addition, and also consistent with the kinetic bilayer trapping mechanism, the polybasic domain in the Src chimera could act as a retention signal on negatively charged endosomes and inner plasma membrane leaflets.
ACKNOWLEDGMENTS
We thank C. Mattar, Z. Yang, R. Ryan, E. Posse de Chaves, T. Hobman, P. Melancon, and R. Rachubinsky for valuable comments on the manuscript. In addition, we thank V. Chlumecky (University of Alberta) and X.J. Sun (Cross Cancer Institute) for help with the confocal analyses. The University of Alberta Faculty of Medicine Confocal Laser Scanning Microscopy Facility is supported in part by funds from the Medical Research Council of Canada (MRC) and the Alberta Heritage Foundation for Medical Research (AHFMR). L.G.B. is a MRC and AHFMR scholar, and J.B.M. is supported by MRC Ph.D. and AHFMR M.D./Ph.D. studentships.
Abbreviations used:
- Ab
antibody
- DRM
detergent-resistant membrane
- GFP
green fluorescent protein
- IgG
immunoglobulin G
- 125I-IC16
16-[125I]iodohexadecanoic acid
- PAT
protein S-acyltransferase
- PTK
protein tyrosine kinase
- TR
Texas Red
REFERENCES
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. New York: Garland; 1994. The compartmentalization of higher cells; pp. 551–553. [Google Scholar]
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;269:16701–16705. [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–28485. doi: 10.1074/jbc.273.43.28478. [DOI] [PubMed] [Google Scholar]
- Berthiaume L, Peseckis SM, Resh MD. Synthesis and use of iodo-fatty acid analogs. Methods Enzymol. 1995;250:455–466. doi: 10.1016/0076-6879(95)50090-1. [DOI] [PubMed] [Google Scholar]
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–20. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- Bijlmakers M-JJE, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56lck to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Cooper S, Dessing M, Yates S, Buder A. Interaction of p59fyn kinase with the dynein light chain, Tctex-1, and colocalization during cytokinesis. J Immunol. 1998;161:1728–1737. [PubMed] [Google Scholar]
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Cavenaugh MM, Whitney JA, Carroll K, Zhang C-J, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Gao T, Perez-Reyes E, Hosey MM. Membrane targeting of L-type calcium channels. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T, Bagrodia S, Shalloway D. Differential localization patterns of myristoylated and nonmyristoylated c-Src proteins in interphase and mitotic c-Src overexpresser cells. J Cell Sci. 1993;105:613–628. doi: 10.1242/jcs.105.3.613. [DOI] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Lutjens R, Pellier V, Stimpson SA, Beuchat M-H, Catsicas S, Grenningloh G. Targeting of SCG10 to the area of the Golgi complex is mediated by its NH2-terminal region. J Biol Chem. 1996;272:5175–5182. doi: 10.1074/jbc.272.8.5175. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein α subunits and p21RAS. J Biol Chem. 1998;273:15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Greentree WK, Manahan CL, Linder ME. G-protein palmitoyltransferase activity is enriched in plasma membranes. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Linder ME. Signaling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436:245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- Fraser IDC, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of Gi1α is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. J Biol Chem. 1999;274:5843–5850. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- Gerdes H-H, Kaether C. Green fluorescent protein: applications in cell biology. FEBS Lett. 1996;389:44–47. doi: 10.1016/0014-5793(96)00586-8. [DOI] [PubMed] [Google Scholar]
- Girotti M, Banting G. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for rat TGN38. J Cell Sci. 1996;109:2915–2926. doi: 10.1242/jcs.109.12.2915. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Linder ME. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI, Duronio RJ, Rudnick DA, Adams SP, Gokel GW. Protein N-myristoylation. J Biol Chem. 1991;266:8647–8650. [PubMed] [Google Scholar]
- Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, Fleming MA, Caron PA, Hsiao K, Navia MA. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1996;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Holub BJ. The biosynthesis of phosphatidylserines by acylation of 1-acyl-sn-glycero-3-phosphoserine in rat liver. Biochim Biophys Acta. 1980;618:255–262. doi: 10.1016/0005-2760(80)90031-4. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Rockwood JM, Kress Y, Steiner A, Shafit-Zagardo B. Molecular and functional characteristics of MAP-2a: ability of MAP-2a versus MAP-2b to induce stable microtubules in COS cells. Cell Motil Cytoskeleton. 1998;40:272–285. doi: 10.1002/(SICI)1097-0169(1998)40:3<272::AID-CM6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–333. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J. 1994;303:749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J, Zhao YH, Murphy D, Sudol M. Differential expression of p62c-yes in normal, hyperplastic and neoplastic human epidermis. Oncogene. 1991;6:933–940. [PubMed] [Google Scholar]
- Ley SC, Marsh M, Bebbington CR, Proudfoot K, Jordan P. Distinct intracellular localization of lck and fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits and H-Ras share a common membrane-anchored scaffolding protein, caveolin. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fisher DA, Storm DR. Analysis of the palmitoylation and membrane targeting domain of neuromodulin (GAP-43) by site-specific mutagenesis. Biochemistry. 1993;32:10714–10719. doi: 10.1021/bi00091a023. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fisher DA, Storm DR. Intracellular sorting of neuromodulin (GAP-43) mutants modified in the membrane targeting domain. J Neurosci. 1994;14:5807–5817. doi: 10.1523/JNEUROSCI.14-10-05807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, et al. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Morales J, Fishburn CS, Wilson PT, Bourne HR. Plasma membrane localization of Gαz requires two signals. Mol Biol Cell. 1998;9:1–14. doi: 10.1091/mbc.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Olson KR, McIntosh JR, Olmsted JB. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol. 1995;130:639–650. doi: 10.1083/jcb.130.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti M, Vigano MA, Newman CMH, Milligan G, Magee AI. A novel N-terminal motif for palmitoylation of G-protein α subunits. Biochem J. 1993;291:349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. GFP in mammalian cells. Trends Genet. 1995;11:326–327. doi: 10.1016/s0168-9525(00)89092-7. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Innerarity TL, Weinstein JN, Mahley RW. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981;1:177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Ralston E. Changes in architecture of the Golgi complex and other subcellular organelles during myogenesis. J Cell Biol. 1993;120:399–409. doi: 10.1083/jcb.120.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochim Biophys Acta. 1993;1155:307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schroeder H, Leventis R, Shahinian S, Walton PA, Silvius JR. Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently S-acylated at the plasma membrane of mammalian cells. J Cell Biol. 1996;134:647–660. doi: 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmend SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL. The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998;17:1463–1468. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- Shahinian S, Silvius JR. Doubly-lipid-modified sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995;34:3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- Sigal T, Zhou W, Buser CA, McLaughlin S, Resh MD. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc Natl Acad Sci USA. 1994;91:12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman L, Resh MD. Lysine residues form an integral component of a novel NH2-terminal membrane targeting motif for myristylated pp60v-src. J Cell Biol. 1992;119:415–425. doi: 10.1083/jcb.119.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol. 1997;43:293–303. [PubMed] [Google Scholar]
- Utsumi T, Kuranami J, Tou E, Ide A, Akimaru K, Hung M-C, Klostergaard J. In vitro synthesis of an N-myristoylated fusion protein that binds to the liposomal surface. Arch Biochem Biophys. 1996;326:179–184. doi: 10.1006/abbi.1996.0063. [DOI] [PubMed] [Google Scholar]
- van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Revery H, Schmidt MFG. Cytoplasmic tail length influences fatty acid selection for acylation of viral glycoproteins. Biochem J. 1996;318:163–172. doi: 10.1042/bj3180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Sachs K, Heckelmann M, Maretzki D, Hofmann KP, Schmidt MFG. Palmitoylation of rhodopsin with S-protein acyltransferase: enzyme catalyzed reaction versus autocatalytic acylation. Biochim Biophys Acta. 1998;1394:90–98. doi: 10.1016/s0005-2760(98)00097-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–601. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Zheng J, Knighton DR, Xoung N-H, Taylor SS, Sowadski JM, Ten Eyck LF. Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993;2:1559–1573. doi: 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Goodman DW, Karns LR, Fishman MC. The neuronal growth-associated protein GAP-43 induces filopodia in nonneuronal cells. Science. 1989;244:1193–1198. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]
- Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]