Summary
The Ig-ITIM family member PECAM-1 is expressed in vascular and endothelial cells, and its functions include suppression of mitochondria-dependent apoptosis. Previous studies have identified distinct PECAM-1 cytoplasmic domain splice variants at the mRNA, but not protein, level. Several relatively abundant mRNA isoforms lack exon 15 (Δ15) and would theoretically encode a protein with a truncated cytoplasmic domain and a unique C-terminal sequence. Using a novel rabbit polyclonal antibody that specifically recognizes Δ15 PECAM-1, we found that the Δ15 PECAM-1 isoform was expressed in human tissues, including brain, testes and ovary. This isoform was also expressed on the cell surface of human platelets, human umbilical vein endothelial cells (HUVECs) and the Jurkat T-cell leukemia, human erythroleukemia (HEL) and U937 histiocytic lymphoma cell lines. Furthermore, murine platelets and lung lysates demonstrated abundant amounts of exon-15-deficient PECAM-1. Functional studies revealed that Δ15 PECAM-1 retains both its homophilic binding capacity and its ability to signal by means of its immunoreceptor tyrosine-based inhibitory motif (ITIM) domains. Δ15 PECAM-1 was unable, however, to protect against apoptosis induced by overexpression of Bax or treatment with the chemotherapy agent etoposide. These studies suggest a novel role for the PECAM-1 C-terminus in cytoprotective signaling and highlight a need for further characterization of expression of PECAM-1 isoforms in normal and malignant tissues.
Keywords: PECAM-1, CD31, Isoforms, Splice variants, Apoptosis
Introduction
Platelet/endothelial cell adhesion molecule (hereafter referred to as PECAM-1; also known as PECA1 or CD31) is a 130-kDa cell-surface protein that is a member of the immunoglobulin immunoreceptor tyrosine-based inhibitory motif (Ig-ITIM)-containing family (Newman, 1999). PECAM-1 is expressed on endothelial cells and a variety of hematopoietic cells, including most human CD34+ hematopoietic progenitor cells (Watt et al., 1993). PECAM-1 expression is maintained at relatively high levels on cells of the myeloid and megakaryocytic lineages but is selectively lost during erythroid and B-cell (Jackson et al., 2000) differentiation. Mature human platelets constitutively express 5000 to 10,000 PECAM-1 molecules per cell (Mazurov et al., 1991; Metzelaar et al., 1991; Newman, 1994), whereas monocytes and neutrophils display 50,000 to 100,000 molecules per cell under resting conditions. PECAM-1 is also expressed in certain T-cell subsets (Tanaka et al., 1992; Torimoto et al., 1992; Zehnder et al., 1992) and is a major constituent of the intercellular junction of endothelial cells (Muller et al., 1989; Albelda et al., 1990; Newman et al., 1990; Newman, 1994). Interestingly, PECAM-1 has also been shown to be expressed in human cytotrophoblasts (Zhou et al., 1997a; Zhou et al., 1997b), spermatozoa (Nixon et al., 2005) and brown adipose tissue (Rosso and Lucioni, 2006).
PECAM-1 functions to negatively regulate immunoreceptor tyrosine-based activating motif (ITAM) signaling pathways in platelets (Patil et al., 2001; Jones et al., 2001) and lymphocytes (Newton-Nash and Newman, 1999; Henshall et al., 2001; Wilkinson et al., 2002). PECAM-1 also contributes to leukocyte transendothelial migration (Muller and Randolph, 1999; O'Brien et al., 2003), modulates integrin-mediated cell adhesion (Tanaka et al., 1992; Leavesley et al., 1994; Berman and Muller, 1995; Berman et al., 1996; Varon et al., 1998; Chiba et al., 1999; Zhao and Newman, 2001; Dangerfield et al., 2002) and plays a role in angiogenesis (DeLisser et al., 1997; Zhou et al., 1999; Cao et al., 2002). At the physiological level, PECAM-1 protects against septic shock (Maas et al., 2005; Carrithers et al., 2005) and modulates reactive oxygen species produced by coronary microvessels (Liu et al., 2006). Recent studies also indicate that PECAM-1 can protect against cell death in a variety of systems, and its presence can influence a number of classical cell-survival pathways (Noble et al., 1999; Bird et al., 1999; Evans et al., 2001; Ferrero et al., 2003; Gao et al., 2003; Limaye et al., 2005; Bergom et al., 2006).
The extracellular domain of full-length (WT) PECAM-1 consists of six immunoglobulin (Ig)-like domains. Ig domain 1 mediates PECAM-1 trans-homophilic binding (Sun, J. et al., 1996; Sun, Q. et al., 1996; Newton et al., 1997), and antibodies against this region diminish transendothelial migration of leukocytes (Muller et al., 1993; Vaporciyan et al., 1993; Bogen et al., 1994; Liao et al., 1995) and angiogenesis in vivo (Zhou et al., 1999). The PECAM-1 cytoplasmic domain is complex (Kirschbaum et al., 1994), in that it is encoded by eight short exons that exhibit differing susceptibilities to alternative splicing (Baldwin et al., 1994). In its full-length, unspliced form, the cytoplasmic domain contains 118 amino acids, including two ITIM domains and several other sites for posttranslational modifications. A plethora of signaling cascades have been found to emanate from events initiated by cytosolic signaling molecules that associate with the cytoplasmic tail of PECAM-1 (Gibbins, 2002; Jackson, 2003; Newman and Newman, 2003).
Previous studies in a number of laboratories have shown the existence of alternatively spliced PECAM1 mRNAs in human and murine hematopoietic cells, murine embryos and tissues, and human tissues and endothelial cells (reviewed in Newman and Newman, 2003). Such mRNA species are relatively abundant at certain developmental stages (Baldwin et al., 1994) and in certain tissues (Sheibani et al., 1997; Sheibani et al., 1999; Robson et al., 2001; Wang and Sheibani, 2002; Li et al., 2005) and have the potential to encode PECAM-1 isoforms that differ markedly in their biological properties; however, the lack of isoform-specific reagents has limited the detection of PECAM-1 isoforms at the protein level. Notably, all of the exons encoding the cytoplasmic domain of PECAM-1 are phase 1 exons – that is, they end with a nucleotide that becomes part of the first triplet in the codon encoded by the following exon (Fig. 1) – with the exception of exon 15, which is a phase 0 exon. Splicing out of exon 15, therefore, results not only in loss of the amino acids normally encoded by exon 15 but also in a change in the reading frame of downstream exon 16 such that it now encodes a novel C-terminal sequence that ends in the amino acids ENGRLP. Because of the potential for variant PECAM-1 isoforms to confer distinct adhesive and signaling properties to the vascular cells in which they are expressed, we sought to determine whether a PECAM-1 isoform that is missing exon 15 (Δ15) is expressed as a protein in human and murine tissues and, if so, whether it functions differently in its ability to protect cells from apoptosis – a property previously shown to require the PECAM-1 cytoplasmic domain (Bergom et al., 2006). We report herein a potential role for the C-terminal region of PECAM-1 in cytoprotective signaling that might have implications for the expression of different PECAM-1 isoforms during embryogenesis, development and the progression of cancer.
Fig. 1.
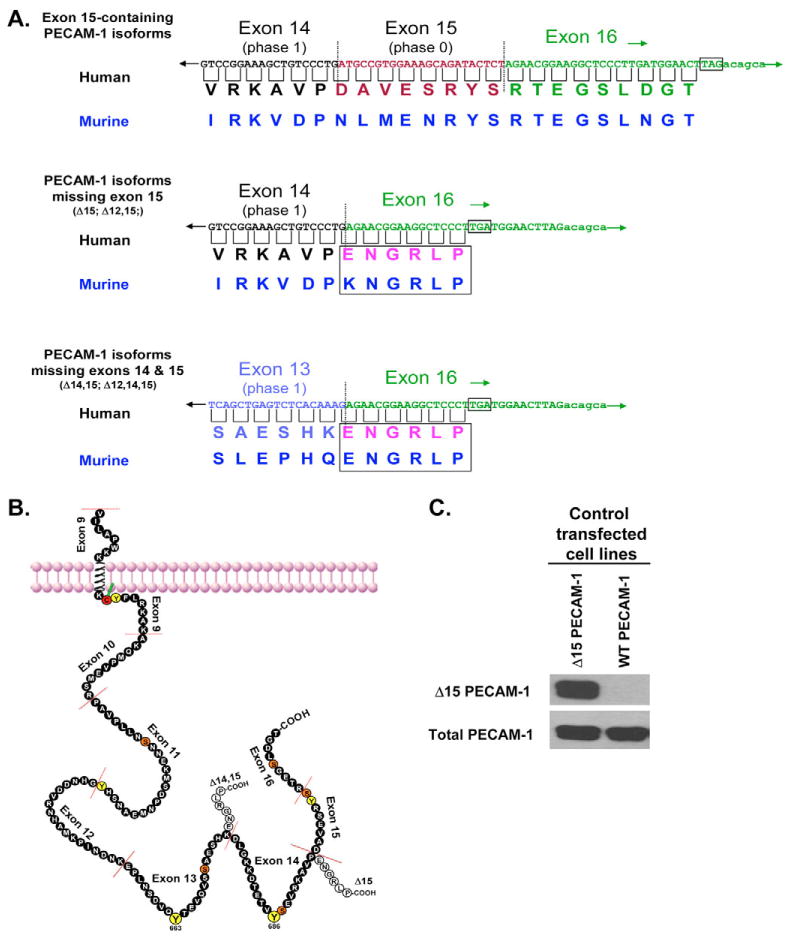
Cytoplasmic domain amino acid sequences for full-length and Δ15-containing isoforms of PECAM-1. (A) Illustration of the cDNA and predicted amino acid sequences for the terminal cytoplasmic tail regions of the PECAM-1 splice variants described in this study. The new C-terminus created by deletion of exon 15 is boxed, and dotted lines indicate splice junctions. Stop codons are also indicated. A schematic diagram of the full-length (filled circles) and alternatively spliced Δ15-containing PECAM-1 cytoplasmic domains (unfilled circles) is shown in (B). Phylogenetically conserved tyrosine residues are colored yellow, whereas conserved serine residues are colored orange. Note that the Δ15 form is missing two serine residues and one tyrosine residue and has a novel six-amino-acid C-terminal sequence. (C) A rabbit polyclonal antibody produced against the novel Δ15 PECAM-1 C-terminal region reacts with human Δ15 PECAM-1, but not full-length WT PECAM-1, in HEK293 cells transfected with specific cDNAs encoding these isoforms. Fig. 1A,B are adapted, with permission, from Newman and Newman (Newman and Newman, 2003).
Results
Δ15 PECAM-1 protein is expressed in a variety of human and murine cell lines and tissues
PECAM1 mRNA splice variants have been reported in a variety of studies (Li et al., 2005; Yan et al., 1995a; Sheibani et al., 1999; Robson et al., 2001; Newman et al., 1987; Goldberger et al., 1994; Baldwin et al., 1994; Wang and Sheibani, 2002; Wang et al., 2003; Wang et al., 2004; Kirschbaum et al., 1994; Aroca et al., 1999), sometimes being present at relatively high levels (Li et al., 2005; Sheibani et al., 1999; Robson et al., 2001; Wang and Sheibani, 2002). We sought to determine whether isoforms lacking exon 15 were also expressed at the protein level in human and murine tissues. Exon 15 is out of phase with its upstream and downstream exons; thus, its deletion generates a novel C-terminal peptide, ENGRLP (Fig. 1A,B). Using this novel C-terminal peptide as an immunogen, we were able to generate a rabbit polyclonal antibody that is highly specific for PECAM-1 isoforms that lack exon 15. As shown in Fig. 1C, this polyclonal antibody against Δ15 PECAM-1 specifically detects the product of an enforced cDNA that encodes Δ15 PECAM-1 but not full-length PECAM-1.
Using this Δ15 PECAM-1-specific antibody, we were able to examine the expression of Δ15 PECAM-1 in human tissues. Lysates prepared from normal human tissues were analyzed by western blot for expression of full-length and Δ15 PECAM-1 isoforms. In addition, using cell-surface immunoprecipitation, we examined the expression of Δ15 PECAM on human platelets, human umbilical vein endothelial cells (HUVECs) and the Jurkat T-cell leukemia, human erythroleukemia (HEL) and U937 histiocytic lymphoma cell lines. As shown in Fig. 2A, lysates of human ovaries exhibit moderate levels of PECAM-1, with only a small percentage being the Δ15 PECAM-1 isoform. By contrast, human brain and cultured HUVECs contain a much greater proportion of Δ15 PECAM-1. Spleen and lung lysates have little or no Δ15 PECAM-1, whereas human testes express a small amount of both Δ15 and WT PECAM-1 isoforms. The hematopoietic cancer cell lines express variable proportions of Δ15 PECAM-1, with Jurkat cells having only a fraction of this isoform, and HEL and U937 cells having a large proportion of Δ15 PECAM-1. Interestingly, whereas human platelets express predominantly WT PECAM-1 (Fig. 2A), murine platelets express abundant amounts of the Δ15 isoform (Fig. 2B). From these data, we conclude that the Δ15 isoform of PECAM-1 is present at the cell surface and is expressed at substantial levels in select human and murine tissues. This led us to examine the physiological consequences, if any, of expressing Δ15 PECAM-1.
Fig. 2.

PECAM-1 isoforms lacking exon 15 are expressed in variable amounts in human and murine tissues. (A) Normal human tissue lysates were immunoblotted for Δ15 and total PECAM-1 protein. To determine Δ15 PECAM-1 expression in platelets, HUVECs and transformed human hematopoietic cells, antibody against PECAM-1 was incubated with whole cells, after which the cells were washed, lysed and cell-bound PECAM-1 was immunoprecipitated. PECAM-1 immunoprecipitates were probed for Δ15 and total PECAM-1 antigen. Note that varying relative amounts of Δ15 PECAM-1 are present in human ovary, brain and testes and that Δ15 PECAM-1 is on the surface of HUVECs, platelets and cancer cells. PECAM-1 antigen migrates with different electrophoretic mobilities in different tissues, probably owing to differences in glycosylation. (B) Murine PECAM-1 immunoprecipitates were probed for expression of total PECAM-1 and Δ15 PECAM-1. Note that a large proportion of the total PECAM-1 expressed in murine platelets and lung tissue is lacking exon 15. All results are representative of at least two independent experiments.
The Δ15 PECAM-1 isoform contains functional ITIM domains and is localized to cell-cell junctions
The PECAM-1 cytoplasmic domain transduces intracellular signals by becoming tyrosine phosphorylated on its paired ITIM domains and recruiting signaling molecules containing Src-homology 2 (SH2) domains, most notably the protein-tyrosine phosphatase SHP-2 (Jackson et al., 1997b; Masuda et al., 1997; Sagawa et al., 1997). Although the Δ15 form of PECAM-1 lacks both exons 15 and 16, it has intact ITIM domains, which are located within exons 13 and 14 (Fig. 1B). As shown in Fig. 3, the Δ15 PECAM-1 isoform becomes tyrosine phosphorylated in response to pervanadate stimulation at least as well as does WT PECAM-1. The absence of tyrosine phosphorylation on the ITIM-less (Y663,668F) form of PECAM-1 indicates that these ITIM tyrosine residues are the only phosphorylated sites on full-length PECAM-1, confirming earlier studies (Jackson et al., 1997a). Fig. 3 also demonstrates that SHP-2 is able to be recruited by both WT and Δ15 forms of PECAM-1 when they are tyrosine phosphorylated. As binding of SHP-2 to PECAM-1 requires that both ITIM tyrosine residues be phosphorylated (Jackson et al., 1997a), these data predict that Δ15 PECAM-1 should be able to transmit ITIM-dependent signals.
Fig. 3.

The Δ15 PECAM-1 isoform becomes tyrosine phosphorylated on its ITIMs and binds to the phosphatase SHP-2. REN mesothelioma cells expressing equivalent amounts of WT, ITIM-less or Δ15 PECAM-1 were stimulated with pervanadate and PECAM-1 was immunoprecipitated. Upon stimulation, WT PECAM-1 became tyrosine phosphorylated, as indicated by the phosphotyrosine immunoblot, and bound to SHP-2. By contrast, the Y663,686F ITIM-less form of PECAM-1 neither becomes tyrosine phosphorylated nor binds to SHP-2. Similar to WT PECAM-1, the Δ15 PECAM-1 isoform was able to become tyrosine phosphorylated and bind to SHP-2.
We have previously reported that the PECAM-1 extracellular domain is all that is required to localize PECAM-1 to cell-cell borders, as neither deletion nor substitution mutations within the cytoplasmic domain had effects on the localization of PECAM-1 to cell-cell junctions (Sun et al., 2000). By contrast, a single lysine-to-alanine mutation within the homophilic-binding Ig domain 1 (K89A) prevents PECAM-1 from accumulating at cell-cell junctions (Sun et al., 2000). Using REN mesothelioma cell lines (Gurubhagavatula et al., 1998; O'Brien et al., 2001) stably transfected with human WT, ITIM-less, Δ15 and K89A forms of PECAM-1, we examined the subcellular localization of PECAM-1 using confocal immunofluorescence microscopy. As shown in Fig. 4, there is no detectable difference in the ability of WT, ITIM-less or Δ15 PECAM-1 to concentrate at cell-cell borders. As expected, because K89A PECAM-1 lacks homophilic binding capability, it is distributed over the entire plasma membrane (Fig. 4D). Thus, Δ15 PECAM-1 retains normal homophilic binding characteristics and junctional localization, becomes tyrosine phosphorylated and recruits SHP-2 upon activation.
Fig. 4.

The Δ15 PECAM-1 isoform localizes to cell-cell borders, similar to full-length PECAM-1. REN mesothelioma cells expressing equivalent amounts of WT, ITIM-less, Δ15 and K89A PECAM-1 were grown to confluence, fixed, permeabilized and then subsequently stained with fluorescently labeled antibodies against PECAM-1 and analyzed using confocal microscopy. The panels on the left show the XY views, whereas panels on the right illustrate the xz cross-sections. As described previously, WT PECAM-1 is concentrated at cell-cell junctions (A), whereas a mutant form (K89A) unable to mediate homophilic binding is distributed along the apical surface of the cells, as illustrated in the xz panels (D). Both the ITIM-less (B) and Δ15 PECAM-1 (C) forms localized normally at cell-cell borders, similar to WT PECAM-1.
Δ15 PECAM-1 is not able to rescue cells from apoptosis induced by overexpression of Bax or chemotherapy treatment
PECAM-1 has previously been shown to confer cytoprotection to cells subjected to the mitochondria-dependent, intrinsic pathway of apoptosis (Gao et al., 2003). However, recent work has shown that, at least in certain systems, the PECAM-1–SHP-2 signaling complex might not fully account for the cytoprotective effects of PECAM-1 (Bergom et al., 2006). To determine whether the changes in the Δ15 cytoplasmic domain alter intracellular survival signals emanating from PECAM-1, we examined whether Δ15 PECAM-1 is able to protect cells efficiently against cell death induced by overexpression of Bax in a HEK293T cell model system (Gao et al., 2003). As shown in Fig. 5A, whereas WT PECAM-1 is able to reduce apoptosis induced by Bax overexpression by ∼40%, Δ15 PECAM-1 failed to protect against Bax-induced cell death. The ITIM-less form of PECAM-1 also failed to protect against cell death, as reported previously (Gao et al., 2003). These data demonstrate that both ITIM-mediated signals as well as the WT C-terminus of PECAM-1 are important for PECAM-1-mediated protection against apoptosis induced by overexpression of Bax in these cells.
Fig. 5.

The PECAM-1 Δ15 isoform fails to mediate full protection against apoptosis induced by overexpression of Bax and by treatment with etoposide. (A) HEK293T cells were transiently transfected with plasmids encoding the indicated proteins. 24 hours after transfection, the cells were lysed and their caspase-3 activity was determined, as described in Materials and Methods. As reported previously, WT PECAM-1 protected against apoptosis induced by overexpression of Bax, but neither the ITIM-less nor the Δ15 form of PECAM-1 provided cytoprotection against overexpression of Bax. (B) REN mesothelioma cells expressing approximately equivalent amounts of WT PECAM-1 or Δ15 PECAM-1 were generated. After 48 hours, caspase-3 activity was determined. Cells expressing Δ15 PECAM-1 provided far less cytoprotection than that afforded by WT PECAM-1 (C). The values shown are the mean±s.d. The results are representative of at least two independent experiments performed in duplicate or triplicate; *P≤0.0002, **P≤0.05, derived using unpaired Student's t test.
Because we have recently found that PECAM-1 expression confers resistance to chemotherapy-induced apoptosis (Bergom et al., 2006), we next examined whether Δ15 PECAM-1 is able to confer resistance to chemotherapy-induced cell death to the same extent as WT PECAM-1. REN cells expressing similar amounts of WT and Δ15 PECAM-1 (Fig. 5B) were stimulated with the genotoxic chemotherapeutic drug etoposide and apoptosis was then assessed. As shown in Fig. 5C, Δ15 PECAM-1 conferred significantly less cytoprotection than did its WT counterpart. Taken together, these data demonstrate that Δ15 PECAM-1 lacks the full anti-apoptotic function conferred by WT PECAM-1, suggesting a novel role for the wild-type C-terminal region of PECAM-1 in cytoprotective signaling.
Discussion
Here, we sought to determine whether the Δ15 PECAM-1 isoform is expressed at the protein level in human and murine tissues and whether human Δ15 PECAM-1 produces different signals compared with WT PECAM-1. Because the Δ15 form of PECAM-1 ends in a unique C-terminus, we were able to develop a novel antibody specific for forms of PECAM-1 that lack exon 15 (Fig. 1).
Using the Δ15 PECAM-1-specific antibody, we demonstrate here that a variety of human and murine tissues do indeed express Δ15 PECAM-1 protein (Fig. 2). Initially, tissue lysates were examined for reactivity with the Δ15 PECAM-1 antibody. The results from these studies merely indicated that this protein was synthesized by cells, but they did not demonstrate that the protein traffics to the cell surface. Therefore, we also immunoprecipitated PECAM-1 from whole cells and subsequently immunoblotted for Δ15 PECAM-1 to determine whether Δ15 PECAM-1 is present at the surface of primary cells and cell lines. Notably, human brain tissue expresses a substantial amount of Δ15 PECAM-1. The cells on which Δ15 PECAM-1 is expressed in the brain could include not only endothelium but also microglial or other central nervous system cells. Human ovary and testes also express Δ15 PECAM-1. When compared with the total amount of PECAM-1 present in human testes tissue, the amount of the Δ15 isoform expressed appears to be proportionally large. The role that PECAM-1 plays in human reproductive tissues is largely unknown, but prior work has demonstrated that PECAM-1 is expressed in human spermatozoa and that PECAM-1 might in part be responsible for capacitation-associated signaling cascades (Nixon et al., 2005). In addition, PECAM-1 expression has been described in subpopulations of cytotrophoblasts (Zhou et al., 1997a; Zhou et al., 1997b). HUVECs also have a relatively small, but easily detectable, amount of Δ15 PECAM-1 protein, and human cancer cell lines can express substantial amounts of Δ15 PECAM-1 on the cell surface. Taken together, these data suggest that the proportion of Δ15 PECAM-1 expressed varies in different vascular beds.
Most strikingly, murine, but not human, platelets and lung tissue from C57BL/6 mice express a considerable proportion of PECAM-1 that lacks exon 15 (Fig. 2B). This might have wide-ranging implications for the way in which prior studies of PECAM-1 function in murine tissues have been interpreted, most of which have been performed in the C57BL/6 strain. Interpretation of these studies was based on the assumption that the majority of PECAM-1 in WT mice is the full-length form. It is not known whether other murine strains also express such large proportions of exon-15-deficient PECAM-1 protein, although, in platelets from FVB/N and C129 strains, the majority of PECAM1 mRNA lacks exon 15 (Wang and Sheibani, 2002). Interestingly, studies examining the effect of PECAM-1 on leukocyte transmigration found that C57BL/6, but not FVB/N, mice are uniquely able to compensate for the loss of PECAM-1 function (Schenkel et al., 2004). It is possible that WT mouse strains express a different array of isoforms of PECAM-1 in leukocytes and/or in endothelial cells, and this in part accounts for the differences in leukocyte transmigration seen between strains. PECAM-1 has also been implicated in modulating platelet signaling (Falati et al., 2006; Jones et al., 2001; Patil et al., 2001), as well as murine megakaryocytopoiesis (Dhanjal et al., 2007; Wu et al., 2007). Once again, it is not known whether certain alternatively spliced isoforms of PECAM-1 are more efficient than others at eliciting these responses. Because the data in Fig. 2 demonstrate that isoforms lacking exon 15 are differentially expressed at the protein level in a variety of human and murine tissues, re-examination of the ability of Δ15 PECAM-1 to signal might be warranted.
Many of the functions of PECAM-1 stem from its ability to bind homophilically to other PECAM-1 molecules and to signal by means of its cytoplasmic ITIMs. Thus, we sought to determine whether the Δ15 isoform of PECAM-1 can similarly perform these functions. Prior studies of murine Δ15 PECAM-1 in Madin-Darby canine kidney (MDCK) cells indicated that it is unable to localize to adherens junctions when compared with murine Δ14,15 PECAM-1 (Sheibani et al., 2000). Unfortunately, these studies did not compare Δ15 PECAM-1 with WT PECAM-1, and the human counterparts of these isoforms were not examined in human cells. In the present investigation, human Δ15 PECAM-1 became concentrated normally at cell-cell borders in human cells, and cells expressing Δ15 PECAM-1 largely exhibited a similar morphology to those cells expressing WT PECAM-1 (Fig. 4). In addition, we determined that human Δ15 PECAM-1 can signal through its ITIM domains (Fig. 3). This suggests that the functions of human PECAM-1 that are mediated merely by extracellular region engagement and/or ITIM-mediated signaling might be largely intact in cells expressing the Δ15 PECAM-1 isoform.
Both ITIM-mediated signals and homophilic binding have previously been shown to be required for PECAM-1 to protect efficiently against programmed cell death (Gao et al., 2003). However, even though Δ15 PECAM-1 retains the ability to both signal through its ITIM domains (Fig. 3) and localize to cell-cell borders (Fig. 4), it surprisingly lacks the full cytoprotective function when compared with WT PECAM-1, when apoptosis is induced both by overexpression of Bax and by treatment with a chemotherapy agent (Fig. 5). This suggests a novel role for the C-terminal region of the PECAM-1 cytoplasmic domain in PECAM-1-mediated cytoprotection. While it is unclear what this role might be, it is tempting to speculate that failure to recruit a cytosolic binding partner that normally associates with an amino acid sequence found in WT, but not Δ15, PECAM-1 accounts for this difference. Identification of such proteins is the subject of current investigations.
A diminished ability to exhibit cytoprotection is one of several potential functional variations between WT and Δ15 PECAM-1. Further studies will be necessary to determine whether expression of Δ15 PECAM-1 has additional functional consequences. Even if further differences are uncovered between these PECAM-1 isoforms, the phenotypic effect might be minimal unless the Δ15 PECAM-1 is expressed at high levels or exhibits dominant-negative or positive effects. However, our results demonstrate that large relative fractions of exon-15-deficient PECAM-1 are expressed in at least a few cell types. It is in these tissues where functional differences are more likely to result in a change of cellular phenotype.
Taken together, the data in the present investigation demonstrate, first, that Δ15 PECAM-1 is expressed in both murine and human tissues and cell lines and, second, that Δ15 PECAM-1 retains homophilic binding characteristics and is able to signal through its ITIM domains, yet, despite this, it fails to offer appreciable cytoprotection against apoptosis when compared with WT PECAM-1. These studies suggest a novel role for the C-terminal region of the PECAM-1 cytoplasmic domain in cytoprotective signaling and highlight a need for further studies on the implications of the expression of this, sometimes abundant, alternatively spliced isoform of PECAM-1. The Δ15-specific antibody against PECAM-1 described here is one tool that can aid in the characterization of the expression of PECAM-1 isoforms in cancers and other tissues.
Materials and Methods
Antibodies and reagents
Human tissue lysates were purchased from Research Diagnostics (Flanders, NJ) and prepared following the manufacturer's instructions. Complete Protease Inhibitor Cocktail tablets were from Roche Applied Science (Indianapolis, IN). Cell culture reagents were purchased from Mediatech (Herndon, VA), and all other reagents were from Sigma-Aldrich (St Louis, MO) unless otherwise specified.
Anti-PECAM-1 monoclonal antibody (mAb) 390 (Baldwin et al., 1994) was a kind gift from Steven M. Albelda (University of Pennsylvania School of Medicine). Rabbit anti-human SHP-2 (C-18) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish-peroxidase-conjugated anti-phosphotyrosine antibody (PY-20) was purchased from Zymed Laboratories (South San Francisco, CA). Rabbit anti-human Bax and β-tubulin antibodies were from Pharmingen/BD Biosciences (Bedford, MA). PECAM-1-specific mAb 1.3 and rabbit polyclonal anti-human PECAM-1 have been extensively characterized and described previously (Jackson et al., 1997a; Yan et al., 1995b).
A murine mAb specific for murine PECAM-1 (muPECAM-1) was prepared by immunizing PECAM-1-deficient mice (Duncan et al., 1999) with purified muPECAM-1. muPECAM-1 was purified on a mAb 390 affinity column from a lysate of muPECAM-1-transfected L-cells (Baldwin et al., 1994). The column was prepared using an ImmunoPure Protein G IgG Plus Orientation Kit from Pierce Biotechnology (Rockford, IL). The immunogen comprised purified muPECAM-1 reduced with 10 mM dithiothreitol (DTT) and alkylated with 30 mM iodoacetamide, mixed with an equal amount of nonreduced muPECAM-1.
Creation of the Δ15 PECAM-1 cDNA construct and specific antibody
A human Δ15 PECAM-1 construct was produced using standard gene splicing by extension overlap PCR techniques. Using PECAM-1 in pcDNA3 as a template, Δ15 PECAM-1 was created using the PECAM-1 primer #1 (5′-GAGAAAAAGAGGCAAACCC-3′) and exon 14-16 reverse primer (5′-AAGGGAGCCTTCCGTTCTCAGGGACAGCTTTCCGGA-3′). A second product was prepared using exons 14-16 (5′ -TCCGGAAAGCTGTCCCTGAGAACGGAAGGCTCCCTT-3′) and PECAM-1 primer #2 (5′-CCCTCTGTATCTCTTTCTAC-3′). The products of both of these reactions were mixed together with PECAM-1 primer #3 (5′-ATGCCAGTGGAAATGTCC-3′) and PECAM-1 primer #2, and a secondary overlap PCR was performed. The final amplified product was digested with Tth111I and Bsu36I (New England Biolabs, Ipswich, MA) into a similarly digested WT PECAM-1 cDNA cloned into pGEM7. This mutated PECAM-1 cDNA was removed from pGEM7 with EcoRI and inserted into the mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA) within the multiple cloning site at EcoRI. The constructs were confirmed by nucleotide sequencing.
In order to create a rabbit polyclonal antibody specific for the unique C-terminus of Δ15 PECAM-1, a peptide was synthesized using standard FMOC protocols on an ABI 433 instrument. Peptide mass was verified by MALDI-TOF mass spectrometry. The peptide sequence, C-GGG-ENGRLP, contained a C for coupling to the hapten carrier maleimide-activated KLH, along with a GGG spacer for increased immunogenicity. The polyclonal antibody serum was prepared by Cocalico Biologicals (Reamstown, PA).
Cell lines
The human HEK293, HEL, U937 and Jurkat cells were obtained from the American Type Culture Collection (Manassas, VA), and the human REN mesothelioma cells (Smythe et al., 1994) were a kind gift from Steven Albelda (Department of Medicine, University of Pennsylvania). Cells were cultured in DMEM (HEK293) or RPMI media containing 10% heat-inactivated fetal bovine serum and 40 mg/ml gentamicin. Human umbilical vein endothelial cells (HUVECs) were cultured as described previously (Maas et al., 2003).
Stable REN cell lines were established by transfecting cells as described previously (Bergom et al., 2006). The PECAM-1-expressing cells were then sorted using fluorescence activated cell sorting (FACS) (FACSDiVa, BD Biosciences) to obtain a mixed population of cells that express PECAM-1.
Immunoblotting and whole-cell immunoprecipitation analysis
Lysates were prepared from HEK293 cells stably transfected with either human PECAM-1 or Δ15 PECAM-1 (Maas et al., 2003). PECAM-1 was immunoprecipitated with PECAM-1.3 (Jackson et al., 1997a) incubated overnight at 4°C. Platelets were isolated as described previously (Rathore et al., 2003). Whole-cell immunoprecipitation was performed on human platelets, HUVECs, Jurkat, HEL and U937 cells. PECAM-1 1.3 mAb was added to cell suspensions at 10 μg/ml and incubated for 90 minutes. The cells were then washed and lysed with Triton X-100 lysis buffer. Murine lungs were harvested from C57BL/6 mice that had been perfused with saline. The lungs were placed in hypotonic lysis buffer (25 mM HEPES, 10 mM EDTA pH 7.5) with protease inhibitors and sonicated. An equal volume of 2× Triton X-100 lysis buffer was added. The sample was mixed for 90 minutes at 4°C and insoluble proteins removed by centrifugation. muPECAM-1 was immunoprecipitated with mAb 390.
Normal human tissue lysates (Research Diagnostics) were prepared according to the manufacturer's instructions, and 50 μg of lysate was loaded per lane. All immunoblotting results are representative of at least two independent experiments.
Confocal microscopic analysis of PECAM-1-expressing REN cells
REN cell lines stably expressing human WT, ITIM-less, K89A or Δ15 PECAM-1 were cultured in Falcon culture slides (BD Biosciences) and allowed to grow to confluence. Cells were prepared as described previously (Maas et al., 2003). Slides were examined with a Leica TCS SP2 confocal microscope with a 100× oil-immersion lens and analyzed with MetaMorph software (Molecular Devices, Sunnyvale, CA).
Stimulation with H2O2 and co-immunoprecipitation
REN cells stably transfected with PECAM-1 variants were harvested with trypsin and washed into RPMI with no serum. Cells were equilibrated at 37°C for 15 minutes and then stimulated with a mixture of 750 μM H2O2 and 1 μM sodium orthovanadate (pervanadate) at 37°C for 5 minutes. Lysates and immunoprecipitates were prepared as described above. PY-20 Ab (Zymed) was used to detect tyrosine phosphorylation.
In vitro induction and quantification of apoptosis
HEK293T cells were seeded in six-well plates and allowed to grow overnight. Bax-overexpression-induced apoptosis assays were then performed as described previously (Gao et al., 2003). A portion of the lysate was also subjected to SDS-PAGE and immunoblotted. Values for caspase activation were normalized by setting the value for vector cells treated with etoposide to 1.0. For pooled data, individual data points from multiple experiments performed in triplicate were combined to determine the mean±s.d. Quantified apoptosis data are shown as the mean±s.d. P values were derived using an unpaired Student's t test.
Examination of etoposide-induced cell death in REN cells was performed as described previously (Bergom et al., 2006). Quantified apoptosis data are shown as the mean±s.d. of representative results of at least three experiments performed in duplicate or triplicate. Values for control-siRNA-expressing cells treated with high-dose etoposide were normalized to 1.0. P values were derived using an unpaired Student's t test.
Acknowledgments
This work was supported by grants HL-40926 (to P.J.N.) from the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by a P.E.O. Scholar Award (to C.B.) from the P.E.O. Foundation.
References
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca F, Renaud W, Bartoli C, Bouvier-Labit C, Figarella-Branger D. Expression of PECAM-1/CD31 isoforms in human brain gliomas. J Neurooncol. 1999;43:19–25. doi: 10.1023/a:1006233816724. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, Newman PJ. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–1707. doi: 10.4161/cbt.5.12.3467. [DOI] [PubMed] [Google Scholar]
- Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Berman ME, Xie Y, Muller WA. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and β2 integrin activation. J Immunol. 1996;156:1515–1524. [PubMed] [Google Scholar]
- Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112:1989–1997. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba R, Nakagawa N, Kurasawa K, Tanaka Y, Saito Y, Iwamoto I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of αvβ3 integrin and enhances β1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood. 1999;94:1319–1329. [PubMed] [Google Scholar]
- Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates α6β1 on transmigrated neutrophils in vivo and plays a functional role in the ability of α6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Dhanjal TS, Pendaries C, Ross EA, Larson MK, Protty MB, Buckley CD, Watson SP. A novel role for PECAM-1 in megakaryocytokinesis and recovery of platelet counts in thrombocytopenic mice. Blood. 2007;109:4237–4244. doi: 10.1182/blood-2006-10-050740. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, et al. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Evans PC, Taylor ER, Kilshaw PJ. Signaling through CD31 protects endothelial cells from apoptosis. Transplantation. 2001;71:457–460. doi: 10.1097/00007890-200102150-00020. [DOI] [PubMed] [Google Scholar]
- Falati S, Patil S, Gross PL, Stapleton M, Merrill-Skoloff G, Barrett NE, Pixton KL, Weiler H, Cooley B, Newman DK, et al. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107:535–541. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E, Belloni D, Contini P, Foglieni C, Ferrero ME, Fabbri M, Poggi A, Zocchi MR. Transendothelial migration leads to protection from starvation-induced apoptosis in CD34+CD14+ circulating precursors: evidence for PECAM-1 involvement through Akt/PKB activation. Blood. 2003;101:186–193. doi: 10.1182/blood-2002-03-0768. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- Gibbins JM. The negative regulation of platelet function: extending the role of the ITIM. Trends Cardiovasc Med. 2002;12:213–219. doi: 10.1016/s1050-1738(02)00164-0. [DOI] [PubMed] [Google Scholar]
- Goldberger A, Middleton KA, Oliver JA, Paddock C, Yan HC, DeLisser HM, Albelda SM, Newman PJ. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J Biol Chem. 1994;269:17183–17191. [PubMed] [Google Scholar]
- Gurubhagavatula I, Amrani Y, Pratico D, Ruberg FL, Albelda SM, Panettieri RA., Jr Engagement of human PECAM-1 (CD31) on human endothelial cells increases intracellular calcium ion concentration and stimulates prostacyclin release. J Clin Invest. 1998;101:212–222. doi: 10.1172/JCI269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall TL, Jones KL, Wilkinson R, Jackson DE. Src homology 2 domain-containing protein-tyrosine phosphatases, SHP-1 and SHP-2, are required for platelet endothelial cell adhesion molecule-1/CD31-mediated inhibitory signaling. J Immunol. 2001;166:3098–3106. doi: 10.4049/jimmunol.166.5.3098. [DOI] [PubMed] [Google Scholar]
- Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540:7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997a;272:24868–24875. doi: 10.1074/jbc.272.40.24868. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem. 1997b;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Jackson DE, Gully LM, Henshall TL, Mardell CE, Macardle PJ. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is associated with a naive B-cell phenotype in human tonsils. Tissue Antigens. 2000;56:105–116. doi: 10.1034/j.1399-0039.2000.560201.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hughan SC, Dopheide SM, Farndale RW, Jackson SP, Jackson DE. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood. 2001;98:1456–1463. doi: 10.1182/blood.v98.5.1456. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- Leavesley DI, Oliver JM, Swart BW, Berndt MC, Haylock DN, Simmons PJ. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+ hemopoietic progenitor cells. J Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- Li ZJ, Wang ZZ, Zheng YZ, Xu B, Yang RC, Scadden DT, Han ZC. Kinetic expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) during embryonic stem cell differentiation. J Cell Biochem. 2005;95:559–570. doi: 10.1002/jcb.20436. [DOI] [PubMed] [Google Scholar]
- Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–3177. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R57–R65. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible PECAM-1 tyrosine phosphorylation and SHP-2 binding. Am J Physiol Heart Circ Physiol. 2003;285:H2336–H2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- Mazurov AV, Vinogradov DV, Kabaeva NV, Antonova GN, Romanov YA, Vlasik TN, Antonov AS, Smirnov VN. A monoclonal antibody, VM64, reacts with a 130 kDa glycoprotein common to platelets and endothelial cells: heterogeneity in antibody binding to human aortic endothelial cells. Thromb Haemost. 1991;66:494–499. [PubMed] [Google Scholar]
- Metzelaar MJ, Korteweg J, Sixma JJ, Nieuwenhuis HK. Biochemical characterization of PECAM-1 (CD31 antigen) on human platelets. Thromb Haemost. 1991;66:700–707. [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci. 1994;714:165–174. doi: 10.1111/j.1749-6632.1994.tb12041.x. [DOI] [PubMed] [Google Scholar]
- Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Doers MP, Gorski J. Molecular cloning of a 130 kD membrane glycoprotein expressed on human platelets, umbilical vein endothelial cells, and human erythroleukemia (HEL) cells. J Cell Biol. 1987;105:53a. meeting abstract. [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–688. [PubMed] [Google Scholar]
- Nixon B, Paul JW, Spiller CM, Attwell-Heap AG, Ashman LK, Aitken RJ. Evidence for the involvement of PECAM-1 in a receptor mediated signal-transduction pathway regulating capacitation-associated tyrosine phosphorylation in human spermatozoa. J Cell Sci. 2005;118:4865–4877. doi: 10.1242/jcs.02604. [DOI] [PubMed] [Google Scholar]
- Noble KE, Wickremasinghe RG, DeCornet C, Panayiotidis P, Yong KL. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J Immunol. 1999;162:1376–1383. [PubMed] [Google Scholar]
- O'Brien CD, Ji G, Wang YX, Sun J, Krymskaya VP, Ruberg FL, Kotlikoff MI, Albelda SM. PECAM-1 (CD31) engagement activates a phosphoinositide-independent, nonspecific cation channel in endothelial cells. FASEB J. 2001;15:1257–1260. doi: 10.1096/fj.00-0467fje. [DOI] [PubMed] [Google Scholar]
- O'Brien CD, Lim P, Sun J, Albelda SM. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003;101:2816–2825. doi: 10.1182/blood-2002-08-2396. [DOI] [PubMed] [Google Scholar]
- Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97:1727–1732. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- Rathore V, Stapleton MA, Hillery CA, Montgomery RR, Nichols TC, Merricks EP, Newman DK, Newman PJ. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102:3658–3664. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- Robson P, Stein P, Zhou B, Schultz RM, Baldwin HS. Inner cell mass-specific expression of a cell adhesion molecule (PECAM-1/CD31) in the mouse blastocyst. Dev Biol. 2001;234:317–329. doi: 10.1006/dbio.2001.0274. [DOI] [PubMed] [Google Scholar]
- Rosso R, Lucioni M. Normal and neoplastic cells of brown adipose tissue express the adhesion molecule CD31. Arch Pathol Lab Med. 2006;130:480–482. doi: 10.5858/2006-130-480-NANCOB. [DOI] [PubMed] [Google Scholar]
- Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated adhesion molecule PECAM-1 (CD31) J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates platelet-endothelial cell adhesion molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sheibani N, Sorenson CM, Frazier WA. Differential modulation of cadherin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol Biol Cell. 2000;11:2793–2802. doi: 10.1091/mbc.11.8.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe WR, Hwang HC, Amin KM, Eck SL, Davidson BL, Wilson JM, Kaiser LR, Albelda SM. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: an effective in vitro drug sensitization system. Cancer Res. 1994;54:2055–2059. [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Sun J, Paddock C, Shubert J, Zhang HB, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell-cell localization. J Cell Sci. 2000;113:1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of β1 integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torimoto Y, Rothstein DM, Dang NH, Schlossman SF, Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992;148:388–396. [PubMed] [Google Scholar]
- Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- Varon D, Jackson DE, Shenkman B, Dardik R, Tamarin I, Savion N, Newman PJ. Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood. 1998;91:500–507. [PubMed] [Google Scholar]
- Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in hematopoietic cells and platelets. J Cell Biochem. 2002;87:424–438. doi: 10.1002/jcb.10321. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su X, Sorenson CM, Sheibani N. Tissue-specific distributions of alternatively spliced human PECAM-1 isoforms. Am J Physiol Heart Circ Physiol. 2003;284:H1008–H1017. doi: 10.1152/ajpheart.00600.2002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Repyak K, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature. Mol Vis. 2004;10:103–111. [PubMed] [Google Scholar]
- Watt SM, Williamson J, Genevier H, Fawcett J, Simmons DL, Hatzfeld A, Nesbitt SA, Coombe DR. The heparin binding PECAM-1 adhesion molecule is expressed by CD34+ hematopoietic precursor cells with early myeloid and B-lymphoid cell phenotypes. Blood. 1993;82:2649–2663. [PubMed] [Google Scholar]
- Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–193. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- Wu Y, Welte T, Michaud M, Madri JA. PECAM-1: a multifaceted regulator of megakaryocytopoiesis. Blood. 2007;110:851–859. doi: 10.1182/blood-2006-05-022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995a;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]
- Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes Commun. 1995b;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- Zehnder JL, Hirai K, Shatsky M, McGregor JL, Levitt LJ, Leung LL. The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J Biol Chem. 1992;267:5243–5249. [PubMed] [Google Scholar]
- Zhao T, Newman PJ. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J Cell Biol. 2001;152:65–73. doi: 10.1083/jcb.152.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997a;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate A strategy for successful endovascular invasion? J Clin Invest. 1997b;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser HM. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]


