Abstract
The Center for Adherence Support Evaluation (CASE) Adherence Index, a simple composite measure of self-reported antiretroviral therapy (ART) adherence, was compared to a standard three-day self-reported adherence measure among participants in a longitudinal, prospective cross-site evaluation of 12 adherence programs throughout the United States. The CASE Adherence Index, consisting of three unique adherence questions developed for the cross-site study, along with a three-day adherence self-report were administered by interviews every three months over a one-year period. Data from the three cross-site adherence questions (individually and in combination) were compared to three-day self-report data and HIV RNA and CD4 outcomes in cross-sectional analyses. The CASE Adherence Index correlated strongly with the three-day self-reported adherence data (p < 0.001) and was more strongly associated with HIV outcomes, including a 1-log decline in HIV RNA level (maximum OR = 2.34; p < 0.05), HIV RNA<400 copies/ml (maximum OR = 2.33; p < 0.05) and performed as well as the three-day self-report when predicting CD4 count status. Participants with a CASE Index score >10 achieved a 98 cell mean increase in CD4 count over 12 months, compared to a 41 cell increase for those with scores ≤10 (p < 0.05). The CASE Adherence Index is an easy to administer instrument that provides an alternative method for assessing ART adherence in clinical settings.
Introduction
Adherence to ART is widely recognized as critical for achieving therapeutic success in the treatment of HIV infection. Adherence has been shown to correlate strongly with both biologic markers of HIV and clinical outcomes, including HIV progression and death (Bangsberg et al., 2001a; Garcia de Olalla et al., 2002; Hogg et al., 2002). The measurement of adherence is problematic, however, as there is currently no widely accepted professional consensus for measuring adherence. In addition, little is known about the optimal method of measuring adherence in clinical or research practice including the use of different instruments in different patient populations. Several methods for measuring adherence have been employed with varying success in both clinical trials and clinical practice. Methods have included both indirect measures (e.g. self-report, electronic monitoring devices, pill counts, medication refill rate and monitoring for an expected therapeutic outcome) and direct measures (e.g. direct observation, therapeutic drug monitoring and biologic markers) (Arnsten et al., 2001; Bangsberg et al., 2001b). Although some of these measures have been useful in adherence research, many are too laborious or impractical for routine application in clinical settings.
The method of self-report offers the advantages of being quick and inexpensive, while providing the opportunity to understand patients’ perspectives on non-adherence (Besch, 1995; Fletcher et al., 1979). Despite indications that self-report may overestimate adherence (Arnsten et al., 2001; Bangsberg et al., 2000; Cramer et al., 1989; Liu et al., 2001; Waterhouse et al., 1993), several studies have demonstrated a strong association between self-reported adherence and virologic, immunologic and clinical outcomes among HIV-infected individuals (Fischl et al., 2002; C. V. Fletcher et al., 2003; Knobel et al., 2002; Mannheimer et al., 2002; Mellors et al., 2002; Montaner et al., 1998; Nieuwkerk et al., 2001; Walsh et al., 2002). Self-reported adherence also has been shown to correlate with plasma concentrations of antiretroviral drugs (Nieuwkerk et al., 2001) and quality of life (Mannheimer et al., 2005).
One widely used format for measuring self-reported adherence is variations on the appropriate use of medications across a fixed period of time as typified by the three-day self-report, a subscale in the Adult AIDS Clinical Trials Group (AACTG) instrument (Chesney et al., 2000). This method of eliciting from the patient the number of missed doses for each medication in each of the prior three days, while strongly associated with clinical outcomes in HIV research, often requires clinic or research staff assistance, is time-consuming and may be limited by poor patient recall. Simpler methods may yield similar results (Giordano et al., 2004; Knobel et al., 2002; Mannheimer et al., 2002; Walsh et al., 2002).
In this analysis, CASE evaluated several methods of assessing self-reported adherence among antiretroviral-experienced participants in a cross-site evaluation study of 12 adherence-support programs. We created an index of ART adherence (CASE Adherence Index) using three standard measures of self-reported adherence that is simple to apply and can be employed by both researchers and clinicians in the field. To assess the new instrument’s validity, we compared the CASE Adherence Index to a three-day self-report modeled after a version found in the AACTG (Chesney et al., 2000). We then compared the power of the CASE Adherence Index against the three-day self-report in predicting contemporaneous measures of HIV RNA levels and CD4 lymphocyte counts and changes in these biologic outcomes across a 12-month period when participants were receiving various adherence support interventions.
Methods
Study background
In 1999, the Department of Health and Human Services, Health Resources and Services Administration (HRSA), HIV/AIDS Bureau and the Special Projects of National Significance (SPNS) funded 12 sites to evaluate interventions designed to improve ART adherence among people living with HIV/AIDS. The interventions primarily targeted under-served populations with high rates of comorbidities and barriers to ART adherence. Program locations were geographically diverse and representative of the AIDS epidemic in the US, including four in the Northeast, two in the Mid-Atlantic, two in the South, one in the Midwest, two in the West and one in the Pacific Northwest.
The adherence support interventions were situated in a range of clinical settings including community health and academic medical centers. Sites designed their own adherence interventions, reflecting both best practice and other common approaches for promoting ART adherence used in the US and abroad. The singular and combined interventions that the sites employed included, but was not restrictive to, readiness training, modified directly observed therapy, stages of change interventions, professional case management, peer counseling and pharmacist monitoring.
Although the specific adherence interventions and populations varied across the 12 sites, the program evaluation strategy was common to all sites including the evaluation design and data collection strategy, the set of core data elements, instruments and protocols for assessment of adherence and clinical outcomes. Cross-site evaluation of the core data elements was coordinated by the New York Academy of Medicine’s (NYAM) Center for Adherence Support Evaluation. The Institutional Review Boards at both NYAM and each participating site approved the study procedures, including the informed consent protocol.
Participant eligibility and enrollment
Patients were eligible for inclusion in the cross-site evaluation if they met the following criteria: HIV-infected; age 18 years or older; prospective enrollment in the site adherence support program (though not necessarily on ART at baseline) and ability to complete an in-person interview in English or Spanish. Site staff recruited all individuals meeting these common eligibility criteria as well as each site’s program-specific criteria. Post-hoc estimates of client intervention refusal rates by site ranged from 10 – 30% of all individuals asked to participate. Those who agreed to enroll in the cross-site evaluation completed a separate informed consent. Participants were enrolled between July 1, 2000 and March 31, 2002.
Data collection
Data collection consisted of individual participant interviews and medical chart abstractions at participating sites. Standardized structured questionnaires developed jointly by the participating SPNS sites and CASE were used to conduct individual participant interviews at baseline and at three, six, nine, and 12 months after enrollment. Follow-up interviews were scheduled at three-month intervals starting from the enrollment interview date. Interviewers from each site received training from CASE staff to ensure standardization. While the majority of interviews and chart abstractions were conducted by healthcare professionals, including primary-care physicians, nurse practitioners, registered nurses, pharmacists, nutritionists and case managers, some data were collected by trained peer counselors and program volunteers.
The core cross-site questionnaire included items about sociodemographics (e.g. age, race/ethnicity, gender, educational level, current housing, employment, income and type of health insurance coverage) and self-reported ART adherence. Chart abstractions from participants’ medical records were conducted at baseline and at three-month follow-up intervals at each site to collect HIV RNA levels and CD4 counts, clinical psychiatric diagnoses, ART medications and adherence to medical appointments. Data collected locally were sent to CASE for data management, quality control and entry into the cross-site database. Data were stored into a Microsoft ACCESS 2000 database. All statistical analyses were performed using SAS software release 8.2 and S-PLUS release 6.2.
Measures
The CASE Adherence Index
Based on the results of correlation and principal components analyses, the CASE Adherence Index was developed as a composite (sum) of three self-reported measures of adherence:
A1
Self-reported frequency of ‘difficulty taking HIV medications on time (no more than two hours before or two hours after the time your doctor told you to take it)’. Responses were: never, rarely, most of the time or all of the time.
A2
Self-reported ‘average number of days per week at least one dose of HIV medications was missed’. Responses were: everyday, 4–6 days per week, 2–3 days per week, once a week, less than once a week or never. (Reverse coded for analysis).
A3
Self-reported ‘last time missed at least one dose of HIV medications’. Responses were: within the past week, 1–2 weeks ago, 3–4 weeks ago, between one and three months ago, more than three months ago or never.
The correlations among these three questions—difficulty taking medications on time, average number of days per week missed at least one dose and last time missed at least one dose—were moderate, suggesting that they partially measured different dimensions of adherence (Table I). In a principal component analysis of the three variables (A1, A2, A3), the first principal component explained 69% of the total variation in the three variables and was equal to 0.51*A1+/0.62*A2+0.59*A3. Since the first principal component showed approximately equal loadings on the three factors, we considered the sum as the CASE Adherence Index, with A1 contributing a possible range of one to four points, and A2 and A3 each contributing one to six points (see Appendix). Composite scores ranged from three to 16 with higher scores indicating better adherence.
Table I.
Correlations among adherence measures.
| Measures | A1 | A2 | A3 | SR3 | CASE Adherence Index |
|---|---|---|---|---|---|
| A1 | 1.000 | 0.501 | 0.407 | 0.260 | 0.642 |
| A2 | 1.000 | 0.698 | 0.373 | 0.861 | |
| A3 | 1.000 | 0.242 | 0.934 | ||
| SR3 | 1.000 | 0.440 |
Note: P > 0.000 for all cells.
Note: A1=Self-reported frequency of taking medications on time.
A2=Self-reported average number of times/week missed at least one dose or did not take the full amount.
A3=Self-reported last time missed at least one dose of HIV medications or did not take the full amount.
SR3=3-Day self-report.
AACTG three-day self-report
We compared the CASE Adherence Index and its individual components to a three-day self-report originally developed by Chesney, et al. (2000) for the AACTG. The three-day self-report consists of a day-by-day recall, assessing the number of doses prescribed and the number of doses missed per medication in the prior three days. An adherence rate was calculated for each ART medication by averaging the number of doses taken divided by doses prescribed for that medication in the prior three days. An overall adherence rate for the regimen (SR3) was obtained by averaging the adherence rates for all of the ART medications in the regimen.
The three-day self-report overall adherence rate was analyzed both continuously and as a dichotomous variable using a cut-off of ≥95% or not, based on findings by Paterson, et al. (2000) and others, of the significant impact on virologic outcome when adherence falls below 95% (Paterson et al., 2000; Mannheimer, 2005).
HIV RNA levels
HIV RNA levels were measured in two ways: (1) as a dichotomous variable of 1-log decrease in log HIV RNA from baseline to follow-up level and (2) as a dichotomous variable of undetectable HIV RNA at follow-up. Undetectable HIV RNA was defined as HIV RNA<400 because all of the sites did not have access to ultra-sensitive HIV RNA testing. Individual cases from any site with HIV RNA levels <400 copies/ml at baseline were excluded from the one-log decrease measure.
CD4 counts
Change in CD4 cell count was measured for each individual participant by taking the difference between the raw quarterly CD4 counts and the raw CD4 baseline measures.
Data analysis
Two steps were taken to assess the CASE Adherence Index’s reliability and validity. In the first step we estimated the CASE Adherence Index’s degree of sensitivity and specificity to changes in the three-day self-report across four cross-sectional time periods. In the second, we compared the CASE Adherence Index and the three-day self-report’s sensitivity to changes in HIV virologic outcomes and CD4 counts across time.
Cross-sectional data from the baseline and three-, six-, nine- and 12-month interviews and quarterly corresponding HIV RNA levels and CD4 laboratory values were used in these analyses. Only participants on ART at the time of the baseline interview were included in these analyses. For each cross-section, only interviews conducted within a 90-day (±45 days) window of the target interview date were included in that quarter’s analysis. Interviews completed more than 45 days after their due date were treated as interviews for the next quarter. Cases with missing quarterly interviews were excluded from that corresponding quarterly analysis. Laboratory data were assessed at baseline or within a quarter if specimens were collected within 45 days prior to or on the day of the baseline interview or within a 30-day window prior to or after the corresponding follow-up interview date. Cases with missing adherence and lab values were excluded from the corresponding quarterly analyses.
Sample available for analyses
Participants on ART at program entry who completed a baseline interview and had corresponding HIV RNA levels and CD4 count laboratory values formed the starting pool of analyzable participants (n = 1,154). A significant decrease in cases available for analyses occurred at follow-up due to a number of factors, including participant attrition before the three-month follow-up interview (n = 252; 22%), not being on ART at the time of the three-month interview (n = 231; 20%), not having an interview in the appropriate time frame (n = 140; 12%) and the lack of corresponding lab values within the specified window period (n = 7; 0.6%). Thus, data from 524 participants were available for the CASE Adherence Index construction; a total of 524, 439, 315, and 305 participants were used to assess the sensitivity and specificity of the CASE Adherence Index to changes in the AACTG three-day self-report at three, six, nine, and 12 months respectively; a total of 405, 328, 248 and 240 cases were used to assess the relationship between the CASE Adherence Index, the three-day self-report and HIV RNA levels at three, six, nine and 12 months, respectively; for the analysis of changes in CD4 counts after baseline measurement 402, 346, 246 and 244 participants were used respectively at three, six, nine and 12 months.
Results
Participant characteristics
The three-month cohort (n = 524) used in these analyses included 35% women, 67% African Americans and the mean age was 40 years of age (Table II). Nearly two-fifths did not complete high school. Heterosexual contact was the most common self-reported mode of HIV transmission (48%), followed by men reporting sex with men and injection drug use. The demographics of this subset were not significantly different from the sample of 1,154 participants who completed a baseline interview or from that of the six-, nine-, or 12-month cross-sections. At baseline the median CD4 count was 216 cells/mm3 and the median log10 viral load was 4.44; 19.5% (225/1,154) had an undetectable viral load (<400 copies/ml) at baseline.
Table II.
Baseline participant characteristics for the 3-month cohort (n = 524).
| 40.13 (SD = 8.6)
|
||
|---|---|---|
| Mean age, years | % | (N) |
| Gender | ||
| Male | 65 | (335) |
| Female | 34 | (179) |
| Transgender | 1 | (1) |
| Race/Ethnicity | ||
| African American | 66 | (337) |
| Latino | 5 | (26) |
| White | 26 | (130) |
| Other | 3 | (13) |
| Education | ||
| Not high school graduate | 41 | (183) |
| High school graduate or GED | 36 | (161) |
| Some college/technical school or beyond | 23 | (106) |
| Self-reported HIV risk behavior | ||
| Men reporting sex with men | 29 | (159) |
| Injecting drug use | 16 | (80) |
| Had sex with men and injected drugs | 2 | (6) |
| Heterosexual contact | 45 | (267) |
| Heterosexual contact and injected drugs | 3 | (28) |
| Blood transfusion, blood components or tissue | 3 | (17) |
| Other | 2 | (11) |
| Mean CD4 count, cells/mm3 | 256 | |
| (SD) | 251 | |
| Median CD4 count, cells/mm3 | 193 | |
| Mean log10 HIV RNA level | 3.99 | |
| (SD) | 1.35 | |
| Median log10 HIV RNA level | 4.27 | |
Note: N = 524 represents participants receiving antiretroviral therapy who had 3-month and baseline interview with corresponding adherence, CD4, and HIV RNA data, missing values result in different total for each variable.
Relationships between CASE adherence index and three-day self-report
The sensitivity and specificity of the CASE Adherence Index with respect to three -day self-reported adherence were calculated at different cut-off scores, as shown in Table III. Based on this analysis, a cutoff score of ten on the CASE Adherence Index was used to dichotomize the CASE Adherence Index in the following analyses to maximize the sensitivity and specificity of the index with respect to the three-day self-report set at 95% adherence.
Table III.
Sensitivity and specificity of CASE Adherence Index with 3-day self-report adherence.
| CASE Adherence Index score | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 5 | 99.28 | 8 |
| 6 | 98.32 | 18 |
| 7 | 96.64 | 30 |
| 8 | 92.57 | 52 |
| 9 | 84.65 | 77 |
| 10 | 74.1 | 99 |
| 11 | 62.83 | 99 |
| 12 | 55.64 | 100 |
| 13 | 48.2 | 100 |
| 14 | 38.61 | 100 |
| 15 | 25.42 | 100 |
Using logistic regression for the four serial cross-sections at three, six, nine, and 12 months after enrollment, the odds of having an AACTG three-day self-reported adherence score >95% was at least 60 times (p < 0.001) more for those with a CASE Adherence Index score >10 compared to those with lower scores. Similar results were found for each of the four cross-sectional analyses.
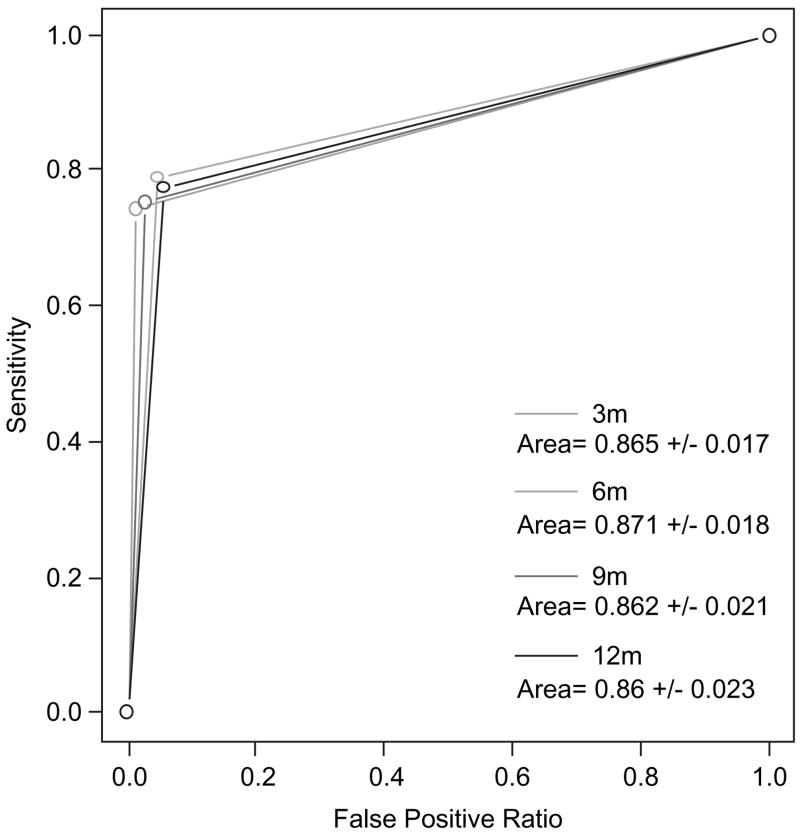
Receiver Operating Characteristic (ROC) curves (Hanley & McNeil, 1982) were then drawn based on the logistic regression results, with the area under the ROC curve (between the x-axis and the curve) providing a quantitative measure of the accuracy of these predictions (Delong et al., 1988). Areas closer to 1.0 reflect better predictive power, whereas areas of 0.5 are no better than chance. Statistical differences between two curves (areas) were tested using a generalized U-statistic (Delong et al., 1988). The strong association between three-day self-report adherence at 95% and the CASE Adherence Index at ten also was reflected in ROC curves (Figure 1), where the areas under the curves were all >0.86.
Figure 1.
ROC curve for CASE Adherence Index as predictor of self-reported adherence at three, six, nine and 12 months, p-value (for H0: Area = 0.5 versus H1: Area = 0.5) <0.05.
Relationship between self-reported adherence measures and HIV RNA
To compare and validate the three-day adherence self-report and the CASE Adherence Index as measures of ART adherence, we examined each instrument’s relationship to HIV virologic outcomes. The 929 (out of 1154) participants with HIV RNA>400 copies/ml at baseline were included in the analysis for modeling the odds of a 1-log decline in HIV RNA, resulting in 405, 328, 248 and 240 participants for this analysis at three, six, nine and 12 months, respectively.
The odds ratios (Table IV) show that the CASE Adherence Index scores >10 were strongly associated with both a 1-log decrease in HIV RNA level and achieving HIV RNA<400 copies/ml at every follow-up time point (p < 0.05 at three, six, nine, and 12 months for both virologic endpoints, except p < 0.10 for a 1-log HIV RNA decline at three months and a HIV RNA<400 copies at 12 months). The association between 95% adherence by three-day self-report with these virologic outcomes was not as strong, with significance only seen at the six-month time point for a 1-log decrease in HIV RNA, and at the six and nine month time point for HIV RNA<400.
Table IV.
Association of change in HIV RNA level with adherence measures.
| OR estimate for viral suppression (1-log decrease in HIV RNA or HIV RNA<400 copies/ml) from baseline
|
||||||
|---|---|---|---|---|---|---|
| Adherence measure | Comparison | HIV RNA measure | 3 Months | 6 Months | 9 Months | 12 Months |
| 3-Day self-report | >95% versus ≤95% | 1-log decrease | 1.23 | 2.26** | 1.53 | 1.16 |
| HIV RNA<400 | 0.97 | 2.3** | 1.66* | 1.23 | ||
| CASE Adherence Index | >10 versus ≤10 | 1-log decrease | 1.52* | 1.9** | 1.76** | 2.13** |
| HIV RNA<400 | 1.6** | 1.68** | 1.87** | 1.6* | ||
Note: p < 0.10
p < 0.05.
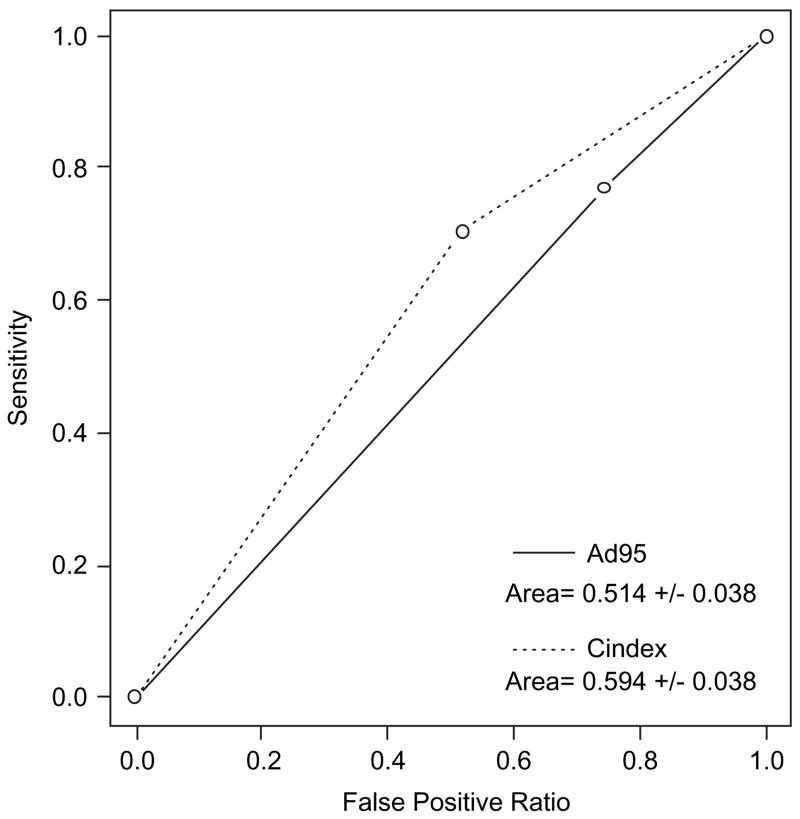
Sensitivity and specificity of the three-day adherence self-report and CASE Adherence Index measures as predictors of a 1-log decrease in HIV RNA from baseline were calculated to obtain ROC curves (Figure 2). The areas under the ROC curves were consistently higher for the CASE Adherence Index than the three-day self-report, suggesting a more robust association between the CASE Adherence Index and virologic outcome. Statistically significant differences in the area under the ROC curves for the two adherence measures, however, were achieved only for the 12-month cross-section (Figure 2).
Figure 2.
ROC for three-day self reported adherence and by CASE Adherence Index at 12 months as predictor of viral suppression (1-log decrease in HIV RNA from baseline). p (H0: Two roc curves are different) = 0.0065.
Relationship between self-reported adherence measures and CD4 cell counts
For the analysis of change in CD4 count from baseline, only clients on ART at baseline (n = 768) were included, resulting in 402, 346, 246 and 244 participants for this analysis at three, six, nine and 12 months, respectively. Change in CD4 cell count was measured for each individual participant by taking the difference between the CD4 count at each time-point and at baseline. Changes from baseline CD4 count at each specific time point were calculated and correlated with adherence measures at baseline. T-tests were used for each cross-section to assess group differences in mean change in CD4 count from baseline to three, six, nine, and 12 months between adherent and non-adherent clients, as assessed by the two adherence measures at each time point. Pooled variance estimates were used to calculate the t-test statistics.
A strong relationship was seen between the CASE Adherence Index score and change in CD4 count from baseline to 12 months (Table V). Participants with baseline CASE Adherence Index scores >10 had a mean CD4 cell count increase of 98 cells over 12 months, compared to a mean increase of 41 cells among the group with baseline CASE Adherence Index scores ≤10 (p = 0.02). The difference in change in CD4 counts over 12 months between high three-day self-report (≥95%) and low three-day self-report (<95%) was not significant (79 versus 64 cells, respectively). The differences from baseline to three, six and nine months were not significant for either of the adherence measures, suggesting a slow rate of change of CD4 cell count over time.
Table V.
Change in CD4 count from baseline to three, six, nine and 12-month follow-up interviews.
| Follow-up time | Adherence measure | Comparison | Mean change in CD4 | Standard error | P-value |
|---|---|---|---|---|---|
| 3 months | 3-day self-report | >95% | 29.86 | 8.61 | 0.88 |
| ≤95% | 32.55 | 15.23 | |||
| CASE Index | >10 | 38.4 | 10.65 | 0.2 | |
| ≤10 | 18.74 | 11.04 | |||
| 6 months | 3-day self-report | >95% | 26.08 | 11.3 | 0.48 |
| ≤95% | 40.65 | 15.65 | |||
| CASE Index | >10 | 24.04 | 13.45 | 0.62 | |
| ≤10 | 33.68 | 13.88 | |||
| 9 months | 3-day self-report | >95% | 55.2 | 9.2 | 0.78 |
| ≤95% | 49.14 | 25.12 | |||
| CASE Index | >10 | 55.25 | 11.43 | 0.51 | |
| ≤10 | 42.92 | 15.82 | |||
| 12 months | 3-day self-report | >95% | 79.31 | 14.03 | 0.57 |
| ≤95% | 64.3 | 21.42 | |||
| CASE Index | >10 | 97.51 | 17.4 | 0.02 | |
| ≤10 | 40.66 | 16.82 |
Discussion
This study identified a new measure of self-reported adherence that was easy to administer and score. It compared the CASE Adherence Index’s and the AACTG-based three-day self-report’s association with virologic markers associated with outcomes of HIV treatment in a variety of clinical settings with a diverse and antiretroviral experienced patient population. The correlation between the CASE Adherence Index scores and the AACTG-based three-day self-report as expressed in both measures’ high degree of sensitivity and specificity indicate that they are concurrently valid.
The CASE Adherence Index is a composite measure composed of three simple questions addressing three different aspects of ART adherence: difficulty taking ART medication on time, frequency of missed ART doses and time since most recent missed ART dose. We found that it was able to predict virologic response and was highly correlated with the previously validated ACTG three-day recall. However, in contrast to the three-day self-report, whose utility is limited by the complexity of the assessment questions, the length of the instrument and a patient’s ability to recall their ART medications accurately, the CASE Adherence Index is brief, not dependent on specific medication recall and requires limited training before it can be implemented in the field by both HIV practitioners and researchers.
Data to test the reliability and validity of the CASE Adherence Index originated in interventions primarily targeting underserved populations with high rates of co-morbidities and barriers to ART adherence living in representative regions of the US. The study was conducted in diverse healthcare settings that designed their own adherence interventions, which included a range of approaches, reflecting both best practice and other common approaches for promoting ART adherence used in the US and abroad. Given the diversity of the patient population, the heterogeneity of support program types and the variety of interventions employed by the sites, our confidence in the generalizability of the findings is strong.
There are several limitations that should be considered in interpreting our findings. First, the study population represents the 1,154 individuals who agreed to participate in the local sites’ adherence programs. Unfortunately, the sites refusal rates and the characteristics of those refusing to participate were not uniformly collected. In addition, although 1,154 participants formed the starting pool of subjects for this analysis, the cross-sections in the analysis consisted of less than half of these participants. Attrition after baseline measurement varied by site and patient characteristics. A preliminary analysis of the data indicates attrition in this sample may be a multivariate function of patient characteristics, prior ART adherence status and service provision type. The issue of program attrition in relationship to measurement error as a function of site, patient and interviewer characteristics will be considered in subsequent papers.
Given that the interventions targeted a population of HIV patients with high rates of barriers to maintaining adherence to care, the rate of program attrition was not unanticipated by the researchers who employed cross-site data pooling to increase the power of their statistical tests. Despite the level of attrition, the participants assessed in these analyses still represented a large, diverse group that reflects the current HIV epidemic in the US.
Sample attrition may not have been the only source of measurement error in this study. Self-reported adherence is subject to upward bias in part due to social desirability, experimenter expectations and increased monitoring. These effects may be just as manifest in the three-day self-report or any other self-reported measure of adherence as in the CASE Adherence Index. However, it is also possible that responses to the generally framed adherence related questions in the CASE Adherence Index may reduce social desirability bias as opposed to focusing on behavior in the three previous days as in the three-day self-report. This may have contributed to the stronger association between the CASE Adherence Index and the virologic markers. The question of response bias associated with the authority of the interviewer, intervention setting and purpose is well worth studying and may be the subject of another analysis.
In our study, the CASE Adherence Index was a better predictor of HIV RNA than three-day self-report. The index also preformed as well as three-day self-report as a predictor of CD4 counts. The CASE Adherence Index’s ease and speed of administration suggest that it is a useful tool for assessing ART adherence as part of routine clinical assessment in standard HIV care. The use of the index in clinical practice will enable health care providers and other treatment staff to provide rapid feedback to support their clients’ efforts to maintain adherence to ART.
Acknowledgments
Source of support: Health Resources and Services Administration, HIV AIDS Bureau, Special Projects of National Significance, Grant #6 H97 HA 00128-03 05, CFDA # 93.928.
Appendix
Case Adherence Index questionnaire
Please ask each question and circle the corresponding number next to the answer, then add up the numbers circled to calculate Index score.
A1. How often do you feel that you have difficulty taking your HIV medications on time? By ‘on time’ we mean no more than two hours before or two hours after the time your doctor told you to take it.
4 Never
3 Rarely
2 Most of the time
1 All of the time
A2. On average, how many days per week would you say that you missed at least one dose of your HIV medications?
1 Everyday
2 4–6 days/week
3 2–3 days/week
4 Once a week
5 Less than once a week
6 Never
7
A3. When was the last time you missed at least one dose of you HIV medications?
1 Within the past week
2 1–2 weeks ago
3 3–4 weeks ago
4 Between 1 and 3 months ago
5 More than 3 months ago
6 Never
INDEX SCORE: _________
>10 = good adherence
≤10 = poor adherence
Footnotes
Publisher's Disclaimer: This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Arnsten J, Demas P, Farzadegan H, Grant R, Gourevitch M, Chang C, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Disease. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: Electronic medication monitors and unannounced pill counts. AIDS and Behavior. 2001;5:275–281. [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load and development of drug resistance in an indigent population. Aids. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Besch CL. Compliance in clinical trials. AIDS. 1995;9:1–10. doi: 10.1097/00002030-199501000-00001. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient care committee & adherence working group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. Journal of the American Medical Association. 1989;261:3273–3277. [PubMed] [Google Scholar]
- Delong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Fischl M, Ribaudo H, Collier A, Erice A, Giuliano M, Dehlinger M, et al. A randomized trial comparing two four-drug antiretroviral regimens with a three-drug regimen in advanced HIV disease. Paper presented at the 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2002. [Google Scholar]
- Fletcher CV, Testa MA, Haubrich R, Brundage R, Jiang H, Ickovics J, et al. Relationships among four measures of medication adherence and virologic response in ACTG 359 (abstract 577). Paper presented at the 10th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003. [Google Scholar]
- Fletcher SW, Pappius EM, Harper SJ. Measurement of medication compliance in a clinical setting. Comparison of three methods in patients prescribed digoxin. Archives of Internal Medicine. 1979;139:635–638. [PubMed] [Google Scholar]
- Garcia de Olalla P, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndrome. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials. 2004;5:74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Hanley J, McNeil B. The meaning and use of the area under a receiver operating characteristics (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O’Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after one year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- Knobel H, Alonso J, Casado JL, Collazos J, Gonzalez J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA Study. AIDS. 2002;16:605–613. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- Mannheimer S, Matts J, Telzak E, Chesney M, Child C, Wu AW, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2004;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical Infectious Diseases. 2002;34:1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- Mellors J, Vaida F, Bennett K, Hellmann NS, DeGruttola V, Hammer S, et al. Efavirenz hypersusceptibility improves virologic response to multidrug salvage regimens in ACTG 398. Paper presented at the 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2002. [Google Scholar]
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine and zidovudine for HIV-infected patients: The INCAS trial. Italy, the Netherlands, Canada and Australia study. Journal of the American Medical Association. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- Nieuwkerk PT, Sprangers MA, Burger DM, Hoetelmans RM, Hugen PW, Danner SA, et al. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Archives of Internal Medicine. 2001;161:1962–1968. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, Gazzard BG. Responses to a one-month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts and microelectronic monitoring. Journal of Clinical Oncology. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]