Abstract
Plasma HDL levels are inversely related to the incidence of atherosclerotic disease. Some of the atheroprotective effects of HDL are likely mediated via preservation of EC function. Whether the beneficial effects of HDL on ECs depend on its involvement in cholesterol efflux via the ATP-binding cassette transporters ABCA1 and ABCG1, which promote efflux of cholesterol and oxysterols from macrophages, has not been investigated. To address this, we assessed endothelial function in Abca1–/–, Abcg1–/–, and Abca1–/–Abcg1–/– mice fed either a high-cholesterol diet (HCD) or a Western diet (WTD). Non-atherosclerotic arteries from WTD-fed Abcg1–/– and Abca1–/–Abcg1–/– mice exhibited a marked decrease in endothelium-dependent vasorelaxation, while Abca1–/– mice had a milder defect. In addition, eNOS activity was reduced in aortic homogenates generated from Abcg1–/– mice fed either a HCD or a WTD, and this correlated with decreased levels of the active dimeric form of eNOS. More detailed analysis indicated that ABCG1 was expressed primarily in ECs, and that these cells accumulated the oxysterol 7-ketocholesterol (7-KC) when Abcg1–/– mice were fed a WTD. Consistent with these data, ABCG1 had a major role in promoting efflux of cholesterol and 7-KC in cultured human aortic ECs (HAECs). Furthermore, HDL treatment of HAECs prevented 7-KC–induced ROS production and active eNOS dimer disruption in an ABCG1-dependent manner. Our data suggest that ABCG1 and HDL maintain EC function in HCD-fed mice by promoting efflux of cholesterol and 7-oxysterols and preserving active eNOS dimer levels.
Introduction
Endothelial dysfunction is a key feature of early atherosclerotic lesions in both humans and animal models (1–3). It is characterized by decreased eNOS activity and NO bioavailability and increased expression of cell adhesion molecules such as VCAM-1 and ICAM-1, promoting atherosclerotic lesion formation, impaired blood flow, and thrombus formation. In animal models, increased dietary cholesterol plays a central role in inducing endothelial dysfunction (4–6). Dietary oxysterols, particularly 7-oxysterols, appear to have a key role in inducing decreased NO-induced vascular relaxation (7, 8). 7-Ketocholesterol (7-KC) is detected at high levels in human atherosclerotic plaques and in the plasma of patients with a high cardiovascular risk, and is abundant in oxidized LDL (9–11). In addition, oxysterols may be present in the diet and incorporated into plasma lipoproteins (12, 13). Dietary sources of oxysterols are cholesterol-rich foods (dairy, egg, meat products), especially those products that are heated in air during processing or are stored for long periods (14, 15). Thus, many foods in the Western diet (WTD) contain cholesterol oxidation products.
Plasma HDL levels are inversely related to the incidence of athero-thrombotic disease (16, 17). A part of the atheroprotective effect of HDL may be related to its role in preserving endothelial function (18, 19). The beneficial effects of HDL on ECs may include stimulation of proliferation, cell survival, migration, and NO synthesis as well as inhibition of the expression of VCAM-1 and ICAM-1 (20–23). HDL may have a specific role in reversing decreased eNOS activity in human ECs treated with oxidized LDL (24) or in reversing the decrease in eNOS-dependent vascular relaxation induced by high-cholesterol diets (HCDs) (4). The ability of HDL to cause relaxation of vascular rings has been reported to be impaired in scavenger receptor B-I–deficient (SR-BI–deficient) mice, and SR-BI expression in cultured cells enables an increase in eNOS activity in response to HDL through a mechanism that depends on the cholesterol efflux properties of HDL (25). While ATP-binding cassette transporters ABCA1 and ABCG1 have a major role in inducing cellular cholesterol efflux (26–28) and are known to be expressed in ECs (29), to our knowledge their role in preserving endothelial function has not been explored. ABCA1 mediates cholesterol efflux to lipid-poor apoA-I but only modestly increases cholesterol efflux to HDL (28, 30, 31). In contrast, ABCG1 promotes macrophage cholesterol efflux to HDL but not to lipid-poor apoA-I (28, 32–34). ABCG1 was recently shown to have a specific role not shared by ABCA1 in promoting efflux of 7-oxysterols from macrophages and transfected cells to HDL (28, 35). To better understand the adverse effects of dietary cholesterol and 7-oxysterols on endothelial function (7, 8), the present study was undertaken to test the hypothesis that ABCG1 and/or ABCA1, by promoting efflux of sterols and oxysterols from ECs, plays a key role in preserving eNOS activity in animals fed HCDs. Our studies show a major role for ABCG1 in defending endothelial eNOS activity in mice fed HCDs regulated to the efflux of cholesterol and 7-oxysterols and the preservation of eNOS dimer.
Results
Impact of ABC transporter deficiency on endothelium-dependent vasorelaxation.
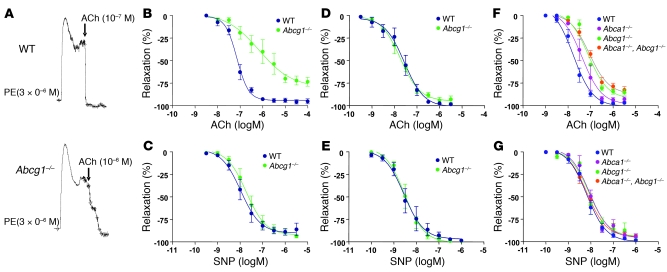
Abcg1–/– and control mice were placed on a HCD (1.25% cholesterol, 7.5% cocoa butter, and 0.5% sodium cholate). After 11 weeks, both groups developed a similar moderate hypercholesterolemia (control, 331 ± 34 mg/dl; Abcg1–/–, 321 ± 46 mg/dl). To test vascular function in these mice, femoral arteries were preconstricted with phenylephrine, and relaxant responses to endothelium-dependent ACh and endothelium-independent sodium nitroprusside (SNP) vasodilating agents were measured. Arterial vasorelaxation in response to ACh was markedly attenuated in Abcg1–/– mice (Figure 1A). The ACh dose-response curve was shifted to the right in Abcg1–/– mice, and the maximum relaxation response was significantly reduced compared with arteries from control mice (P < 0.01; Figure 1B). In contrast, there was no significant difference in relaxation in response to SNP (Figure 1C). There was also no significant difference in ACh-induced or SNP-induced arterial relaxation in WT and Abcg1–/– mice fed the chow diet (Figure 1, D and E). We also assessed ACh-induced vascular relaxation in WTD-fed mice with single or combined deficiencies of ABCA1 and ABCG1 (Figure 1, F and G). This revealed a similar severe defect in vascular relaxation in Abcg1–/– and Abca1–/–Abcg1–/– mice (EC50, 79.6 ± 13.0 vs. 88.3 ± 14.7 nM), while the response of Abca1–/– mice (EC50, 42.1 ± 11.8 nM) was intermediate between these groups and that of the controls (17.4 ± 2.7 nM) (Figure 1F). There was no difference in relaxation in response to SNP in any of the groups (Figure 1G). These findings suggest that while both transporters may be involved in preserving vascular relaxation responses, ABCG1 has a more prominent role than ABCA1.
Figure 1. Response to vasoconstrictive agents in the femoral arteries from control (WT), Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– mice.
(A–C) WT and Abcg1–/– mice (n = 4 per group) were put on a HCD (1.25% cholesterol, 7.5% cocoa butter and 0.5% sodium cholate) for 11 weeks. (A) Original trace recordings showing vessel tension increase after addition of 3 μM phenylephrine (PE) and relaxation in response to different concentrations of ACh. ACh-induced vasorelaxation occurred at the indicated concentrations in control and Abcg1–/– mice. (B) Vasorelaxation in response to ACh was markedly attenuated in Abcg1–/– mice. (C) There was no significant difference in relaxation in response to SNP. (D and E) WT and Abcg1–/– mice (n = 5 per group) were put on a chow diet. There was no difference between the groups in the response to ACh (D) or SNP (E). (F and G) WT, Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– (n = 5 per group) were put on a WTD (0.25% cholesterol and 21% milk fat) for 12 weeks. (F) There was a similar severe defect in vascular relaxation response to ACh in Abcg1–/– and Abca1–/–Abcg1–/– mice, while the response of Abca1–/– mice was intermediate between these groups and the controls. (G) There was no significant difference in relaxation in response to SNP. The results are represented as mean ± SEM.
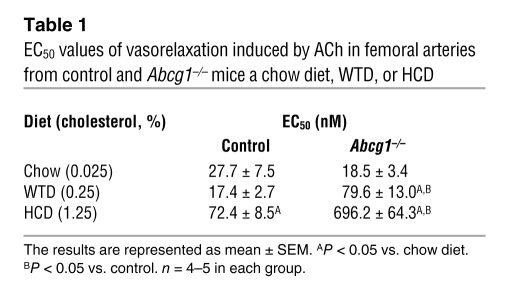
Table 1 summarizes the effect of diet on vascular relaxation parameters in WT and Abcg1–/– mice. In the control group, the response to ACh was similar on the chow or WTD (Table 1) but impaired in response to the HCD (Table 1). There was a progressive, more marked impairment with increasing dietary cholesterol content in the Abcg1–/– mice (Table 1). The EC50 value for the response to ACh was approximately 4-fold greater than control in Abcg1–/– mice on the WTD, and 10-fold greater on the HCD, while there was no difference in response to the chow diet (Table 1). The data indicate that ABCG1 plays a progressively more important role in maintaining endothelium-dependent vasorelaxation as dietary cholesterol content is increased.
Table 1 .
EC50 values of vasorelaxation induced by ACh in femoral arteries from control and Abcg1–/– mice a chow diet, WTD, or HCD
Cholesterol and 7-KC accumulation in aorta: effects of diet and genotype.
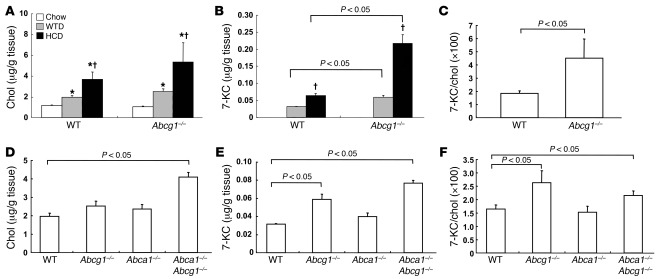
In view of the specific role of ABCG1 in efflux of 7-oxysterols from cells (35), we next measured the content of cholesterol and 7-KC in non-atherosclerotic thoracic and abdominal aortas excluding the proximal aorta. Total cholesterol content was increased by the HCDs in a dietary cholesterol concentration–dependent manner (Figure 2A). However, there was no significant difference in cholesterol content between the control and Abcg1–/– mice (Figure 2A). 7-KC was not detectable in aortas of chow-fed mice but accumulated in response to the HCD (Figure 2B) and WTD (Figure 2, B and E). Accumulation of 7-KC was more prominent in Abcg1–/– mice (Figure 2, B and E). The ratio of 7-KC to cholesterol was significantly higher in Abcg1–/– mice than in controls in response to HCD (Figure 2C) and WTD feeding (Figure 2F). We also compared aortic cholesterol and 7-KC contents in Abcg1–/– mice with those in Abca1–/– and Abca1–/–Abcg1–/– mice in response to the WTD. There was no significant difference in cholesterol content between the control and Abcg1–/– or Abca1–/– mice (Figure 2D), while in Abca1–/–Abcg1–/– mice cholesterol content was significantly higher than in the controls (Figure 2D). In contrast, 7-KC was significantly increased in Abcg1–/– and Abca1–/–Abcg1–/– mice compared with controls, although no difference was found between Abcg1–/– and Abca1–/–Abcg1–/– (Figure 2E). The ratio of 7-KC to cholesterol was also significantly increased in Abcg1–/– and Abca1–/–Abcg1–/– mice (Figure 2F). Thus, we conclude that deficiency of both ABCA1 and ABCG1 results in increased cholesterol accumulation compared with accumulation associated with a single deficiency of the transporters, while accumulation of 7-KC specifically reflects deficiency of ABCG1. The latter finding parallels the impairment of vasodilatory responses and suggests that the impaired ACh-induced vascular relaxation in Abcg1–/– mice could be brought about by aortic accumulation of 7-oxysterols.
Figure 2. Cholesterol and 7-KC contents in thoracic and abdominal aortas: effects of diet and genotype.
(A–C) WT and Abcg1–/– mice were put on a chow diet, WTD, or HCD (n = 4–5 per group). (D–F) WT, Abca1–/–, Abcg1–/–, and Abca1–/–Abcg1–/– mice were put on the WTD (n = 5 per group). (A and D) Cholesterol (Chol), (B and E) 7-KC, and (C and F) the ratio of 7-KC to cholesterol were measured. The results are represented as mean ± SEM. *P < 0.05 versus chow diet; †P < 0.05 versus WTD.
eNOS protein expression and dimerization in aorta.
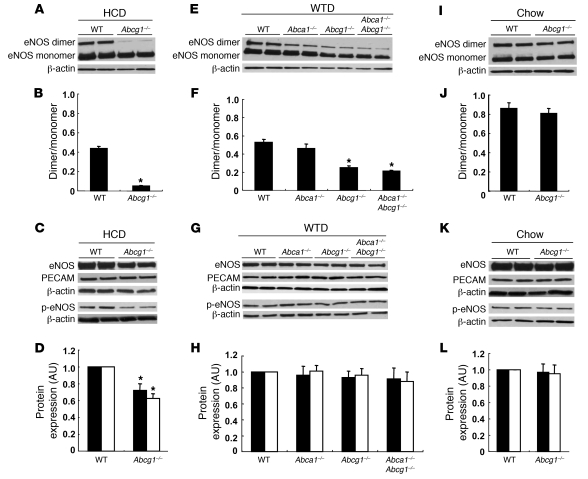
Previous studies have shown that the formation of eNOS homodimers is necessary for eNOS activity (36, 37). In response to the HCD, eNOS dimer levels were dramatically reduced in Abcg1–/– mice (Figure 3, A and B). Total eNOS and phospho-eNOS levels were also moderately decreased in Abcg1–/– mice (Figure 3, C and D), but the ratio of phospho-eNOS to eNOS did not change. On the WTD, Abca1–/–, Abcg1–/–, and Abca1–/–Abcg1–/– mice exhibited decreased eNOS dimer levels in aortas (Figure 3, E and F). This reduction was most prominent in Abcg1–/– and Abca1–/–Abcg1–/– mice (Figure 3, E and F). There was no difference in eNOS or phospho-eNOS levels between the groups (Figure 3, G and H). In aortas of chow-fed mice, there was no significant difference in eNOS dimer levels, eNOS, or phospho-eNOS levels between the control and Abcg1–/– mice (Figure 3, I–L). PECAM levels were not changed in any groups or diets (Figure 3, C, G, and K), indicating an intact endothelium. These data suggest that endothelial dysfunction induced by ABCG1 deficiency in response to the HCD resulted from the reduction of eNOS dimer levels.
Figure 3. Western blot for eNOS protein of mouse aorta.
(A–D) Aortas from HCD-fed WT and Abcg1–/– mice. (E–H) Aortas from WTD-fed WT, Abca1–/–, Abcg1–/–, and Abca1–/–Abcg1–/– mice. (I–L) Aortas from chow-fed WT and Abcg1–/– mice. (A, E, and I) Western blot for eNOS dimer levels. (B, F, and J) Quantification of eNOS dimer/monomer levels. (C, G, and K) Western blot for eNOS and phospho-eNOS. (D, H, and L) Quantification of eNOS (filled bars) and phospho-eNOS (open bars). The results are represented as mean ± SEM. *P < 0.05 versus control.
ABCG1 expression and accumulation of 7-KC in aorta.
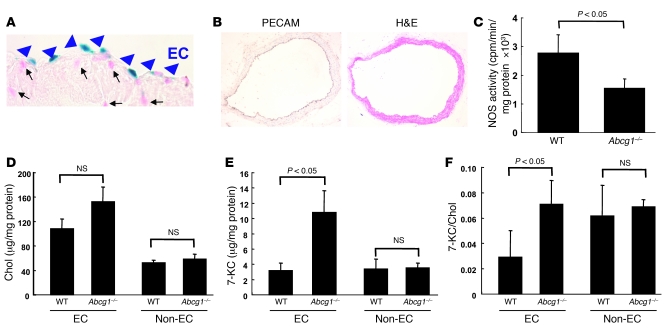
To further evaluate the role of ABCG1 in endothelium-dependent vasorelaxation, we investigated ABCG1 expression in non-atherosclerotic aorta of Abcg1–/– mice that harbor a lacZ cassette insertion at the Abcg1 locus. Blue nuclear lacZ expression was detected specifically in ECs (Figure 4A, arrowheads), but not in other cells indicated by nuclear fast red staining (Figure 4A, arrows). We also carried out PECAM staining in aorta of WTD-fed Abcg1–/– mice, which indicated an intact endothelium (Figure 4B). As expected, these segments of abdominal and thoracic aorta did not show any evidence of atherosclerosis or macrophage accumulation (data not shown). We also measured NOS activity using aortic lysates in WTD-fed WT and Abcg1–/– mice. The NOS activity in Abcg1–/– mice was significantly decreased (Figure 4C). These data are consistent with the reduction of eNOS dimer levels in aorta (Figure 3, E and F). We also isolated ECs from aorta in WTD-fed WT and Abcg1–/– mice using an affinity column with anti-PECAM antibody. After isolation of ECs, PECAM, eNOS, and Abcg1 mRNA levels were increased by 15- to 20-fold compared with the non-endothelial fraction (data not shown). There was no significant difference in cholesterol content between WT and Abcg1–/– mice (Figure 4D). 7-KC levels (Figure 4E) and the 7-KC/cholesterol ratio (Figure 4F) were significantly increased in the ECs isolated from Abcg1–/– mice, but not in the non-EC fraction. These findings suggest that lack of ABCG1 in ECs leads to 7-KC accumulation and reduced eNOS dimer levels and that decreased eNOS activity is responsible for impaired vascular relaxation in mice fed HCDs.
Figure 4. LacZ expression in Abcg1–/– mice and NOS activity and sterol mass in WTD-fed WT and Abcg1–/– mice.
(A) LacZ expression in endothelium of aorta in Abcg1–/– mouse. Blue nuclear lacZ expression was detected specifically in ECs (arrowheads) but not in other cells indicated by nuclear fast red (arrows). (B–F) WT and Abcg1–/– mice were put on a WTD for 12 weeks (n = 4 per group). (B) PECAM-immunostained aorta. Original magnification, ×200 (A), ×100 (B). (C) Aortic NOS activity. (D) Cholesterol mass, (E) 7-KC mass, and (F) 7-KC/cholesterol ratio in ECs and non-ECs from mouse aortas. The results are represented as mean ± SEM.
Effects of HDL and 7-KC on eNOS dimer and NOS activity.
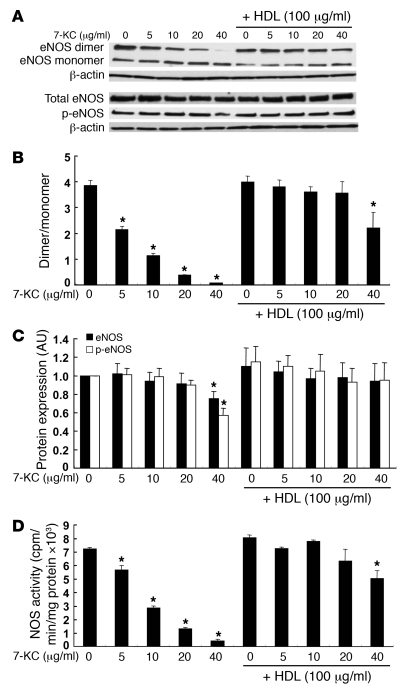
To further investigate the role of HDL and ABCG1 in promoting efflux of 7-oxysterols and preserving eNOS dimer levels and activity, we carried out experiments using human aortic ECs (HAECs), which are known to have a high level of ABCG1 (29). We first tested the effects of different concentrations of 7-KC (5–40 μg/ml) and HDL (100 μg/ml). 7-KC (5–40 μg/ml) significantly reduced eNOS dimer levels (Figure 5, A and B). Treatment of cells with HDL (100 μg/ml) following exposure to 7-KC prevented disruption of eNOS dimer levels by 7-KC (Figure 5, A and B). Only treatment with a high concentration of 7-KC (40 μg/ml) reduced eNOS and phospho-eNOS levels (Figure 5, A and C), and this did not change the ratio of phospho-eNOS to eNOS. The 7-KC concentration of 40 μg/ml also reduced eNOS mRNA (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI35470DS1) and induced apoptosis (Supplemental Figure 1B), but this was not observed at lower concentrations. Notably the concentration range of 5–10 μg/ml led to 7-KC levels that were comparable with those in isolated ECs from Abcg1–/– mice (see below). HDL treatment completely preserved eNOS dimer levels up to a concentration of 7-KC of 20 μg/ml (Figure 5, A and C). Increasing doses of 7-KC also progressively impaired eNOS activity, and this effect was reversed by HDL (Figure 5D). These data demonstrate a strong correlation between decreased eNOS dimer levels and NOS activity in response to increasing doses of 7-KC and show that both effects are reversed by HDL. 7-KC did not affect inflammatory gene expression such as Il6 or Mcp1 (Supplemental Figure 2). Insig1 and LDL receptor mRNA levels, which are regulated by SREBP-2, were reduced by 7-KC (Supplemental Figure 2). The reduction of these mRNAs most likely reflected intracellular accumulation of 7-KC.
Figure 5. Effects of HDL on eNOS dimer, eNOS, and phospho-eNOS levels, and NOS activity in HAECs.
HAECs were incubated with 7-KC (5–40 μg/ml) in the presence or absence of HDL (100 μg/ml) for 16 h, and cell lysates were analyzed by western blotting. (A) Western blot for eNOS dimer and monomer, total eNOS and phospho-eNOS. (B) Quantification of eNOS dimer/monomer levels. (C) Quantification of eNOS (filled bars) and phospho-eNOS (open bars). (D) NOS activity. The results are represented as mean ± SEM of 3 individual experiments. *P < 0.05 versus control.
Cholesterol and 7-KC mass efflux.
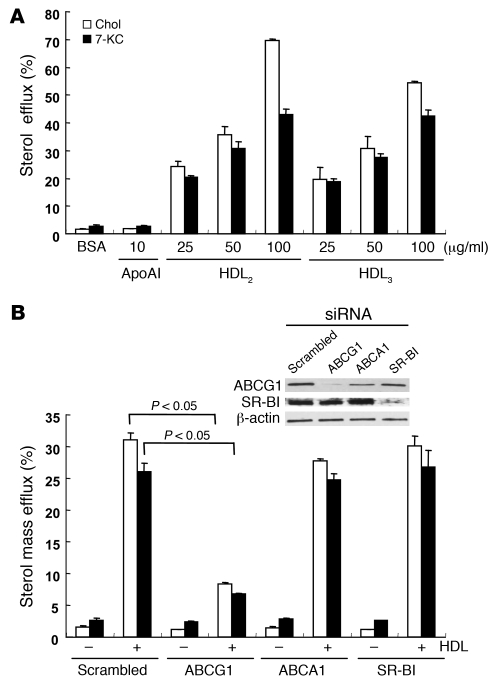
Next, we measured cholesterol and 7-KC mass efflux to different acceptors in HAECs. HAECs were loaded with cholesterol (5 μg/ml) and 7-KC (5 μg/ml) for 24 h. Before starting efflux, intracellular cholesterol and 7-KC contents were measured to determine the baseline contents for the control dishes. Intracellular 7-KC contents were around 10 μg/mg protein and corresponded to the content in the isolated ECs from aortas in WTD-fed Abcg1–/– mice (Figure 4E).
HDL2 (25–100 μg/ml) and HDL3 (25–100 μg/ml) stimulated both cholesterol and 7-KC mass efflux, whereas apoA-I did not (Figure 6A). We examined the effect of suppression of ABCG1, ABCA1, and SR-BI by siRNA transfection on HDL-mediated cholesterol and 7-KC mass efflux. Western blotting showed effective suppression of ABCG1 and SR-BI; however, ABCA1 was not readily detected in these non–liver X receptor agonist–treated cells (Figure 6B, inset). Suppression of ABCG1 significantly reduced both cholesterol and 7-KC mass efflux (Figure 6B). Neither suppression of ABCA1 nor SR-BI affected cholesterol or 7-KC mass efflux (Figure 6B). These data indicate that the ABCG1-mediated sterol efflux pathway is predominant in HAECs.
Figure 6. Effects of ABCG1 and HDL on sterol efflux in HAECs.
(A) HAECs were loaded with cholesterol or 7-KC mixture (each 5 μg/ml) for 24 h. HAECs were washed with PBS and incubated with different acceptors for 16 h. (B) HAECs were transfected with scrambled, ABCG1, ABCA1, or SR-BI siRNA. Twenty-four hours after transfection, HAECs were incubated with cholesterol (5 μg/ml) or 7-KC (5 μg/ml) for 24 h. HAECs were then washed with PBS and incubated with or without HDL2 (50 μg/ml) for 16 h. Results are represented as mean ± SEM of 3 individual experiments. Inset: Western blot for ABCG1 and SR-BI.
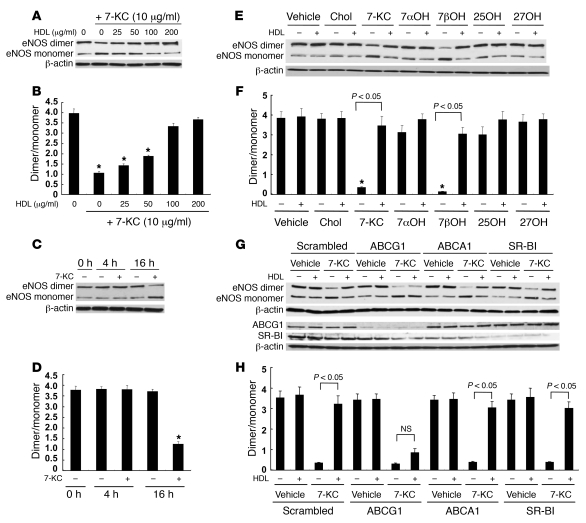
Effects of ABCG1 and HDL on eNOS dimer levels.
We examined the effects of different concentrations of HDL on eNOS dimer disruption by 7-KC. HDL treatment protected the disruption of eNOS dimer levels in a concentration-dependent manner with concentrations between 25 and 100 μg/ml (Figure 7, A and B). The reduction of eNOS by 7-KC required a relatively long incubation time (>4 h) (Figure 7, C and D). We have previously reported a specific role of ABCG1 in the efflux of 7-oxysterols (35). To further evaluate the role of ABCG1 on eNOS dimer levels, we tested the effects of different oxysterols and cholesterol (each 10 μg/ml) in similar experiments. 7β-Hydroxycholesterol as well as 7-KC significantly decreased eNOS dimer levels (Figure 7, E and F). HDL treatment prevented 7β-hydroxycholesterol–induced eNOS dimer disruption. Cholesterol, 7α-hydroxycholesterol, 25-hydroxycholesterol, or 27-hydroxycholesterol did not affect eNOS dimer levels (Figure 7, E and F). This pattern of predominant effects of 7-oxysterols on eNOS dimer levels parallels the specific role of ABCG1 in promoting efflux of these oxysterols compared with other sterols (35).
Figure 7. Effects of HDL concentrations, incubation time with 7-KC, different oxysterols, and ABCG1 expression on eNOS dimer levels.
(A and B) Effects of HDL concentrations on eNOS dimer disruption by 7-KC. HAECs were incubated with 7-KC (10 μg/ml) and HDL (25–200 μg/ml) for 16 h. (A) Western blot for eNOS dimer and monomer. (B) Quantification of the eNOS dimer/monomer. (C and D) Effects of incubation time with 7-KC on eNOS dimer disruption. (C) Western blot for eNOS dimer and monomer. (D) Quantification of the eNOS dimer/monomer. *P < 0.05 compared with no 7-KC at same time point. (E and F) Effects of different oxysterols on eNOS dimer disruption. HAECs were incubated with 10 μg/ml cholesterol or oxysterols in the presence or absence of HDL (100 μg/ml) for 16 h. 7αOH, 7α-hydroxycholesterol; 7βOH, 7β-hydroxycholesterol; 25OH, 25-hydroxycholesterol; 27OH, 27-hydroxycholesterol. (E) Western blot for eNOS dimer and monomer. (F) Quantification of the eNOS dimer/monomer. (G and H) HAECs were transfected with scrambled, ABCG1, ABCA1, or SR-BI siRNA. Forty-eight hours after transfection, HAECs were incubated with 7-KC (10 μg/ml) in the presence or absence of HDL (100 μg/ml) for 16 h. (G) Western blot for eNOS dimer and monomer. (H) Quantification of the eNOS dimer/monomer. The results are represented as mean ± SEM of 3 individual experiments. *P < 0.05 versus control.
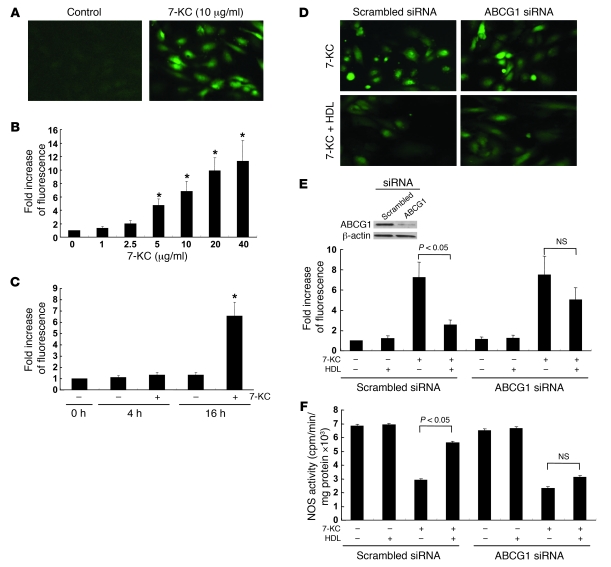
To assess the specific role of ABCG1 in 7-KC–induced disruption of eNOS dimer levels, we knocked down expression of ABCG1 by siRNA. In ABCG1 siRNA-transfected HAECs, the protective effect of HDL was abolished (Figure 7, G and H). In contrast, suppression of neither ABCA1 nor SR-BI affected the ability of HDL to protect against disruption of eNOS dimer levels by 7-KC (Figure 7, G and H). These experiments show a specific requirement for ABCG1 in the ability of HDL to promote 7-KC efflux and to protect ECs from eNOS dimer disruption induced by 7-KC.
Effects of ABCG1 and HDL on ROS production.
Previous studies have shown that disruption of eNOS dimer levels can be mediated by peroxynitrite (ONOO–), which is generated from superoxide (O2–) and NO (38). To investigate the hypothesis that HDL and ABCG1 reverse the effects of 7-KC on ROS production, we used the cell-permeable reagent 6-carboxy-2,7-dichlorodihydrofluorescein diacetate, diacetoxymethyl-ester (CM-H2DCFDA) (39). In HAECs, 7-KC (5–40 μg/ml) induced ROS formation in a dose-dependent manner (Figure 8, A and B). The ROS production by 7-KC required more than 4 h incubation (Figure 8C). The concentration dependence and the time-course response paralleled those of eNOS dimer disruption induced by 7-KC (Figure 7, A–D). 7β-Hydroxycholesterol (10 μg/ml) also significantly increased ROS (P < 0.01), whereas cholesterol, 7α-hydroxycholesterol, 25-hydroxycholesterol, or 27-hydroxycholesterol did not (data not shown). Incubation with HDL (100 μg/ml) significantly reduced ROS production by 7-KC (10 μg/ml) (Figure 8, D and E). This HDL protection was virtually abolished by ABCG1 knockdown (Figure 8, D and E). Similar protective effects of HDL and ABCG1 on ROS production by 7-KC were observed in primary mouse aortic ECs isolated in a manner similar to the studies described in Figure 4, D–F (see also Supplemental Figure 3). We also measured NOS activity in the similar experiments. HDL preserved the reduction of NOS activity by 7-KC, whereas the HDL protection was abolished by ABCG1 siRNA transfection (Figure 8F). These experiments suggest HDL preserves eNOS dimer levels and activity by promoting efflux of 7-KC via ABCG1 and thus reducing ROS formation.
Figure 8. Effects of ABCG1 and HDL in ROS production by 7-KC.
(A and B) HAECs were incubated with 7-KC (1–40 μg/ml) for 16 h. Intracellular ROS was determined after 30 min of pulse, using CM-H2DCFDA. (A) Fluorescence of CM-H2DCFDA in HAECs. (B) Quantification of CM-H2DCFDA fluorescence. (C) Fold increase in fluorescence over time with 7-KC treatment. *P < 0.05 compared with no 7-KC at same time point. (D–F) HAECs were transfected with scrambled or ABCG1 siRNA. Forty-eight hours after transfection, HAECs were treated with 7-KC (10 μg/ml) in the presence or absence of HDL (100 μg/ml) for 16 h. (D) Fluorescence of CM-H2DCFDA. (E) Quantification of CM-H2DCFDA fluorescence. Inset: Western blot for ABCG1. (F) NOS activity. The results are represented as mean ± SEM of 3 individual experiments. Original magnification, ×200. *P < 0.05 versus control.
Effects of antioxidants and NG-nitro-l-arginine methyl ester on eNOS dimer levels.
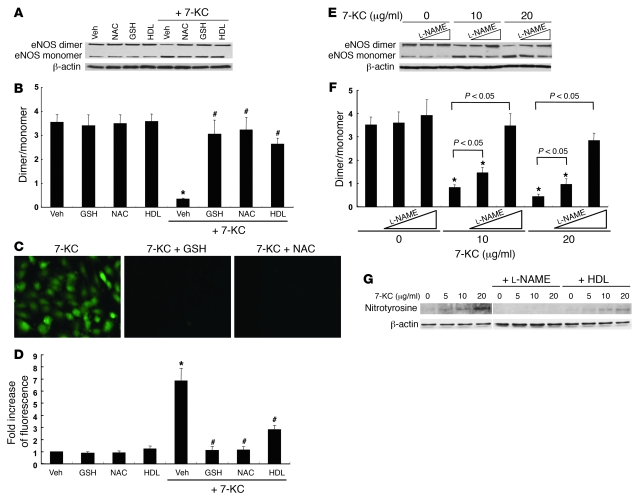
To confirm that 7-KC–induced eNOS dimer disruption is mediated by ROS, we also evaluated the effects of 2 potent antioxidants, glutathione (GSH) and N-acetylcysteine (NAC). Both GSH and NAC showed protective effects on eNOS dimer disruption (Figure 9, A and B) and ROS production (Figure 9, C and D). To determine the site of 7-KC–induced intracellular ROS production, HAECs were also incubated with carbonyl cyanide m-chlorophenylhydrazone (CCCP), an uncoupler of oxidative phosphorylation that abolishes the mitochondrial membrane proton gradient. CCCP did block the ability of 7-KC to induce ROS, suggesting that mitochondria were the source of ROS production by 7-KC (Supplemental Figure 4). By contrast, knockdown of NADPH oxidases Nox1, Nox2, and Nox4, did not affect 7-KC–induced ROS production (data not shown).
Figure 9. Effects of antioxidants and NOS inhibitor on eNOS dimer disruption by 7-KC.
(A–D) HAECs were incubated with 7-KC (10 μg/ml) in the presence of GSH (10 mM), NAC (10 mM), or HDL (100 μg/ml) for 16 h. (A) Western blot for eNOS dimer and monomer. (B) Quantification of the eNOS dimer/monomer ratio. (C) Fluorescence of CM-H2DCFDA. Original magnification, ×200. (D) Quantification of CM-H2DCFDA fluorescence. (E and F) HAECs were incubated with 7-KC in the presence or absence of l-NAME for 16 h. (E) Western blot for eNOS dimer and monomer. (F) Quantification of the eNOS dimer/monomer ratio. (G) HAECs were incubated with 7-KC (5–20 μg/ml) in the presence of l-NAME or HDL (100 μg/ml) for 16 h. Western blot for nitrotyrosine. All lanes were run on the same gel but were noncontiguous between 7-KC and 7-KC + l-NAME. The results are represented as mean ± SEM of 3 individual experiments. *P < 0.05 versus vehicle control; #P < 0.05 versus 7-KC alone. Veh, vehicle.
To further analyze the mechanism of eNOS dimer disruption by 7-KC, we investigated the effect of NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME). l-NAME treatment prevented eNOS dimer disruption by 7-KC in a dose-dependent manner (Figure 9, E and F). We also investigated the effect of 7-KC on protein tyrosine nitrosylation, since nitrotyrosine formation is considered an indicator for ONOO– production. Treatment with 7-KC significantly increased the detection of nitrotyrosine-positive protein (Figure 9G). In addition, either the presence of l-NAME or HDL significantly reduced the level of nitrotyrosine formation (Figure 9G). These data strongly suggest that 7-KC induces formation of O2–, which reacts with eNOS-generated NO to form ONOO–, which in turn leads to eNOS oxidation.
Effect of apoA-I transgene expression in endothelial function.
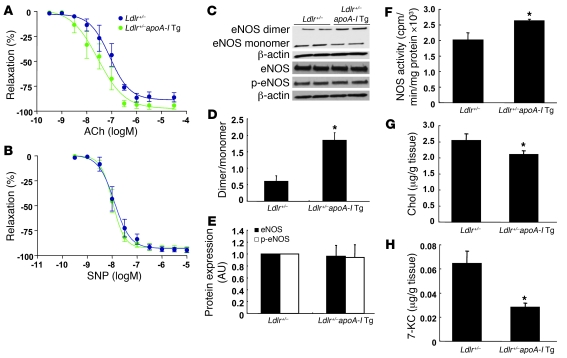
To further evaluate the role of HDL in endothelial function, we also investigated the effect of apoA-I transgene expression on endothelium-dependent vasorelaxation in HCD-fed Ldlr+/– mice. ApoA-I transgene expression significantly improved endothelium-dependent vasorelaxation (EC50: Ldlr+/–, 84.4 ± 11.6 vs. Ldlr+/–apoA-I Tg, 23.4 ± 6.5 nM; P < 0.05) (Figure 10A). There was no difference between the groups in the response to SNP (Figure 10B). ApoA-I transgene expression also significantly increased eNOS dimer levels (Figure 10, C and D) and NOS activity (Figure 10F). There was no difference between the groups in eNOS and phospho-eNOS levels (Figure 10, C and E). We also measured cholesterol and 7-KC contents in the aortas. In Ldlr+/–apoA-I Tg mice, both cholesterol (Figure 10G) and 7-KC (Figure 10H) contents were significantly decreased, but the magnitude of 7-KC reduction was more pronounced. These data suggest that increased HDL levels resulting from apoA-I transgene expression promote efflux of 7-KC from the aorta, contributing to preservation of eNOS dimer levels and activity.
Figure 10. Effect of apoA-I transgene expression in endothelial function in HCD-fed Ldlr+/– mice.
Ldlr+/– and Ldlr+/–apoA-I Tg mice (n = 6 per group) were put on a HCD for 6 weeks. (A) ACh-induced vasorelaxation. (B) SNP-induced vasorelaxation. (C) Western blot for eNOS dimer and monomer, eNOS, and phospho-eNOS in aorta. (D) Quantification of the eNOS dimer/monomer ratio. (E) Quantification of eNOS and phospho-eNOS. (F) Aortic NOS activity. (G) Cholesterol and (H) 7-KC contents in aorta. The results are represented as mean ± SEM. *P < 0.05 versus control.
Discussion
One of the most important athero-protective functions of HDL is thought to be the stimulation of macrophage cholesterol efflux, and recent studies have highlighted the key roles of ABCA1 and ABCG1 in reversing macrophage foam cell formation (40) and atherosclerosis (41, 42). HDL has also been shown to exert a variety of beneficial actions that are independent of macrophage cholesterol efflux. For example, HDL inhibits LDL oxidation, smooth muscle cell migration, and platelet aggregation and reverses endothelial dysfunction (20–22). Our studies revealed a non-redundant role of ABCG1 and a lesser role of ABCA1 in preserving endothelial eNOS activity in mice fed HCDs, and our results suggest that this may be a major mechanism underlying the ability of HDL to defend endothelial NO activity in response to such diets. The ability of ABCG1 to preserve endothelial function appears to be at least partly related to its role in promoting efflux of 7-oxysterols such as 7-KC to HDL.
HDL has consistently been shown to increase eNOS-dependent NO activity in cultured ECs (24, 25) and aortic rings (43) and in human forearm blood flow studies (44, 45). In humans, HDL levels are correlated with flow-mediated vasodilation responses of the brachial artery (18, 19) and with decreased coronary vasoconstrictor responses (44). Importantly, infusion of recombinant phospholipid/apoA-I particles into Tangier disease heterozygotes with isolated low HDL levels reversed defective forearm blood flow measurements (46). Deckert et al. (7, 8) showed that 7-oxysterols can produce decreased eNOS activity in rabbits and HUVECs. In apoE–/– mice fed a chow diet, arterial eNOS activity was preserved but became impaired when mice were challenged with a HCD (4). Importantly, apoA-I transgene expression reversed the decrease in eNOS activity induced by the HCD (4). The current study in HCD-fed Ldlr+/– mice reproduced preservation of endothelium-dependent aortic relaxation by apoA-I transgene overexpression (Figure 10), and we show that this effect is associated with preservation of eNOS dimer levels and eNOS activity and reduced aortic 7-KC levels.
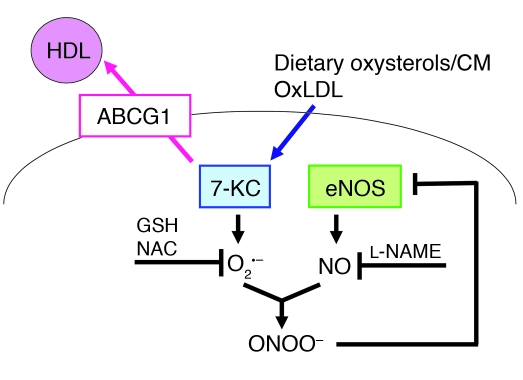
Our studies extend these important earlier observations (4, 7, 8) and suggest that the underlying mechanism by which increased or basal HDL levels protect the endothelium involves efflux of dietary sterols, especially 7-oxysterols from ECs to HDL, mediated principally by ABCG1 (Figure 11). It is most likely that dietary oxysterols are normally incorporated into chylomicrons, cleared by the liver, converted into bile acids, and excreted (13). However, when there is delayed clearance of chylomicron remnants, as occurs in apoE–/– mice or in humans with increased coronary heart disease risk (47), the vascular endothelium has increased exposure to dietary oxysterols, and ABCG1 and HDL likely have a key role in excluding or promoting efflux of 7-oxysterols from ECs. Indeed, we found that ABCG1 was expressed specifically in endothelium in non-atherosclerotic mouse aorta (Figure 4A) and 7-KC also accumulated in ECs isolated from the aorta in Abcg1–/– mice (Figure 4E). Even though HDL may have a variety of different antioxidant properties in different settings (48, 49), the ability of HDL to promote efflux of 7-KC, reduce ROS production, and preserve eNOS dimer levels and activity were all dependent on ABCG1 expression, indicating that the underlying mechanism involves ABCG1-mediated oxysterol efflux. Notably, while 7-KC was readily detected in non-lesioned arteries from mice fed HCDs, it was not measurable in arteries from mice fed chow diets (Figure 2), making it unlikely that 7-KC was artifactually formed during sample processing. Moreover, 7-KC was specifically increased as a result of ABCG1 deficiency (Figure 2), consistent with the role of ABCG1 and not ABCA1 in promoting efflux of this oxysterol to HDL (35).
Figure 11. Diagram illustrating the sequence of events triggered by 7-KC and involved in eNOS dimer disruption and the inhibitory effect of HDL and ABCG1.
CM, chylomicron; oxLDL, oxidized LDL.
In the present study in HAECs, disruption of eNOS dimer levels was induced by a 7-KC concentration of 5 μg/ml, which might be equivalent to levels found in human plasma after a fat-rich meal (50). Intracellular 7-KC content in HAECs treated with the relevant concentrations of 7-KC (5–10 μg/ml) were around 10 μg/mg protein, approximating the concentration found in isolated ECs from aortas in WTD-fed Abcg1–/– mice (Figure 4), in which eNOS dimer levels were reduced (Figure 3). Thus, these 7-KC concentrations are likely sufficient to induce endothelial dysfunction. The current findings agree with the notion that endothelial dysfunction is a key feature of early atherosclerosis (1) and also occurs transiently in the postprandial state (51).
Our parallel studies in mice and in HAECs suggest that ABCG1 mediates the efflux of 7-oxysterols from ECs to HDL, resulting in decreased ROS formation and preservation of the active dimeric form of eNOS (Figure 9). O2– is known to inactivate NO and generate ONOO– (37, 38). ONOO– can disrupt eNOS dimers through oxidation and displacement of the zinc metal ion (37, 52). Our studies also demonstrate that both l-NAME and antioxidants reversed the disruption of eNOS dimer levels by 7-KC (Figure 9). These data strongly suggest that 7-KC induced O2– and ONOO– production through interaction with NO, resulting in eNOS oxidation (Figure 11). There is considerable evidence that increased ROS can inhibit eNOS dimer formation and produce endothelial dysfunction in vivo, for example in diet-induced diabetic mice (53, 54) or in apoptosis signal–regulating kinase-1–deficient mice (55).
A number of different mechanisms have been proposed to account for the ability of HDL to preserve or increase arterial eNOS activity. HDL appears to be moderately effective in inducing eNOS-dependent vascular relaxation when directly added to aortic rings isolated from rats or mice (43, 56). However, the effect is very rapid (within a few minutes) and is saturated at very low concentrations of HDL (10 μg/ml), far below that normally bathing the endothelium (43). The response to added HDL is defective in vascular rings isolated from chow diet–fed SR-BI–/– mice (43), and from mice lacking the lysophospholipid S1P3 receptor (56). The direct effect of HDL on induction of eNOS activity has also been attributed to minor components such as lysophospholipids (56) or estrogen (57), but the concentrations of these components may not be sufficiently high to be physiologically relevant (25). While SR-BI may not have a major role in mediating net cellular cholesterol efflux to HDL in vivo, it is likely that ABCA1 and ABCG1 do mediate net efflux (27, 42, 58). Finally, our study has not assessed the role of ABC transporters in efflux to HDL of oxidized phospholipids, which are also likely to be important in endothelial dysfunction (59–61). Further studies are required to assess the relative roles of these different potential mechanisms in HDL-induced eNOS activity in vivo. Our study conclusively demonstrates the essential role of the ABC transporters, and especially ABCG1, in this process and delineates one mechanism involving efflux of 7-oxysterols and preservation of eNOS dimer levels.
Therapies that increase HDL levels, such as niacin and cholesteryl ester transfer protein inhibitors, probably activate the ABCG1-cholesterol/oxysterol efflux pathway not only in macrophages (26–28, 35) but also in ECs, likely with beneficial effects on endothelial function. Importantly, niacin therapy has been shown to improve NO-mediated vascular relaxation in humans (62). Our studies suggest that the underlying mechanism may involve increased efflux of cholesterol and 7-oxysterols via the ABCA1 and ABCG1 pathway.
Methods
Materials.
The ROS-sensitive fluorescent probe CM-H2DCFDA and nuclear fast red were from Invitrogen. Anti-eNOS and anti–phospho-eNOS (S1177) antibodies were obtained from BD Transduction Laboratories. Anti-ABCG1, anti-PECAM, and anti-nitrotyrosine antibodies were purchased from Abcam. SR-BI antibody was from Santa Cruz Biotechnology Inc. Anti–β-actin antibody, X-gal (5-bromo-4-chloro-3-indolyl β-d-galactopyranoside), lipoprotein-deficient serum, NAC, GSH, phenylephrine, ACh, SNP, cholesterol, 7-KC, 7β-hydroxycholesterol, and 25-hydroxycholesterol were purchased from Sigma-Aldrich. 27-Hydroxycholesterol was obtained from Steraloids. l-NAME was purchased from Cayman Chemical. Human apoA-I was obtained from Biodesign International. HDL (density 1.063–1.210 g/ml), HDL2 (density 1.063–1.125 g/ml), and HDL3 (density 1.125–1.210 g/ml) were isolated by preparative ultracentrifugation from normolipidemic human plasma and stored in PBS.
Mouse studies.
Abcg1–/–, Abca1–/–, and Abca1–/–Abcg1–/– mice have been previously described (41). We performed studies with a chow diet (0.025% cholesterol), a HCD (1.25% cholesterol, 7.5% cocoa butter, and 0.5% sodium cholate; catalog no. TD88051; Harlan Teklad) and a WTD (21% milk fat, 0.2% cholesterol; catalog no. TD88137; Harlan Teklad).
C57BL/6 Ldlr–/– mice and C57BL/6 apoA-I Tg mice (63) were obtained from the Jackson Laboratory and crossed to generate Ldlr+/–apoA-I Tg mice. Next, these animals were crossed with DBA/1LacJ mice (The Jackson Laboratory) to obtain the genetically uniform F1 generation. F1 hybrid C57BL/6 × DBA Ldlr+/–apoA-I Tg mice were put on the HCD.
Animals had ad libitum access to both food and water. Animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University.
Tissue collection.
Mice were anesthetized with an intraperitoneal injection of ketamine. The chest and peritoneal cavity were opened and the circulatory system was perfused via the left ventricle with PBS. Aortas were removed and processed for all assays. For vascular studies, the left superficial femoral artery was removed and immediately placed in ice-cold physiologic salt solution.
Vascular function studies.
Femoral arteries with intact endothelium and similar dimensions were mounted on a small vessel wire myograph (Danish MyoTechnology) as described previously (53). Vessels were bathed in physiologic salt solution at 37°C and aerated continuously with 5% CO2/95% O2 to achieve pH 7.4. The startup protocol and evaluation of vessel viability was conducted as described previously (53). Concentration response curves were performed for ACh (endothelium dependent) and SNP (endothelium-independent NO-releasing agent). Wall tension was expressed as mN/mm of artery length. Sensitivity to the agonist was expressed as the negative log of EC50 (–log EC50). Sensitivity was calculated from each concentration response curve by fitting the Hill equation using Prism (GraphPad Software).
Isolation of ECs from aorta.
Mice aortas were perfused with PBS and digested in RPMI 1640 medium containing collagenase D (2 mg/ml; Roche Applied Science) at 37°C for 45 min. The digest was sequentially filtered through 100-μm, 70-μm, and 40-μm cell strainers and was washed with PBS. The cells were incubated with anti-PECAM biotin-conjugated antibody (Millipore) at 4°C for 15 min and were washed with PBS. Next, the cells were labeled with streptavidin microbeads (Miltenyi Biotec) and aortic ECs were separated by MACS column (Miltenyi Biotec) according to the manufacturer’s instructions. Isolated aortic ECs were used for sterol mass measurement.
LacZ expression and PECAM immunostaining.
The tissues were snap-frozen in OCT and stored at –80°C. Frozen sections 10 μm long were prepared. To determine β-galactosidase activity, the glass slides were incubated for 16 h in the presence of X-gal. The slides were counterstained with nuclear fast red. PECAM immunostaining was carried out as previously described (64).
Cell culture.
HAECs and the culture medium EMG-2 were purchased from Lonza. The cells were grown in EMG-2 at 37°C in humidified 5% CO2 and used for experiments between passages 3 and 5. All siRNAs were purchased from Invitrogen or Santa Cruz Biotechnology Inc. HAECs were transfected with siRNA using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s protocol. Forty-eight hours after transfection, HAECs were treated with 7-KC in the presence or absence of HDL.
Sterol mass analysis.
The lipid fractions of abdominal aortas, isolated ECs or non-ECs from aorta, and HAECs were extracted using hexane/isopropanol (3:2 vol/vol) in presence of stigmasterol added as the internal standard. Total cholesterol and 7-KC were determined after saponification by gas-liquid chromatography (26, 35).
Sterol mass efflux assay.
HAECs were incubated in EGM-2 plus 5% lipoprotein-deficient serum with cholesterol (5 μg/ml) and 7-KC (5 μg/ml) for 24 h. The next day, cells were washed with PBS and then incubated in EGM-2 plus 5% lipoprotein-deficient serum alone or supplemented with human apoA-I or HDL for 16 h. After the efflux period, media and cells were collected separately and lipids were extracted with hexane/isopropanol (3:2 vol/vol) with stigmastanol as the internal standard. Sterol mass of media and cells was determined using gas chromatography. Percentages of sterol mass efflux were calculated by the ratio of sterol mass in the medium to total (medium plus cellular) sterol mass.
NOS activity assay.
The NO synthesizing activity was determined by quantifying the rate of the conversion of [3H]l-arginine to [3H]l-citrulline with kits obtained from Calbiochem-Novabiochem according to the manufacturer’s instructions (52).
Western blotting.
Protein was resolved on 4%–20% SDS-PAGE reducing gels (Bio-Rad). Protein was transferred to PVDF membranes and probed with primary antibodies overnight. For detection of eNOS dimer levels, we performed low-temperature SDS-PAGE (4°C) (52, 53). Mice aorta lysates and HAEC lysates were heated to 55°C and room temperature, respectively, for 30 min in the presence of SDS and 2.5% β-mercaptoethanol.
Quantification of intracellular ROS.
The generation of intracellular ROS was estimated by incubating CM-H2DCFDA (1 μM) with cells 30 min before determination, as described previously (39).
Statistics.
Statistical analysis was performed using the Student’s t test. Bonferroni post-hoc tests were utilized. Results are represented as means ± SEM.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (HL 54591).
Footnotes
Nonstandard abbreviations used: CM-H2DCFDA, 6-carboxy-2,7-dichlorodihydrofluorescein diacetate, diacetoxymethyl-ester; GSH, glutathione; HAEC, human aortic EC; HCD, high-cholesterol diet; 7-KC, 7-ketocholesterol; l-NAME, NG-nitro-l-arginine methyl ester; NAC, N-acetylcysteine; SNP, sodium nitroprusside; SR-BI, scavenger receptor B-I; WTD, Western diet.
Conflict of interest: A.R. Tall has received consulting fees from Pfizer, Merck, AstraZeneca, and Roche; lecture fees from Merck; and grant support from Merck and Pfizer.
Citation for this article: J. Clin. Invest. 118:3701–3713 (2008). doi:10.1172/JCI35470
References
- 1.Zeiher A.M., Drexler H., Wollschlager H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 2.Bossaller C., et al. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5ι-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forstermann U., Mtigge A., Alheid U., Haverich A., Frdlich J.C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ. Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Deckert V., et al. Impairment of endothelium-dependent arterial relaxation by high-fat feeding in ApoE-deficient mice. Circulation. 1999;100:1230–1235. doi: 10.1161/01.cir.100.11.1230. [DOI] [PubMed] [Google Scholar]
- 5.Minor R.L., Jr., Myers P.R., Guerra R., Jr., Bates J.N., Harrison D.G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J. Clin. Invest. 1990;86:2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimokawa H., Vanhoutte P.M. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ. Res. 1989;64:900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- 7.Deckert V., et al. Inhibitors of arterial relaxation among components of human oxidized low-density lipoproteins. Cholesterol derivatives oxidized in position 7 are potent inhibitors of endothelium-dependent relaxation. Circulation. 1997;95:723–731. doi: 10.1161/01.cir.95.3.723. [DOI] [PubMed] [Google Scholar]
- 8.Deckert V., Katan M. Inhibition by cholesterol oxides of NO release from human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:1054–1060. doi: 10.1161/01.atv.18.7.1054. [DOI] [PubMed] [Google Scholar]
- 9.Brown A.J., Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. . 1999;142:1–28. doi: 10.1016/S0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 10.Myoishi M., et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q., Wasowicz E., Handler B., Fleischer L., Kummerow F.A. An excess concentration of oxysterols in the plasma is cytotoxic to cultured endothelialcells. Atherosclerosis. 2000;149:191–197. doi: 10.1016/S0021-9150(99)00343-3. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson M.S. Cholesterol oxides in Indian ghee: possible cause of unexplained high risk of atherosclerosis in Indian immigrant populations. Lancet. 1987;2:656–658. doi: 10.1016/s0140-6736(87)92443-3. [DOI] [PubMed] [Google Scholar]
- 13.Vine D.F., Mamo J.C.L., Beilin L.J., More T.A., Croft K.D. Dietary oxysterols are incorporated in plasma triglyceride-rich lipoproteins, increase their susceptibility to oxidation and increase aortic cholesterol concentration of rabbits. J. Lipid Res. 1998;39:1995–2004. [PubMed] [Google Scholar]
- 14.Sander B.D., Smith D.E., Addis P.B., Park S.W. Effects of prolonged and adverse storage conditions on levels of cholesterol oxidation products in dairy products. J. Food Sci. 1989;54:874–879. doi: 10.1111/j.1365-2621.1989.tb07903.x. [DOI] [Google Scholar]
- 15.van de Bovenkamp P., Kosmeijer-Schuil T.G., Katan M.B. Quantification of oxysterols in Dutch foods: egg products and mixed diets. Lipids. 1988;23:1079–1085. doi: 10.1007/BF02535656. [DOI] [PubMed] [Google Scholar]
- 16.Gordon D.J., Rifkind B.M. High-density lipoprotein-the clinical implications of recent studies. N. Engl. J. Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 17.Assmann G., Gotto A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(Suppl. 1):III-8–III-14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 18.Li X.P., et al. Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int. J. Cardiol. 2000;73:231–236. doi: 10.1016/S0167-5273(00)00221-7. [DOI] [PubMed] [Google Scholar]
- 19.Kuvin J.T., et al. Relation between high-density lipoprotein cholesterol and peripheral vasomotor function. Am. J. Cardiol. 2003;92:275–279. doi: 10.1016/S0002-9149(03)00623-4. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell B.J., Genest J., Jr. High-density lipoproteins and endothelial function. Circulation. . 2001;104:1978–1983. doi: 10.1161/hc3901.096667. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer L., Hersberger M., von Eckardstein A. High density lipoproteins in the intersection of diabetes mellitus, inflammation and cardiovascular disease. Curr. Opin. Lipidol. . 2004;15:269–278. doi: 10.1097/00041433-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Mineo C., Deguchi H., Griffin J.H., Shaul P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 23.Collins T., Cybulsky M.I.2001NF-kappaB: pivotal mediator or innocent bystander in atherogenesis ? J. Clin. Invest. 107255–264. 10.1172/JCI10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uittenbogaard A., Shaul P.W., Yuhanna I.S., Blair A., Smart E.J. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J. Biol. Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 25.Mineo C., Deguchi H., Griffin J.H., Shaul P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura F., Wang N., Chen W., Jiang X.C., Tall A.R. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J. Clin. Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yvan-Charvet L., et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 2007;27:1132–1138. doi: 10.1161/ATVBAHA.106.138347. [DOI] [PubMed] [Google Scholar]
- 28.Tall A.R., Yvan-Charvet L., Terasaka N., Pagler T., Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell B.J., Denis M., Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation. . 2004;110:2881–2888. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 30.Wang N., Silver D.L., Costet P., Tall A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. . 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 31.Oram J.F., Lawn R.M., Garvin M.R., Wade D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 32.Wang N., Lan D., Chen W., Matsuura F., Tall A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy M.A., et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan A.M., Oram J.F. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. . 2005;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 35.Terasaka N., Wang N., Yvan-Charvet L., Tall A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Crespo I., Moenne-Loccoz P., Loehr T.M., Ortiz de Montellano P.R. Endothelial nitric oxide synthase: modulations of the distal heme site produced by progressive N-terminal deletions. Biochemistry. 1997;36:8530–8538. doi: 10.1021/bi970192j. [DOI] [PubMed] [Google Scholar]
- 37.Forstermann U., Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Xie Z., Reece R., Pimental D., Zou M.H. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2006;26:2688–2695. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 39.Robbesyn F., et al. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J. 2003;17:743–745. doi: 10.1096/fj.02-0240fje. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yvan-Charvet L., et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rader D.J. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuhanna I.S., et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 44.Zeiher A.M., Schachlinger V., Hohnloser S.H., Saurbier B., Just H. Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation. 1994;89:2525–2532. doi: 10.1161/01.cir.89.6.2525. [DOI] [PubMed] [Google Scholar]
- 45.Bisoendial R.J., et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 46.Bisoendial R.J., et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 47.Bansal S., et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 48.Nicholls S.J., et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–1550. doi: 10.1161/01.CIR.0000159351.95399.50. [DOI] [PubMed] [Google Scholar]
- 49.Tall A.R. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. . J. Intern. Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 50.Emanuel H.A., Hassel C.A., Addis P.B., Bergman S.D., Zavoral J.H. Plasma cholesterol oxidation products (oxysterols) in human subjects fed a meal rich in oxysterols. J. Food Sci. 1991;56:843–847. doi: 10.1111/j.1365-2621.1991.tb05396.x. [DOI] [Google Scholar]
- 51.Vogel R.A., Corretti M.C., Plotnick G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997;79:350–354. doi: 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 52.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molnar J., et al. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ. Res. . 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 54.Hink U., et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita T., et al. Apoptosis signal-regulating kinase-1 is involved in vascular endothelial and cardiac remodeling caused by nitric oxide deficiency. Hypertension. 2007;50:519–524. doi: 10.1161/HYPERTENSIONAHA.107.092049. [DOI] [PubMed] [Google Scholar]
- 56.Nofer J.R., et al. HDL induces NO dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong M., et al. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J. Clin. Invest. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yvan-Charvet L., et al. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J. Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Watson A.D., et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 60.Rikitake Y., et al. Inhibition of endothelium-dependent arterial relaxation by oxidized phosphatidylcholine. Atherosclerosis. 2000;152:79–87. doi: 10.1016/S0021-9150(99)00453-0. [DOI] [PubMed] [Google Scholar]
- 61.Navab M., et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Kuvin J.T., et al. A novel mechanism for the beneficial vascular effects of highdensity lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- 63.Rubin E.M., Ishida B.Y., Clift S.M., Krauss R.M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc. Natl. Acad. Sci. U. S. A. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welch C.L., et al. Spontaneous atherothrombosis and medial degradation in Apoe–/–, Npc1–/– mice. . Circulation. . 2007;116:2444–2452. doi: 10.1161/CIRCULATIONAHA.107.701276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.