Abstract
Egg activation, which is the first step in the initiation of embryo development, involves both completion of meiosis and progression into mitotic cycles. In mammals, the fertilizing sperm delivers the activating signal, which consists of oscillations in free cytosolic Ca2+ concentration ([Ca2+]i). Intracytoplasmic sperm injection (ICSI) is a technique that in vitro fertilization clinics use to treat a myriad of male factor infertility cases. Importantly, some patients who repeatedly fail ICSI also fail to induce egg activation and are, therefore, sterile. Here, we have found that sperm from patients who repeatedly failed ICSI were unable to induce [Ca2+]i oscillations in mouse eggs. We have also shown that PLC, zeta 1 (PLCZ1), the sperm protein thought to induce [Ca2+]i oscillations, was localized to the equatorial region of wild-type sperm heads but was undetectable in sperm from patients who had failed ICSI. The absence of PLCZ1 in these patients was further confirmed by Western blot, although genomic sequencing failed to reveal conclusive PLCZ1 mutations. Using mouse eggs, we reproduced the failure of sperm from these patients to induce egg activation and rescued it by injection of mouse Plcz1 mRNA. Together, our results indicate that the inability of human sperm to initiate [Ca2+]i oscillations leads to failure of egg activation and sterility and that abnormal PLCZ1 expression underlies this functional defect.
Introduction
After the luteinizing hormone surge, fully grown mammalian oocytes resume meiosis and undergo maturation, advancing to the second meiotic metaphase (MII), where exit from meiosis is halted. Following ovulation, fertilization relieves this arrest and induces a succession of concurrent events that result in the completion of meiosis and preparation of the newly formed zygote for the first mitotic cell cycle; these changes are collectively referred to as egg activation (1). Easily observable signs of egg activation, such as the release of the second polar body and pronucleus (PN) formation, which precedes the initiation of zygotic DNA synthesis, are commonly used in the laboratory and in the clinic to assess successful fertilization.
It has been known for some time that the biochemical changes that underlie mammalian egg activation are induced by repeated elevations of the intracellular concentration of free cytosolic Ca2+ ([Ca2+]i), here referred to as oscillations (2, 3). Similarly, the participation of the phosphoinositide pathway, which involves production of inositol 1,4,5-trisphosphate (IP3) by PLC and binding of IP3 to its cognate receptor/channel in the ER, is a well-established feature of fertilization in almost all species studied to date (4). Despite the widely conserved role of the phosphoinositide pathway and Ca2+ release in egg activation, the mechanism or mechanisms by which sperm induce IP3 production remain largely unknown and may ultimately be phylum/class-specific.
In mammals, growing experimental evidence supports the notion that, following fusion of the gametes, a factor from sperm is responsible for inducing [Ca2+]i oscillations and stimulating IP3 production (5). Initial evidence stemmed from injection of cytosolic sperm extracts into eggs that reproduced the [Ca2+]i responses associated with fertilization regardless of the eggs’ species of origin (6, 7). Subsequent biochemical characterization of the extracts revealed that the active component contained a protein moiety (6) that possesses PLC-like activity capable of inducing production of IP3 (8, 9) and that the PLC activity was highly sensitive to Ca2+ (10). A screen of expressed sequenced tags from testes identified a sperm-specific PLC (PLC zeta 1 [PLCZ1]), the presence of which correlated with Ca2+ activity in cytosolic sperm extracts (11). Moreover, injection of eggs with the recombinant protein (12) or with the encoding mRNA induced fertilization-like oscillations (11), whereas depletion of PLCZ1 from the extracts with specific antisera abrogated PLC activity (13) and the [Ca2+]i oscillatory activity of the extracts (11, 13). Nonetheless, whether PLCZ1 represents the sole [Ca2+]i oscillation–inducing factor in mammalian sperm and how its absence has an impact on male fertility has not been conclusively established.
Male factor infertility affects a large proportion, by most accounts up to 30%, of couples seeking specialized assistance to conceive (14). The advent of intracytoplasmic sperm injection (ICSI) has overcome many male factor conditions, especially those caused by severe oligospermia, asthenospermia, teratospermia, and/or a combination thereof, which until recently were deemed to result in sterility (15). Since that first report, ICSI has proven to be a safe and highly effective treatment for the most severe cases of male factor infertility. Nevertheless, there are some unexplained conditions in which ICSI does not result in fertilization or development. Overall, only 50% to 70% of the eggs that undergo ICSI show signs consistent with fertilization (16). More importantly, fertilization failure after ICSI occurs in up to 3% of couples, and certain couples never achieve fertilization rates greater than 50% (16, 17). Presently, the molecular underpinning or underpinnings that limit the success of ICSI in these individuals remain unknown, although it is remarkable that nearly 80% of the oocytes unsuccessfully fertilized by ICSI do not exit the MII stage (18, 19), suggesting that failure of egg activation is the responsible defect.
While the etiology underlying the poor success of ICSI is likely to be multifactorial and might involve egg factors (20), it is noticeable that the success of ICSI is consistently reduced when sperm with certain morphological defects, round-headed sperm, for example, are used (21, 22). Moreover, several studies report that activation and development to term can be restored in some of these couples if an external activation stimulus, such as Ca2+ ionophore, is used at the time of sperm injection (22, 23). Collectively, these data suggest that a defective male-factor component may underlie failure of egg activation, fertilization, and conception in some patients. In humans, as expected, the signal for egg activation appears to be common with other mammals, as both in vitro fertilization and ICSI initiate typical fertilization-like [Ca2+]i oscillations (24, 25). Furthermore, injection of human PLCZ1 mRNA into human eggs evokes [Ca2+]i oscillations and egg activation (26). Therefore, given that the assumed sole function of PLCZ1 is to induce [Ca2+]i oscillations and egg activation, it is reasonable to hypothesize that sperm from patients who fail ICSI may have reduced levels or harbor a dysfunctional form of the PLCZ1 protein. Accordingly, we have attempted to determine whether the sperm from these patients show reduced ability to initiate [Ca2+]i responses and whether this is associated with abnormal distribution or expression of the PLCZ1 protein.
Results
Fertilization success after ICSI depends on the ability of the sperm to induce [Ca2+]i oscillations.
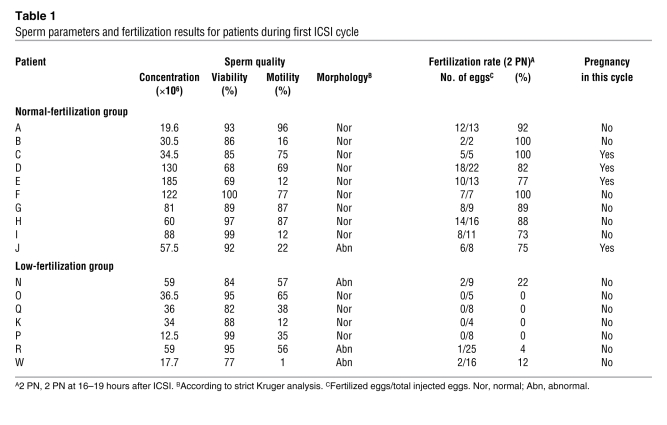
ICSI is a highly successful assisted reproductive technique capable of overcoming the most severe cases of infertility associated with male factors (15). Nonetheless, a few males consistently fail to fertilize after this procedure, and the underlying molecular mechanisms remain unknown. We therefore examined fertilization and pregnancy data from patients who had been recommended to undergo ICSI. The advice to undergo ICSI was made on the basis of a history of poor fertilization after an in vitro fertilization cycle(s) or poor semen analysis results. Based on fertilization results from this ICSI cycle, consenting patients meeting our selection criteria were grouped into a normal-fertilization group, in which more than 60% of the surviving injected eggs showed signs of normal fertilization, and into a low-fertilization group, in which 25% or less of the eggs showed PN formation after the ICSI procedure (Table 1). Unsurprisingly, 4 out of 10 women in the normal-fertilizing group conceived during this cycle, while 0 out of 7 women in the low-fertilization group became pregnant (Table 1).
Table 1 .
Sperm parameters and fertilization results for patients during first ICSI cycle
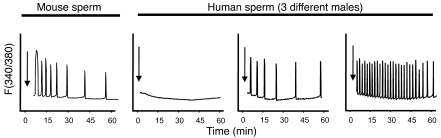
To ascertain whether the observed differences in fertilization after ICSI could be attributed to differences in the [Ca2+]i oscillation–inducing activity among our patients, we injected mouse eggs with patients’ sperm and monitored their [Ca2+]i responses. Previous studies have shown that injection of human sperm results in activation of mouse eggs, and these results were used to surmise the presence of the egg-activating factor; succinctly, successful egg activation was interpreted to mean that the sperm contained the egg-activating factor, and failure of egg activation led to the opposite assumption (27, 28). Consistent with these results, later studies found that injection of human sperm initiated [Ca2+]i oscillations in mouse eggs (25). Nonetheless, whether human sperm with distinct morphologies and from different patients could initiate consistent [Ca2+]i oscillations and whether this capacity could be related to the ability to fertilize after ICSI has not been examined. Our results show that while injection of mouse sperm induced highly similar [Ca2+]i oscillations regardless of male of origin (Figure 1; see also ref. 29), injection of the patients’ sperm evoked highly variable [Ca2+]i responses, which ranged from a complete lack of Ca2+ release to initiation of high-frequency oscillations (Figure 1).
Figure 1. Injection of mouse and human sperm into mouse eggs induces [Ca2+]i oscillations.
Mouse sperm (left panel) induce consistent oscillations (representative of at least 10 sperm per male and of at least 10 different males), whereas human sperm induce highly variable responses according to male of origin (3 right panels). Each panel corresponds to a different patient and is representative of the activity of the sperm for the particular male. Arrows denote the time of sperm injection. [Ca2+]i monitoring commenced within 10 minutes of injection. These experiments were repeated at least 5 times. F, fluorescence ratio.
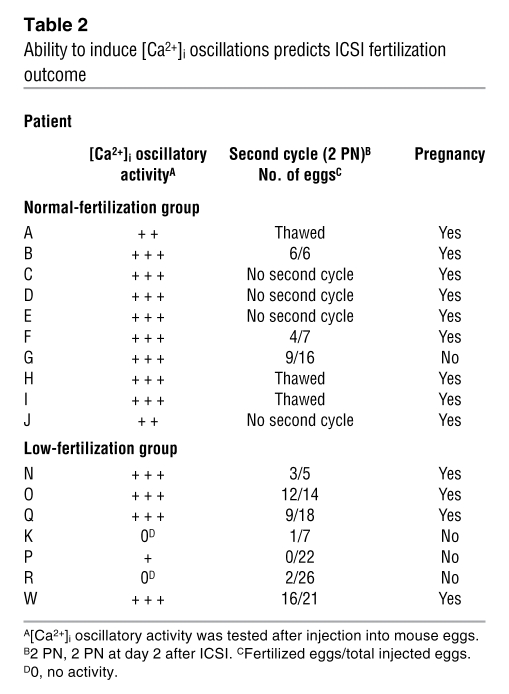
In light of this variable [Ca2+]i oscillation–inducing activity and to ascertain whether consistently low fertilizing success after ICSI could be associated with inability to induce [Ca2+]i oscillations, we tested the sperm of all 17 patients in the study and then related our results to the fertilization success and pregnancy outcome of a second ICSI cycle (Table 2). Regardless of the initial fertilizing group classification, the sperm of 14 out of 17 patients were able to induce robust [Ca2+]i oscillations, receiving scores of ++ or +++, meaning that they induced at least 6 (++) or more than 10 (+++) [Ca2+]i rises within the first 60 minutes of monitoring (Figure 2). Remarkably, 3 patients in the low-fertilization group received scores of 0 or +, which means that they induced 0 or fewer than 2 [Ca2+]i rises in the same monitoring period. As expected, these couples showed low fertilization rates and remained childless even after the second ICSI cycle. Conversely, 13 out of 14 couples with the higher [Ca2+]i scores became pregnant within the 2 ICSI cycles (Table 2). Together, these results show that the sperm’s inability to induce [Ca2+]i oscillations may predict ICSI failure.
Table 2 .
Ability to induce [Ca2+]i oscillations predicts ICSI fertilization outcome
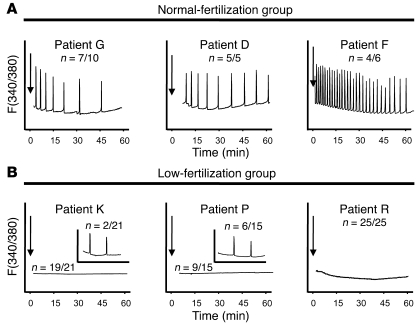
Figure 2. Sperm from patients with different ICSI fertilization rates initiate widely dissimilar [Ca2+]i oscillations in mouse eggs.
(A) [Ca2+]i profiles of 3 patients with high fertilization rates are shown. (B) Profiles belong to 3 patients who failed or showed low fertilization rates following ICSI. Insets in panels for patients K and P depict representative patterns of [Ca2+]i responses observed in the few eggs that initiated oscillations following sperm injection. Arrows denote time of sperm injection. n, numerator indicates the number of eggs that displayed the [Ca2+]i profile shown, whereas denominator denotes the total number of eggs tested.
Fertilization success after ICSI and capacity to induce [Ca2+]i oscillations require the presence of PLCZ1.
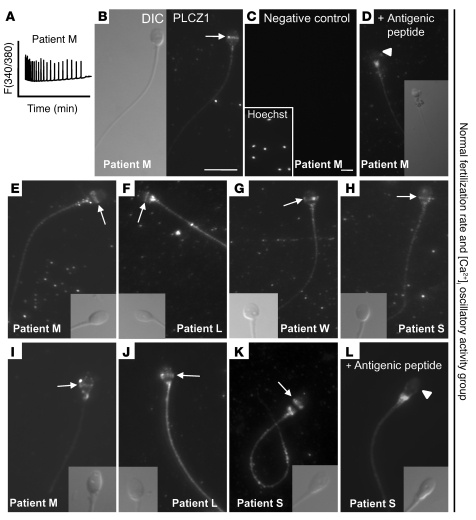
Mounting evidence in mammals suggests that PLCZ1, the only known sperm-specific PLC, is the sole molecule responsible for inducing [Ca2+]i oscillations and egg activation (30). In humans, studies showing that the fertilization-associated [Ca2+]i oscillations (24) can be closely replicated by injection of human PLCZ1 mRNA (26) support a similar role for PLCZ1 in this species. Nonetheless, the expression and distribution of PLCZ1 protein in human sperm has not been carefully examined. Toward this end, we raised 2 antibodies, MI-305 and PF-354, against different peptide sequences of human PLCZ1 and used them to localize the protein in human sperm. Using the MI-305 antibody, we found that PLCZ1 localized to the equatorial region of the sperm head (Figure 3, B–H), although in some sperm the distribution of PLCZ1 seemed to extend into the upper part of the postequatorial region (Figure 3, B and E); we attributed this seemingly variable localization to the variable shape of human sperm, even within single ejaculates. We confirmed that this distribution was specific, as similar localization of PLCZ1 in the same set of males was detected using the PF-354 antibody (Figure 3, I–L). In addition, the equatorial/postacrosomal PLCZ1-reactive band was abolished by incubation with the appropriate antigenic peptide for each antibody (Figure 3, D and L). The PF-354 antibody, which was not affinity purified, showed additional reactivity to the sperm tail (Figure 3, J and K). We interpreted this reactivity to be nonspecific, as it was not blocked by the corresponding antigenic peptide (Figure 3L) and was weaker when the MI-305 antibody was used (i.e., patient S; Figure 3, H and K). Both antibodies showed variable reactivity on the base of the sperm head and/or on the head/tail junction, although this staining was also deemed nonspecific, as the respective antigenic peptides failed to block it (Figure 3, D and L).
Figure 3. PLCZ1 localizes to the equatorial/postacrosomal region of human sperm.
Two different antibodies, MI-305 (B–H) and PF-354 (I–L), and sperm from different patients capable of inducing [Ca2+]i oscillations were used to characterize PLCZ1 localization. In B–D, localization of PLCZ1 and specificity of the MI-305 antibody were characterized using patient M’s sperm, which induced high-frequency oscillations (A). (B) Left panel shows bright field image, whereas right panel shows the corresponding immunofluorescent image. Arrows denote PLCZ1 localization. Negative controls were incubated in the absence of primary antibody (C) or after incubation with antigenic peptide (D). (C) Inset shows Hoechst 33342–stained sperm nuclei. Original magnification, ×630. (D) Inset shows reduced (50%) bright field image of fluorescent image in panel. (D) White arrowheads denote the loss of the PLCZ1 band in the presence of antigenic peptide. (E–H) Staining of different patients’ sperm (insets show corresponding bright field images). (I–L) Staining of same patients’ sperm but using the PF-354 antibody. Insets show corresponding bright field images. Scale bars: 10 μm. Scale bar for B also applies to parts D–L.
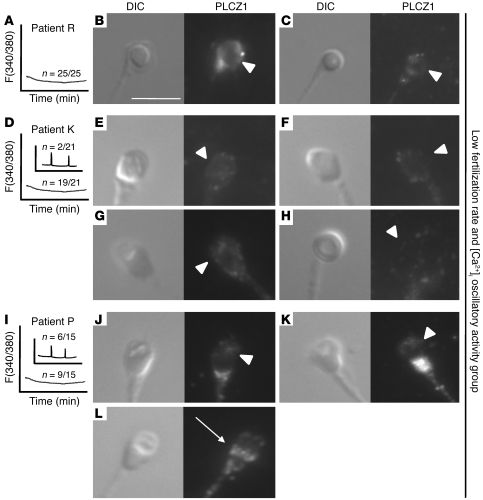
Having demonstrated the localization of PLCZ1 in human sperm capable of inducing [Ca2+]i oscillations and fertilization, we determined whether or not PLCZ1 had the same distribution in the sperm of patients who failed ICSI in the clinic and who were unable to initiate normal [Ca2+]i responses in mouse eggs. As shown in Figure 4, the sperm of patient R, nearly all of which presented a round-headed sperm shape, were devoid of PLCZ1 (Figure 4, B and C). PLCZ1 was also absent from the sperm of patient K, although only 25% of the sperm showed round-headed morphology (Figure 4, E–H). Last, patient P, who had seemingly normal sperm morphology although sperm presented widely visible vacuoles over the acrosomal area, lacked the expected equatorial PLCZ1 reactivity in the majority of sperm (Figure 4, J and K). Remarkably, approximately 30% of this patient’s sperm showed PLCZ1 reactivity in the equatorial region (Figure 4L), which is consistent with our findings that a similar percentage of this patient’s sperm induced marginal [Ca2+]i responses (Figure 4I).
Figure 4. Reduced expression of PLCΖ1 in the sperm of patients who fail ICSI and lack [Ca2+]i oscillatory ability.
MI-305 antibody and sperm from 3 patients who failed ICSI and lacked [Ca2+]i oscillatory activity (A, D, and I) were used to examine PLCZ1 localization. (B and C) Left panels show bright field images of sperm from patient R; right panels show the corresponding immunofluorescent images. White arrowheads point to the expected location of PLCZ1. (E–H and J–L) Sperm from patients K and P were examined, respectively, using the MI-305 antibody. (L) Arrow denotes PLCZ1 localization. Scale bar: 10 μm.
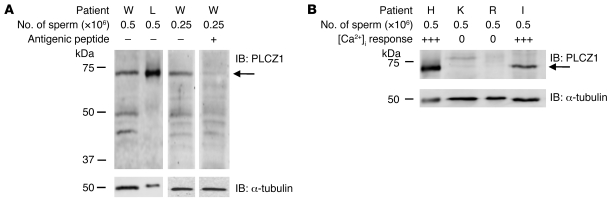
To confirm the previous immunofluorescence results, we performed Western blotting on sperm samples from patients in our study. We first determined that the MI-305 antibody was able to detect PLCZ1 under these conditions. To test this, we selected 2 patients whose sperm had been shown to have high [Ca2+]i oscillatory activity (Table 1) and/or display equatorial localization of PLCZ1 (Figure 3). As shown in Figure 5A, a band of approximately 70 kDa, which is the expected MW of human PLCZ1 (31), was detected in the samples of both patients. Moreover, this PLCZ1 signal was abolished when the antibody was incubated with the corresponding antigenic peptide (Figure 5A), confirming the specificity. We then determined whether the sperm of patients who failed ICSI and lacked [Ca2+]i oscillatory activity also were without PLCZ1 immunoreactivity. Following Percoll purification, we were able to recover a sufficient number of sperm from the samples of 2 of the 3 patients with those characteristics (K and R). Remarkably, Western blotting revealed that sperm of these patients lacked PLCZ1 immunoreactivity (Figure 5B), although sperm samples from patients with known oscillatory activity showed prominent PLCZ1 reactive bands (Figure 5B). We confirmed that these differences were not due to unequal sample sizes since, besides including equal numbers of sperm per sample, simultaneous detection of α-tubulin in each of the membranes revealed similar immunoreactivity for this protein (Figure 5, A and B). Together, these results show that PLCZ1 is present in the equatorial/postacrosomal region of human sperm heads and that its absence compromises the sperm’s ability to fertilize and initiate embryo development.
Figure 5. Expression of PLCZ1 by IB in sperm of patients capable of inducing [Ca2+]i oscillations and/or fertilization and in sperm of patients who failed ICSI.
(A) Band of approximately 70 kDa was detected in sperm extracts of patients whose sperm showed [Ca2+]i oscillatory activity; arrow on the right side of the panel denotes the expected MW of PLCZ1. The intensity of the 70-kDa band was reduced by preincubation of the MI-305 antibody with the appropriate antigenic peptide. (B) Expression of PLCΖ1 was detected in the extracts of 2 other patients with successful ICSI outcomes, but it was absent in patients K and R, who failed ICSI. IB of α-tubulin was used as a loading control and shows equal loading per lane.
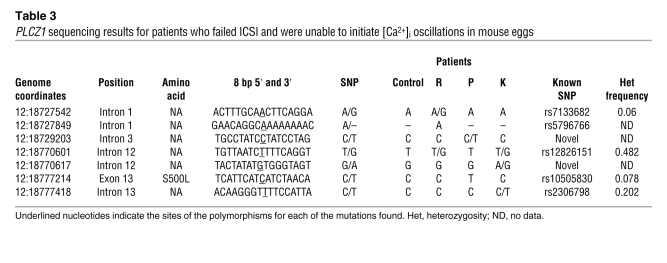
The undetectable levels of PLCZ1 protein in the sperm of 3 patients in our study could be due to several factors. We therefore determined whether abnormalities in the sequence of the PLCZ1 gene could be undermining the production of an intact, full-length protein. We sequenced genomic DNA from 1 control (proven fertile) and the 3 patients in question. The human PLCZ1 gene consists of 15 exons and is localized on chromosome 12 (31). PCR amplicons were designed including each exon of the PLCZ1 mRNA (Ensembl transcript ID ENST00000266505) and included at least 50 bases of intronic sequence upstream and downstream of exon/intron boundaries. Sequencing of PCR products revealed 8 polymorphisms among our patients (Table 3). Two of the SNPs in this region were, to our knowledge, novel (introns 3 and 12; Table 3), and 1 (dbSNP:rs10505830) resulted in an amino acid change at position 500 (S500L; Table 3). This serine-to-leucine missense mutation has been previously documented at a very low frequency (0.078). Furthermore, only heterozygous individuals have been identified. Interestingly, we found that patient P is homozygous for this mutation, which is surprising given the aforementioned frequency of heterozygosity. Whether or not this mutation has an impact on the function of human PLCZ1 remains to be demonstrated, although this and 2 adjacent serine residues are missing in the bovine PLCZ1 sequence, whose mRNA is able to induce oscillations when injected into bovine or mouse eggs (32).
Table 3 .
PLCZ1 sequencing results for patients who failed ICSI and were unable to initiate [Ca2+]i oscillations in mouse eggs
Egg activation failure after injection of human sperm into mouse eggs can be rescued by injection of mouse Plcz1 mRNA.
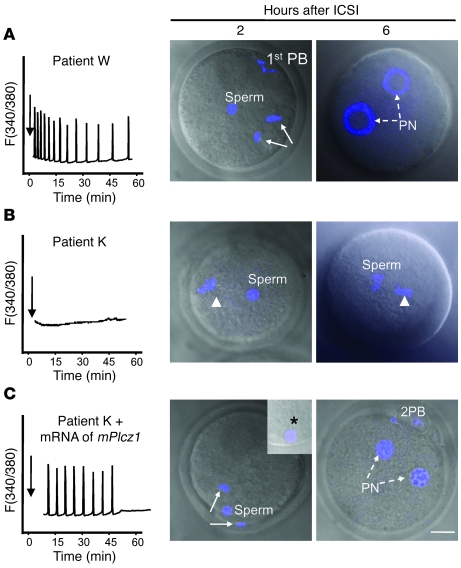
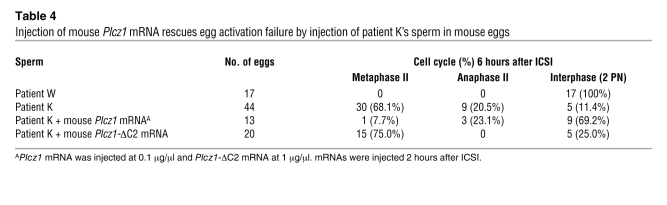
Besides abnormal PLCZ1 expression, the sperm function from patients who failed to fertilize in our study may be compromised by other unknown molecular defects that might also undermine the ability to support embryo development. Nonetheless, the fertilization phenotype of these sperm was uniform and consisted of egg activation failure (Table 1). We therefore presumed a defect in the generation of [Ca2+]i oscillations, and we found abnormal levels and localization of PLCZ1 in the sperm of these patients. Nevertheless, conclusive evidence that the egg activation failure is exclusively due to lack of PLCZ1 observed in these patients requires a rescue experiment. Accordingly, we injected mouse eggs with the sperm of patients and observed them for signs of activation. As reported by others (27, 28), we found that human sperm induce resumption of meiosis (Figure 6A), extrusion of the second polar body, and PN formation in mouse eggs (Figure 6A; Table 4). In contrast, injection of sperm from patient K failed to induce resumption of meiosis, even when examined 6 hours after ICSI (Figure 6B; similar results were seen for patient R, data not shown). Importantly, patient K’s sperm were able to undergo normal PN formation after sperm injection only if eggs were injected with mouse Plcz1 mRNA (Figure 6C; Table 4). Importantly, injection of Plcz1-ΔC2 mRNA, which lacks [Ca2+]i oscillatory activity (13), failed to induce egg activation in the majority of eggs fertilized with patient K’s sperm (Table 4). Collectively, these results indicate that abnormal PLCZ1 expression underlies the egg activation failure of these patients’ sperm and possibly the infertility of male patients who repeatedly fail ICSI.
Figure 6. Injection of mouse Plcz1 mRNA rescues egg activation failure after ICSI in mouse eggs.
(A) Injection of a sperm from patient W, with normal [Ca2+]i oscillatory activity (left panel), induces resumption of meiosis after 2 hours (arrows denote anaphase II) and PN formation (broken arrows) by 6 hours after injection. (B) Injection of sperm from patient K, which is unable to initiate [Ca2+]i oscillations (left panel), fails to induce egg activation by either 2 hours or 6 hours (arrowhead denotes MII chromatin). (C) Injection of mouse Plcz1 mRNA (0.1 μg/μl) 2 hours after injection of sperm initiates fertilization-like oscillations and rescues egg activation, as shown by the resumption of meiosis (2 hours) and PN formation (6 hours; 8 hours after injection of patient K’s sperm). 1st PB, first polar body; 2PB, second polar body. TO-PRO-3 staining (blue) was used to stain the chromatin. Asterisk in inset points to the persistence of the human sperm tail in mouse eggs. Scale bar: 10 μm.
Table 4 .
Injection of mouse Plcz1 mRNA rescues egg activation failure by injection of patient K’s sperm in mouse eggs
Discussion
It is widely acknowledged that in all species studied to date, an increase in the concentration of [Ca2+]i underlies egg activation and initiation of development. Nonetheless, how the steps upstream of Ca2+ release unfold during fertilization and whether or not a deficiency in 1 or more of the components of the Ca2+ release–signaling pathway results in infertility/sterility are issues that remain unresolved. Research shows that human males who repeatedly fail ICSI are consistently unable to induce egg activation. We therefore sought to associate these findings with a possible inability of these patients’ sperm to induce [Ca2+]i oscillations and to link this failure to PLCZ1, the sperm protein proposed to induce [Ca2+]i oscillations in mammals. We found that patients whose sperm are unable to initiate [Ca2+]i oscillations consistently fail ICSI. We also found that PLCZ1 protein is located in the equatorial region of human sperm. Importantly, both immunofluorescence and Western blot analyses indicate an absence of PLCZ1 in the sperm of patients who failed ICSI and who were unable to induce [Ca2+]i oscillations. Last, we reproduced in mice the defining phenotype of these patients, failure of egg activation, and we rescued it by injection of mouse Plcz1 mRNA. Collectively, our results show that the sperm’s inability to initiate [Ca2+]i oscillations during fertilization leads to egg activation failure and sterility and that abnormal PLCZ1 expression underlies this functional defect.
PLCZ1 protein is the mammalian sperm Ca2+ oscillator.
Since the realization that Ca2+ release and egg activation in mammals is downstream of gamete fusion (33), several sperm proteins have emerged as candidate molecules responsible for egg activation. The supporting evidence for 2 of these proteins, truncated c-Kit (34) and PAWP (35), is mainly based on the findings that their injection into mammalian and nonmammalian eggs induces activation. Nevertheless, their role in this process remains unconfirmed, as neither of these proteins has been shown to induce [Ca2+]i oscillations in eggs and the ability of PAWP to activate mouse eggs as well as the developmental capacity of zygotes activated by it remain to be ascertained. Opposite to these findings is the evidence that injection of PLCZ1 mRNA/protein induces sperm-like [Ca2+]i oscillations in eggs of all species studied to date (30) and that zygotes activated by injection of its mRNA develop to the blastocyst stage at rates comparable to those induced by in vitro fertilization (11, 32). In total, these studies support the notion that PLCZ1 is the sperm molecule responsible for initiating [Ca2+]i oscillations and is sufficient to induce complete egg activation. Nonetheless, whether PLCZ1 is necessary for egg activation requires demonstration that its absence affects egg activation and fertility. Toward this end, a transgenic RNA interference approach was used in the mouse to reduce PLCZ1 expression (36). These sperm displayed marginally decreased [Ca2+]i oscillatory ability, but mating of the male founders resulted in litters of fewer pups and, significantly, none of the offspring inherited the transgene, suggesting that reduced expression of PLCZ1 prevented embryo development. Here, we show in a more direct manner that sperm of patients who consistently fail ICSI are unable to trigger [Ca2+]i oscillations and egg activation and have reduced/absent expression of PLCZ1, clearly linking the sperm’s ability to initiate [Ca2+]i oscillations with PLCZ1 expression.
It could be argued that since the absence of PLCZ1 in the sperm of these patients is not caused by mutation to the PLCZ1 locus, although patient P showed an amino acid substitution, mutations to other genes may be responsible for the Ca2+ and/or infertility phenotype reported here. Several cogent arguments can be raised against this supposition. Regarding the lack-of–[Ca2+]i oscillation phenotype, it is worth noting that PLCZ1 is the only sperm-specific molecule capable of replicating in a concentration- and in a spatiotemporal-equivalent manner the fertilization-associated [Ca2+]i oscillations observed across mammalian species (30). In addition, PLCZ1 depletion from complete sperm extracts abrogates both the PLC and [Ca2+]i oscillatory activity from these fractions (11, 13). Regarding the infertility phenotype, we cannot rule out that a protein(s) absent in these sperm besides PLCZ1 may undermine the ability of these embryos to develop to term. Nonetheless, the uniform lack-of–egg activation phenotype observed in these patients, despite the widely dissimilar morphology of the patients’ sperm, points to an abnormal [Ca2+]i signal as the initial cause of infertility. While we also cannot rule out an “egg factor” defect, it is remarkable that we reproduced the lack-of–[Ca2+]i oscillation/activation phenotype in mouse eggs by injecting the sperm of the affected patients. Moreover, the activation failure of PLCZ1-defective sperm in these eggs was rescued by injection of mouse Plcz1 mRNA. Last, a male factor defect exclusively associated with the sperm’s inability to induce egg activation appears to underlie the failure of round-headed, globozoospermic sperm to fertilize eggs after ICSI, because fertility in several of these patients can only be restored by application of an external activation stimulus after sperm injection (37). Therefore, the simplest and most logical interpretation of our findings is that the egg activation function of the sperm of patients who repeatedly fail ICSI is undermined by the abnormal expression of PLCZ1.
The molecular underpinning or underpinnings of this atypical PLCZ1 protein expression in the subset of patients in our study are not known. Our examination of the genomic sequence of PLCZ1 in these patients revealed no major abnormalities, as only 1 patient, P, evidenced an amino acid substitution on the C2 domain of the protein. While the impact of this substitution will need to be investigated further and, curiously, some of this patient’s sperm showed some PLCZ1 localization that is consistent with the marginal [Ca2+]i oscillatory activity observed, the majority of this patient’s sperm and the sperm of 2 other patients had minimal/absent PLCZ1 expression. Although speculative, it is possible that a transcription factor regulating PLCZ1 expression may be mutated in these patients. Alternatively, the possibility exists that even though the protein is produced, it is degraded/discarded during the late stages of spermatogenesis. Although not feasible in our patients, analysis of mRNA levels in developing sperm would elucidate whether the defects are transcriptional or translational in nature. Future studies in the mouse will address the regulation of Plcz1 gene expression during spermatogenesis and the factors that contribute to the molecular localization and stability of PLCZ1 in the mature sperm.
Egg activation, [Ca2+]i oscillations, and fertility.
While the focus of this study was on cases of ICSI failure associated with extreme reduction of PLCZ1 expression, we also observed decreased, rather than absent, expression levels of PLCZ1 in the sperm of 1 patient (P), who was the only patient without round-headed sperm morphology to fail ICSI. These results raise the prospect that other infertile or subfertile males may have decreased expression of the PLCZ1 protein and that the subsequent less-than-optimal activation stimulus may affect embryo development in a more subtle manner. We raise this possibility based on recent evidence showing that the Ca2+-activation stimulus not only has an impact on the recruitment of maternal RNAs at the zygote stage (38) but also the gene expression profile at later stages of preimplantation embryo development (39, 40). Importantly, in the latter study, besides the gene expression anomalies, some of the embryos experienced developmental defects. For example, blastocysts generated by fewer than normal [Ca2+]i rises displayed lower implantation rates, which correlated with reduced expression of cell adhesion genes, some of which are involved in the process of implantation (41). It would be interesting therefore to determine whether similar changes in gene expression may occur in young couples that suffer unexplained but recurrent implantation failure (42). Research shows that the 2 most noticeable defects in these couples are the presence of a male factor and a decreased fertilization rate after ICSI, which here we show is associated with decreased expression of PLCZ1. It is therefore possible that suboptimal PLCZ1 expression and the subsequent abnormal [Ca2+]i oscillatory pattern may have a more insidious phenotype than previously expected. Importantly, our results show that PLCZ1 supplementation in the form of mRNA injection can reproduce the normal [Ca2+]i oscillatory pattern and therefore possibly restore fertility.
In conclusion, we show that repeated ICSI failure associated with egg activation failure is linked to the inability of the sperm to initiate [Ca2+]i oscillations and that this deficiency can be functionally associated with reduced/absent expression of PLCZ1 in the sperm of these patients. We also present evidence that injection of PLCZ1-deficient sperm into mouse eggs reproduces the ICSI failure phenotype, which can be rescued by injection of mouse Plcz1 mRNA. The data presented unambiguously link PLCZ1 protein with the ability to induce [Ca2+]i oscillations and fertility. Future studies are necessary to examine the prevalence of PLCZ1- and [Ca2+]i oscillation–deficient sperm in larger cohorts of ICSI failure cases and in other cases of undiagnosed male infertility.
Methods
Patient selection.
This study was conducted on patients enrolled in the assisted reproduction program at Baystate Medical Center from September 2005 to January 2008. During this period, 119 couples consented to participate in the study, and 17 of these couples met enrollment criteria. The criteria required (a) a female partner who was 38 years old or younger; and (b) a previous in vitro fertilization cycle with either total fertilization failure, lower fertilization rate (<25%), or normal fertilization rate (>60%) but without conception, or semen analyses that revealed less than 30% motility, and/or less than 20 × 106 sperm/ml, and/or a sample(s) with morphology grades of 2 or lower according to strict Kruger analysis (43). Based on these parameters, ICSI was recommended to all couples in the study. Sperm samples from consenting patients not enrolled in the study but with optimal sperm parameters were used on occasion as additional controls. The Institutional Review Board at Baystate Medical Center approved the study.
Ovarian stimulation and egg retrieval.
Prior to follicle-stimulating hormone stimulation (GONAL-f; Merck Serono), female patients underwent pituitary desensitization with either leuprolide acetate (Lupron; TAP Pharmaceuticals) or Ganirelix (Organon). Ovulation was induced by human chorionic gonadotropin (Ovidrel; Merck Serono). Cumulus-oocyte complexes were retrieved 36 hours after administration of human chorionic gonadotropin by surgical aspiration under anesthesia and were collected using Quinn’s Advantage HTF medium supplemented with HEPES (all human media from Sage BioPharma). Following isolation, the cumulus-oocyte complexes were transferred into Quinn’s Advantage Fertilization medium and incubated at 37°C under 5% CO2 until and after ICSI. ICSI was performed 4 hours after the retrieval. Prior to ICSI, oocytes were stripped of their cumulus cells in hyaluronidase-supplemented medium; stripped mature oocytes are referred to as eggs.
Sperm preparation, ICSI procedure (human), assessment of fertilization, embryo transfer, and pregnancy detection.
Freshly ejaculated semen was collected and washed by centrifugation on a 40%/80% PureCeption Percoll gradient (Sage BioPharma). The recovered pellet was washed and subjected to 1 to 2 hours swim-up, after which sperm were analyzed for motility, concentration, viability, and morphology. Motile sperm were placed into polyvinylpyrrolidone (PVP) (7%; MW = 360 kDa; Sage BioPharma) and selected by strict Kruger morphology prior to injection. Sperm were introduced into the ooplasm essentially as described by Palermo et al. (15) using Narishige micromanipulators mounted on an inverted Olympus 1X70 microscope (Zander Medical Supplies). ICSI was performed approximately 6 hours after ejaculation. Fertilization was assessed 16–19 hours after ICSI by searching eggs for evidence of PN formation. Zygotes with 2 PN were cultured and transferred into the uterus. Clinical pregnancy was established by the presence of a gestational sac and heartbeat using transvaginal ultrasound performed at 6 to 7 weeks after embryo transfer.
After the completion of ICSI, the remaining patients’ sperm were washed in Dulbecco’s PBS (DPBS) and prepared for injection into mouse eggs or for immunofluorescence or Western blotting (see below for technical details).
Preparation of mouse eggs.
MII eggs were obtained from superovulated 6- to 10-week-old B6D2F1 (C57BL/6J × DBA/2J) female mice as described by our laboratory (29). Eggs were collected in a HEPES-buffered Tyrode’s-lactate solution (TL-HEPES) (ref. 44) supplemented with 5% heat-treated FCS (GIBCO; Invitrogen). Cumulus cells were removed with 0.1% bovine testes hyaluronidase (Sigma-Aldrich) and eggs incubated in CZB medium (45) at 36.5°C under 5% CO2 until ICSI. All animal studies were approved by the Animal Care and Use Committee, University of Massachusetts.
ICSI of mouse eggs.
MII eggs were injected with either mouse sperm or human sperm as described by Kimura and Yanagimachi (46) and by our laboratory (29, 47) using a piezo micropipette-driven unit (PiezoDrill; Burleigh Instruments Inc.). Mouse sperm were collected from the cauda epididymis of 10- to 12-week-old B6D2F1 male mice, washed with microinjection buffer (MIB) (75 mM KCl and 20 mM HEPES, pH 7.0), and placed in MIB supplemented with 12% PVP (Sigma-Aldrich) prior to injection. Spare sperm from each patient were washed with MIB and frozen at –80.0°C until injection, which was performed within a week of collection. Prior to ICSI, human sperm were thawed and mixed 1:1 with MIB containing 12% PVP. ICSI was performed in HEPES-buffered CZB medium supplemented with 0.1% polyvinyl alcohol (MW = 30–80 kDa). For human sperm, whole sperm were injected whereas only heads of mouse sperm (separated by piezo pulses) were injected.
[Ca2+]i monitoring.
[Ca2+]i monitoring was carried out as described by our laboratory (47). In brief, eggs loaded with fura-2-acetoxymethyl ester (Molecular Probes; Invitrogen) were subjected to ICSI and immediately transferred into TL-HEPES microdrops placed on a monitoring dish (MatTek Corp.) under mineral oil. Eggs were monitored simultaneously using a ×20 objective on an inverted microscope (Nikon) outfitted for fluorescence measurements and with a temperature-controlled stage (20-20 Technologies). The excitation wavelength was alternated between 340 nm and 380 nm by a filter wheel (Ludl Electronic Products Ltd.), and fluorescence ratios were taken every 20 or 30 seconds. After passing through a 510-nm barrier filter, the emitted light was collected by a CoolSNAP ES digital camera (Roper Scientific). Images were analyzed by SimplePCI software 5.2.1 (Hamamatsu). [Ca2+]i values are reported as the ratio of 340 nm:380 nm fluorescence in the whole egg, and [Ca2+]i oscillatory activity of human sperm was scored from 0 to +++, according to the number of [Ca2+]i rises within 1 hour of monitoring.
Messenger RNA preparation and microinjection.
cDNA encoding for full-length mouse Plcz1 (GenBank Accession number AF435950; a gift from K. Fukami, Tokyo University of Pharmacy and Life Science, Tokyo, Japan) was amplified by PCR and cloned into the pCS2+ vector (48). A mutant, inactive version of this construct, Plcz1-ΔC2, was previously generated by our laboratory (13). The linearized cDNAs were in vitro transcribed and purified using the mMessage/mMachine SP6 Kit and MEGAclear Kit, respectively (Ambion; Applied Biosystems), as reported earlier by our laboratory (13). Microinjection procedures were as described by our laboratory (13), and mRNAs were delivered by pneumatic pressure using a picoinjector (PLI-100, Harvard Apparatus). The injection volume was 5 to 10 pl, which is approximately 1%–3% of the total egg volume.
Primary antibodies.
Two polyclonal antisera were raised in rabbits against peptide sequences of the human PLCZ1 protein. The MI-305 antibody was raised against a 15-mer–peptide sequence (305KETHERKGSDKRGDN319) located within the linker domain and was affinity purified (Zymed; Invitrogen). The second antibody, PF-354, was raised against a 21-mer–peptide sequence (354IYTKAEKFKSFQHSRLYQQFC373C) located within the Y domain of the protein (QCB). The anti–α-tubulin monoclonal antibody was purchased from Sigma-Aldrich.
Western blotting.
Patients’ sperm were diluted to appropriate concentrations in ×2 sample buffer (49) and kept at –80°C until use. Thawed samples were boiled for 3 minutes and loaded into 7.5% SDS-PAGE, and resolved polypeptides were transferred onto PVDF membranes (Millipore) using a Mini Trans-Blot Cell (Bio-Rad). The membranes were blocked in 6% nonfat dry milk in PBS–0.1% Tween and incubated overnight at 4°C with the MI-305 antibody (1:500); this was followed by 1 hour of incubation with a horseradish peroxidase–labeled secondary antibody (Bio-Rad). Immunoreactivity was detected using chemiluminescence per manufacturer’s instructions (PerkinElmer) using a Kodak Image Station 440CF. Western blotting procedures were repeated at least 3 times per sample.
Immunofluorescence.
Sperm for immunofluorescence were fixed in freshly made 3.7% PFA-DPBS and kept at 4°C in DPBS until use, when the suspension was spotted onto 0.1% poly l-lysine (Sigma-Aldrich) precoated multi-well slides (Thermo Scientific). After attachment, sperm were permeabilized with 0.1% (v/v) Triton X-100–DPBS (Triton X-100; Sigma-Aldrich) for 5 minutes on ice. Slides were blocked in 5% normal goat serum–DPBS (normal goat serum; GIBCO, Invitrogen) and incubated overnight at 4°C with MI-305 (1:30) or PF-354 (1:100) in 5% normal goat serum. Washes were performed with 0.1% (v/v) Tween 20–DPBS, followed by 1 hour incubation at room temperature with Alexa Fluor 555–labeled goat anti-rabbit (1:200) as secondary antibody. Samples were counterstained with 5 μg/ml Hoechst 33342 and mounted using mounting media (Vector Laboratories). Fluorescence images were captured with a Zeiss Axiovert 200M microscope outfitted with a ×63 oil immersion objective and a Hamamatsu Orca-AG cooled CCD Camera controlled by AxioVision software 4.6 (Zeiss). For mouse eggs/zygotes, nuclear staining was accomplished after fixation with 1 μM TO-PRO-3 iodide (642/661 nm; Invitrogen). Slides were examined with a confocal laser-scanning microscope (LSM 510 META; Zeiss) using an Axiovert 2 microscope outfitted with a ×63 1.4 numerical aperture oil-immersion objective lens. Whole-egg images were reconstructed and projected from Z-stack images using LSM 510 META software, further processed using Photoshop (Adobe), and assembled in PowerPoint (Microsoft).
Genomic DNA sequencing.
DNA samples were recovered from whole blood or ejaculated sperm by standard proteinase K lysis and phenol chloroform extraction protocols. PCR primers were designed using Primer3 software (50), spanning each exon of human PLCZ1 (ENSG00000139151) and extending at least 50 bases in both the 5′ and 3′ directions from the exon-intron junction (200 bases 5′ to exon 1). RubyTaq 2X master mix (USB) was used in order to generate each amplicon using a touchdown PCR strategy. Amplicons were run on 1% agarose gel and extracted. Automated sequencing was performed by GENEWIZ, and results were analyzed using CodonCode Aligner 2.0.4 software (CodonCode Corp.). Primers were as follows: exon 1, F-5′-TGTCTGGCATTTTTCCATGA-3′ and R-5′-TGGTTGCAACAGAAGCAAAG-3′; exon 2, F-5′-CAGAACAAACAGGGAAAAGCA-3′ and R-5′-TGGAAAAATGCCAGACATCA-3′; exon 3, F-5′-TGTTAGCATTTCTGTATGCTCAGG-3′ and R-5′-TGTGCTATGCCCTTTCATACC-3′; exon 4, F-5′-GACCCATCCAAAAACGGTAA-3′ and R-5′-CCAATGTAATTTTCATCTTCTTTCAA-3′; exon 5, F-5′-TGCACTCTTGCCTACTGAGG-3′ and R-5′-TGCTAATGGTATTTTTGCATCC-3′; exon 6, F-5′-CAACATGGATTTCACTGCCTA-3′ and R-5′-CCAAGCCTTGTATGGAGAGC-3′; exon 7, F-5′-CCCTAGGCAACATTGCAAAA-3′ and R-5′-ACAGTTGTGAGCCACTGAGC-3′; exons 8 and 9, F-5′-TCCACCATCGATGTTTTCAA-3′ and R-5′-TGTGAATAGGGGTATGGGAAA-3′; exon 10, F-5′-CCACATCGGACATTCTCAATC-3′ and R-5′-ATCAAATGGTGTTTGAGGAGA-3′; exon 11, F-5′-CAATGTCCATTTGGGTTTCC-3′ and R-5′-TGCAAATTTTTGCCAAGACA-3′; exon 12, F-5′-TGGGCTGAACTGAGACCTGT-3′ and R-5′-CATTTGGGGACATAGAATCCA-3′; exon 13, F-5′-AAATGCCTTCTTAATTCGGTGA-3′ and R-5′-TCAATGTTTGTGGGAGCTGA-3′; exon 14, F-5′-GAGCTATTTGGTATGTCAAAATGTG-3′ and R-5′-CCAAACTTTTTCTTTTCAACCC-3′; exon 15, F-5′-ATGCTGGGTTAGGACCCTCT-3′ and R-5′-GGTGTCTCCAATAGCCTTGC-3′.
Acknowledgments
We thank Changli He for technical assistance. This work was supported in part by a grant from the USDA National Research Initiative competitive grant program (2007-35203-17840 to R.A. Fissore) and by grants from the Gustavus and Louise Pfeiffer Research Foundation and the Baystate Medical Center–UMass-Amherst Collaborative Biomedical Research Program (to R.A. Fissore, P.E. Visconti, and D. Grow). Gustavus and Louise Pfeiffer Research Foundation funds were used for studies that did not involve research animals. Work in the laboratory of P.E. Visconti was supported in part by grants from the NIH (HD38082 and HD44044). Work in the laboratory of J.B. Cibelli was supported by grants from the office of the Vice President for Research and Graduate Studies at Michigan State University and the Michigan State University Foundation. Work in the laboratory of J. Parrington was supported by a grant from the British Medical Research Council. The University of Massachusetts–Amherst Central Microscopy Facility, where the confocal microscope is housed, was supported by a grant from the National Science Foundation (BBS-8714235).
Footnotes
Nonstandard abbreviations used: [Ca2+]i, free cytosolic Ca2+ concentration; DPBS, Dulbecco’s PBS; ICSI, intracytoplasmic sperm injection; IP3, inositol 1,4,5-trisphosphate; MIB, microinjection buffer; MII, second meiotic metaphase; PLCZ1, PLC, zeta 1; PN, pronucleus (pronuclei); PVP, polyvinylpyrrolidone.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3671–3681 (2008). doi:10.1172/JCI36942
Sook-Young Yoon and Teru Jellerette contributed equally to this work.
References
- 1.Schultz R.M., Kopf G.S. Molecular basis of mammalian egg activation. Curr. Top. Dev. Biol. 1995;30:21–62. doi: 10.1016/S0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- 2.Kline D., Kline J.T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 1992;149:80–89. doi: 10.1016/0012-1606(92)90265-I. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki S., et al. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev. Biol. 1986;118:259–267. doi: 10.1016/0012-1606(86)90093-X. [DOI] [PubMed] [Google Scholar]
- 4.Stricker S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki S., Ito M. Calcium signals for egg activation in mammals. J. Pharmacol. Sci. 2006;100:545–552. doi: 10.1254/jphs.CPJ06003X. [DOI] [PubMed] [Google Scholar]
- 6.Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 1990;110:1295–1302. doi: 10.1242/dev.110.4.1295. [DOI] [PubMed] [Google Scholar]
- 7.Wu H., He C.L., Fissore R.A. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol. Reprod. Dev. 1997;46:176–189. doi: 10.1002/(SICI)1098-2795(199702)46:2<176::AID-MRD8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Jones K.T., Cruttwell C., Parrington J., Swann K. A mammalian sperm cytosolic phospholipase C activity generates inositol trisphosphate and causes Ca2+ release in sea urchin egg homogenates. FEBS Lett. 1998;437:297–300. doi: 10.1016/S0014-5793(98)01254-X. [DOI] [PubMed] [Google Scholar]
- 9.Wu H., et al. Sperm factor induces intracellular free calcium oscillations by stimulating the phosphoinositide pathway. Biol. Reprod. 2001;64:1338–1349. doi: 10.1095/biolreprod64.5.1338. [DOI] [PubMed] [Google Scholar]
- 10.Rice A., Parrington J., Jones K.T., Swann K. Mammalian sperm contain a Ca(2+)-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev. Biol. 2000;228:125–135. doi: 10.1006/dbio.2000.9929. [DOI] [PubMed] [Google Scholar]
- 11.Saunders C.M. , 2002PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 1293533–3544. [DOI] [PubMed] [Google Scholar]
- 12.Kouchi Z., et al. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa M., et al. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev. Biol. 2007;312:407–418. doi: 10.1016/j.ydbio.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forti G., Krausz C. Clinical review 100: Evaluation and treatment of the infertile couple. J. Clin. Endocrinol. Metab. 1998;83:4177–4188. doi: 10.1210/jc.83.12.4177. [DOI] [PubMed] [Google Scholar]
- 15.Palermo G., Joris H., Devroey P., Van Steirteghem A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty S.P., Payne D., Matthews C.D. Fertilization failures and abnormal fertilization after intracytoplasmic sperm injection. Hum. Reprod. 1998;13(Suppl. 1):155–164. doi: 10.1093/humrep/13.suppl_1.155. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty S.P., Payne D., Swann N.J., Matthews C.D. Assessment of fertilization failure and abnormal fertilization after intracytoplasmic sperm injection (ICSI). Reprod. Fertil. Dev. 1995;7:197–210. doi: 10.1071/RD9950197. [DOI] [PubMed] [Google Scholar]
- 18.Sousa M., Tesarik J. Ultrastructural analysis of fertilization failure after intracytoplasmic sperm injection. Hum. Reprod. 1994;9:2374–2380. doi: 10.1093/oxfordjournals.humrep.a138455. [DOI] [PubMed] [Google Scholar]
- 19.Mahutte N.G., Arici A. Failed fertilization: is it predictable? Curr. Opin. Obstet. Gynecol. 2003;15:211–218. doi: 10.1097/00001703-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 20.van der Westerlaken L., Helmerhorst F., Dieben S., Naaktgeboren N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil. Steril. 2005;83:612–617. doi: 10.1016/j.fertnstert.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Dam A.H., et al. Globozoospermia revisited. Hum. Reprod. Update. 2007;13:63–75. doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- 22.Battaglia D.E., Koehler J.K., Klein N.A., Tucker M.J. Failure of oocyte activation after intracytoplasmic sperm injection using round-headed sperm. Fertil. Steril. 1997;68:118–122. doi: 10.1016/S0015-0282(97)81486-0. [DOI] [PubMed] [Google Scholar]
- 23.Rybouchkin A.V., Van der Straeten F., Quatacker J., De Sutter P., Dhont M. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil. Steril. 1997;68:1144–1147. doi: 10.1016/S0015-0282(97)00378-6. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C.T., Lawrence Y.M., Kingsland C.R., Biljan M.M., Cuthbertson K.S. Oscillations in intracellular free calcium induced by spermatozoa in human oocytes at fertilization. Hum. Reprod. 1993;8:2174–2179. doi: 10.1093/oxfordjournals.humrep.a137999. [DOI] [PubMed] [Google Scholar]
- 25.Morozumi K., Shikano T., Miyazaki S., Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17661–17666. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers N.T., et al. Phospholipase Czeta causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction. 2004;128:697–702. doi: 10.1530/rep.1.00484. [DOI] [PubMed] [Google Scholar]
- 27.Rybouchkin A., Dozortsev D., Pelinck M.J., De Sutter P., Dhont M. Analysis of the oocyte activating capacity and chromosomal complement of round-headed human spermatozoa by their injection into mouse oocytes. Hum. Reprod. 1996;11:2170–2175. doi: 10.1093/oxfordjournals.humrep.a019071. [DOI] [PubMed] [Google Scholar]
- 28.Yanagida K., et al. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum. Reprod. 1999;14:1307–1311. doi: 10.1093/humrep/14.5.1307. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa M., Fissore R.A. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol. Hum. Reprod. 2003;9:523–533. doi: 10.1093/molehr/gag072. [DOI] [PubMed] [Google Scholar]
- 30.Swann K., Saunders C.M., Rogers N.T., Lai F.A. PLCzeta(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Cox L.J., et al. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- 32.Ross P.J., et al. Parthenogenetic activation of bovine oocytes using bovine and murine phospholipase C zeta. BMC Dev. Biol. 2008;8:16. doi: 10.1186/1471-213X-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence Y., Whitaker M., Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- 34.Sette C., et al. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development. 1997;124:2267–2274. doi: 10.1242/dev.124.11.2267. [DOI] [PubMed] [Google Scholar]
- 35.Wu A.T., et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J. Biol. Chem. 2007;282:12164–12175. doi: 10.1074/jbc.M609132200. [DOI] [PubMed] [Google Scholar]
- 36.Knott J.G., Kurokawa M., Fissore R.A., Schultz R.M., Williams C.J. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol. Reprod. 2005;72:992–996. doi: 10.1095/biolreprod.104.036244. [DOI] [PubMed] [Google Scholar]
- 37.Heindryckx B., Van der Elst J., De Sutter P., Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum. Reprod. 2005;20:2237–2241. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 38.Ducibella T., et al. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev. Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- 39.Rogers N.T., et al. The absence of a Ca2+ signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction. 2006;132:45–57. doi: 10.1530/rep.1.01059. [DOI] [PubMed] [Google Scholar]
- 40.Ozil J.P., Banrezes B., Toth S., Pan H., Schultz R.M. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev. Biol. 2006;300:534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 41.Armant D.R. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev. Biol. 2005;280:260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farhi J., et al. Male factor infertility, low fertilisation rate following ICSI and low number of high-quality embryos are associated with high order recurrent implantation failure in young IVF patients. Acta Obstet. Gynecol. Scand. 2008;87:76–80. doi: 10.1080/00016340701743074. [DOI] [PubMed] [Google Scholar]
- 43.Kruger T.F., et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil. Steril. 1986;46:1118–1123. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 44.Parrish J.J., Susko-Parrish J., Winer M.A., First N.L. Capacitation of bovine sperm by heparin. Biol. Reprod. 1988;38:1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- 45.Chatot C.L., Lewis J.L., Torres I., Ziomek C.A. Development of 1-cell embryos from different strains of mice in CZB medium. Biol. Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y., Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 47.Yoon S.Y., Fissore R.A. Release of phospholipase C zeta and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction. 2007;134:695–704. doi: 10.1530/REP-07-0259. [DOI] [PubMed] [Google Scholar]
- 48.Turner D.L., Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 49.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 50.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]