Abstract
Valproic acid, a drug commonly used to treat seizures and other psychiatric disorders, causes neural tube defects (NTDs) in exposed fetuses at a rate 20 times higher than in the general population. Failure of the neural tube to close during development, results in exencephaly or anencephaly, and spina bifida. In mice, non specific activation of the maternal immune system can reduce fetal abnormalities caused by diverse etiologies, including diabetes induced NTDs. We hypothesized that nonspecific activation of the maternal immune system with IFNγ and GM-CSF could reduce valproic acid (VA) induced defects as well. Female CD-1 mice were given immune stimulant pre-breeding; either IFNγ or GM-CSF. Approximately half of the control and immune stimulated pregnant females were then exposed to 500 mg/kg VA on the morning of gestational day 8. The incidence of developmental defects was determined on gestational day 17 from at least 8 litters in each of the following treatment groups: Control, VA only, IFNγ only, IFNγ+VA, GM-CSF only, GM-CSF+VA. NTDs were significantly reduced from 18% in VA exposed fetuses to 3.7% in IFNγ+VA, and 2.9% in GM-CSF+VA treatment groups. Ocular defects were also significantly reduced from 28.0% in VA exposed groups to 9.8% in IFNγ+VA and 12.5% in GM-CSF+VA groups. The mechanisms responsible for the reduction in birth defects by maternal immune stimulation remain unclear, but may involve maternal or fetal production of cytokines or growth factors which protect the fetus from the dysregulatory effects of teratogens.
Keywords: maternal immune stimulation, valproic acid, neural tube defects, development
INTRODUCTION
Birth defects usually result from a multifactorial etiology, where embryonic development of a genetically susceptible fetus is altered by environmental factors. Neural tube defects (NTDs) are among the most prevalent and disabling of all the congenital malformations with as many as 10 to 20 infants per 10,000 births being affected (DeSesso et al. 1999). NTDs are major malformations of the central nervous system, in which the canal of the malformed brain or spinal cord is persistently open to the outside environment, resulting in anencephaly cranially and spina bifida caudally. These developmental defects result in a severely impaired brain or local disruption of vertebrae and spinal axonal pathways.
A multifactorial model for NTD etiology has been proposed where susceptible genotypes are affected by a number of extrinsic factors such as maternal age, nutrition, socioeconomic influence, and exposure to NTD–inducing toxicants or drugs during pregnancy (Ehlers et al., 1996; DeSesso et al., 1999). The antiepileptic drug Valproic acid (VA) has been implicated as a human teratogen in both retrospective and prospective studies. VA use during early pregnancy may induce NTDs, cardiovascular, urogenital, craniofacial (fetal valproate syndrome), digital and skeletal abnormalities. Women taking VA have a 1–2 % absolute risk of having a child with a neural tube defect, compared to 0.06% for the general population, a 15 to 30 fold increase in incidence (Padmanabhan and Shafiullah, 2003). Valproic acid is being used increasingly to manage conditions other than epilepsy, particularly bipolar and other affective disorders that do not respond to conventional therapies (Kennedy and Koren, 1998). The demographics of the populations affected by these psychiatric conditions are such that more women of childbearing age are likely to be exposed to this teratogenic drug. Valproic acid exposure also causes NTDs in susceptible strains of mice. The mouse model of VA induced NTDs is well characterized and has been used for many years as a research tool to investigate the causes of human NTDs both in general and specific to VA.
Non-specific stimulation of the maternal immune system in mice during the peri-conception period appears to have a broad spectrum efficacy for reducing teratogen induced birth defects from a variety of sources including chemical agents, hyperthermia, x-rays, and diabetes mellitus (Nomura et al., 1990; Holladay et al., 2000; Punareewattana et al., 2003; Punareewattana and Holladay, 2004; Hrubec et al., 2006a, 2006b). Maternal immune stimulation reduced or blocked digit and limb defects (Prater et al., 2004), tail malformations, cleft palate (Sharova et al., 2002), craniofacial defects (Hrubec et al., 2006b) and neural tube defects (Torchinsky et al., 1997; Punareewattana et al., 2003; Punareewattana and Holladay, 2004). The operating mechanisms by which such immune stimulation reduces fetal dysmorphogenesis are unknown; however, the collective literature suggests that immunoregulatory cytokines of maternal or placental origin may be effector molecules that normalize dysregulated apoptosis or timing of cell proliferation in the fetus (Sharova et al., 2000; Punareewattana and Holladay, 2004; Hrubec et al., 2006a). The protective effect of maternal immune stimulation appears to be independent of the manner of stimulation. Diverse methods including intraperitoneal (IP) injection of inert particles, intrauterine injection of xenogenic lymphocytes and intrauterine or IP injection of immunostimulatory cytokines have all been effective in reducing defect rates (reviewed by Hrubec et al., 2006a).
Recently in our laboratory, we demonstrated that maternal immune stimulation with the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) or interferon-γ (IFNγ)reduced the incidence of diabetes induced craniofacial, ocular and neural tube defects (Punareewattana and Holladay, 2004; Hrubec et al., 2006b). We therefore hypothesized that maternal immune stimulation with these same cytokines would reduce the incidence of VA induced NTDs.
MATERIALS AND METHODS
Six to eight-week-old CD-1 mice were obtained from Charles River Laboratories (Portage, MI) and were acclimated for at least one week prior to use in an experiment. The CD-1 strain does not produce spontaneous NTDs but is sensitive to VA and is often used in the VA induced NTD model. The mice were maintained under the following controlled conditions: temperature, 22.0 C, humidity, 40–60%, light 14/10 h light/dark cycle, food (Rodent Diet, Harlan Teklad, Madison, WI) and fresh water were provided ad libitum. All procedures involving mice were reviewed by and conducted in compliance with the guidelines of the Virginia Tech Animal Care and Use Committee at the VA-MD Regional College of Veterinary Medicine, an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility.
Female mice were divided randomly into 7 groups: Control, GM-CSF only, IFNγ only, VA only, VA + GM-CSF 2000, VA + GM-CSF 8000, and VA + IFNγ as described in Table 1. Following the procedure previously developed in our lab to prevent NTDs in diabetic pregnancies, mice were injected IP with either saline or immune stimulant ten days prior to breeding and again at 5 days prior to breeding (Punareewattana and Holladay, 2004). Two females were added to each male overnight and checked for vaginal plugs the following morning. The presence of a vaginal plug indicated mating and was designated day 0 of gestation. Consistent with standard methods to induce NTDs, mice received either saline or VA (sodium valproate, Sigma–Aldrich, St. Louis, MO) dissolved in sterile distilled water at 500 mg/kg IP on the morning of gestational day 8, the day the neural tube begins to close. In a previous dose range study, treatment of the dam with 500 mg/kg gave the highest rate of NTDs in the offspring and the lowest rate of embryolethality including resorptions (data not shown). Dams in all groups were not visibly affected by any of the immune stimulant or saline treatments. Approximately half of the dams receiving VA injection became slightly ataxic and lethargic for approximately 3 hours. There was no association between dams that became lethargic and rate of NTDs in the offspring (data not shown). Dams in all treatment groups appeared healthy through out pregnancy and were euthanized by cervical dislocation on gestational day 17.
Fetuses and placentae from each dam were collected and weighed. The number of live and dead fetuses, fetal resorptions, and externally visible fetal malformation were recorded for each litter. Fetuses and placentae were placed in cold 10% neutral buffered formalin for 24 hours and then transferred to fresh formalin at room temperature.
To demonstrate alterations in bone and cartilage formation, one normal fetus from a control dam and one fetus exhibiting exencephaly from a VA treated dam were fixed in 100% ethanol for 1 week then stained and cleared as follows. Fetal heads were stained with 0.15% alcian blue in a 4:1 solution of 95% ethanol to glacial acetic acid for 48 hours. Tissues were cleared in 1% KOH for 3 days until translucent and then counterstained with 0.01% alizarin red in 0.02% KOH for 12 hours. Heads were further cleared in 0.5% KOH until transparent, approximately one week, and then transferred through a graded KOH:glycerine series over the next week until in 100% glycerine. Fetuses were photographed using an Olympus Zoom Stereo Microscope SZX7 (Olympus America Inc., Melville, NY) equipped with a CFW 1310 Scion camera (Scion Corporation, Frederick, MD).
Placentae from two complete litters chosen at random from each treatment were processed for histopathological analysis. Placentae were placed on their endometrial surface and sectioned longitudinally through the umbilical region. Bisected placentae were embedded in parafin, processed for routine histopathology and mid longitudinal 5μm sections were stained with hematoxylin and eosin.
Statistical analysis
Data were analyzed with SAS (SAS Institute, Cary, NC). In all analyses, the mother was used as the treatment unit. Only live fetuses were included in the analysis, with the exception of the investigation of fetal death and resorption. For different treatment groups, a generalized linear model was used to test differences in the probabilities that a fetus would exhibit exencephaly or an ocular defect. Measurements of fetal and placental weights were analyzed via analysis of variance with repeated measures. Pairwise differences in fetal and placental weights between treatment groups were tested. The Tukey-Kramer procedure was used when dealing with multiple comparisons (Tukey 1953; Kramer 1956). For the different treatment groups, a generalized linear model was used to test differences in the probability that a fetus from a dam would be dead or resorbed.
RESULTS
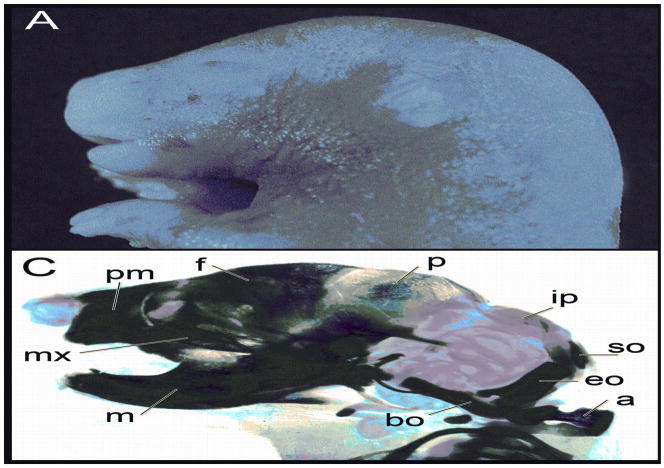
Valproic acid, given at 500 mg/kg on the morning of gestational day 8, induced exencephaly and open eye defects in some of the fetuses (Fig. 1). Clearing and staining of the fetal heads revealed alterations in development of both cranial and facial skeletal elements with changes to the maxillae, premaxillae, mandibles, and abnormal (or absent) frontal, parietal and occipital bones (Fig. 1). A small percentage of the fetuses exposed to VA also had a reduced curled tail.
Figure 1.
Fetal heads at gestational day 17 demonstrating characteristic lesions associated with valproic acid treatment. A, Control; B, Exencephaly and open eye. These heads were then cleared and stained with alcian blue for cartilage and alizarin red for bone. C, Control; D, Exencephalic fetus demonstrating abnormalities in most of the craniofacial skeleton. The following structures are labeled: m, mandible; mx, maxilla; pm, premaxilla; f, frontal; p, parietal; ip, interparietal; so, supraoccipital; eo, exoccipital; bo, basioccipital; a, atlas.
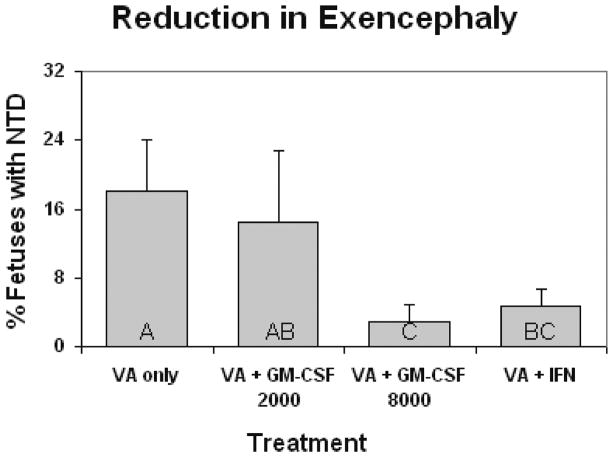
There were no cases of exencephaly in the fetuses of control dams treated only with saline or immune stimulant (data not shown). Valproic acid induced exencephaly (NTD) in 18 % of the fetuses per litter (Fig. 2). GM-CSF at 8000 IU in conjunction with VA reduced the incidence of NTDs in the litters to 2.9 %. In dams treated with both VA + IFNγ NTDs were reduced to 3.7 % (Fig. 2). Treatment of the dam with 2000 IU GM-CSF did not significantly reduce the incidence of NTD.
Figure 2.
Incidence of exencephaly (± SE) seen in gestational day 17 mouse embryos caused by maternal valproic acid exposure (500 mg/kg IP), and reduction in these defects with maternal immune stimulation. Dams were immune stimulated with either GM-CSF (at 2000 or 8000 units, IP injection), or IFN-γ (1000 units, IP injection). Bars with shared letters are not significantly different (p ≥ 0.05).
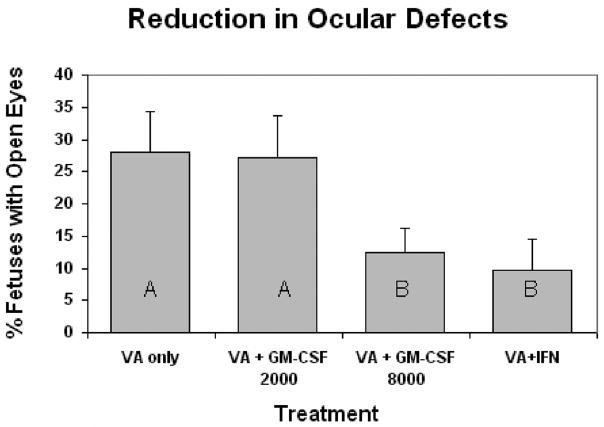
There were no cases of open eyes in the fetuses of control dams treated only with saline or immune stimulant (data not shown). Valproic acid induced open eyes (manifest as either one or both eyes open) in 28.0% of the fetuses per litter (Fig. 3). Treatment of the dam with VA + GM-CSF at 8000 IU reduced the incidence of open eyes in pups to 12.5%, while treatment with VA+IFNγ reduced the incidence to 9.8% (Fig. 3). Treatment of the dam with 2000 IU GM-CSF did not significantly reduce the incidence of open eyes.
Figure 3.
Incidence of open eyes (± SE) seen in gestational day 17 mouse embryos caused by maternal valproic acid exposure (500 mg/kg IP), and reduction in these defects with maternal immune stimulation. Dams were immune stimulated with either GM-CSF (at 2000 or 8000 units, IP injection), or IFN-γ (1000 units, IP injection). Bars with shared letters are not significantly different (p ≥ 0.05).
Treatment of the dam with immune stimulant alone did not alter fetal weight from saline injected controls (Fig. 4). Fetal weight was significantly reduced 20% by VA exposure (Fig. 4). Maternal immune stimulation in conjunction with VA exposure did not prevent the fetal weight loss. Treatment of the dam with immune stimulant alone did not significantly increase placental weight from saline injected controls (Fig. 5). Placenta weight was reduced approximately 10% by VA exposure. Maternal immune stimulation of VA treated dams did not prevent placental weight loss, although there was a trend toward larger placentas in the immune stimulated groups, particularly with GM-CSF (Fig. 5). Histological examination of the placentae did not demonstrate an effect of immune stimulant or VA exposure on a specific placental cell compartment; VA placentae were histologically indistinguishable from unexposed placentae.
Figure 4.
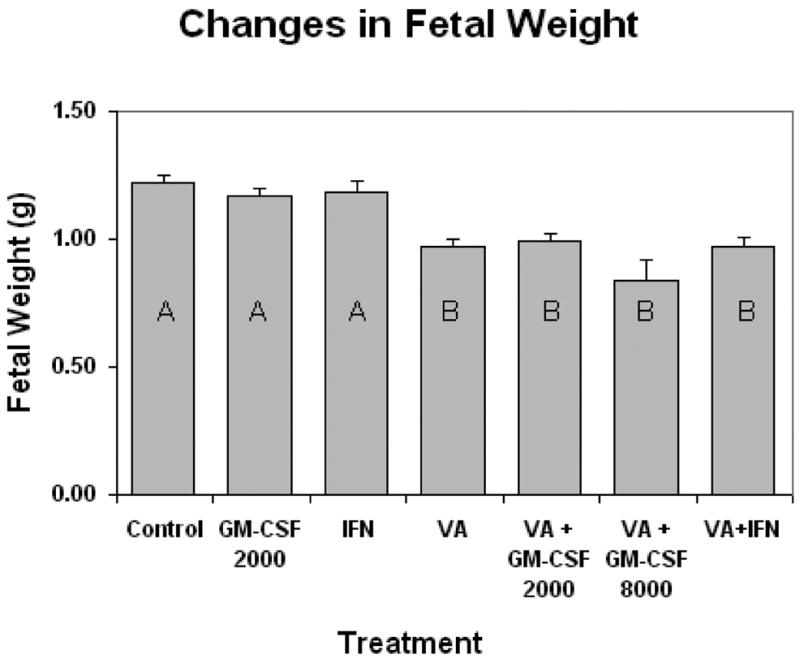
Alterations in fetal weight (± SE) seen in gestational day 17 mouse embryos caused by maternal valproic acid exposure (500 mg/kg IP), and maternal immune stimulation. Dams were immune stimulated with either GM-CSF (at 2000 or 8000 units, IP injection), or IFN-γ (1000 units, IP injection). Bars with shared letters are not significantly different (p ≥ 0.05).
Figure 5.
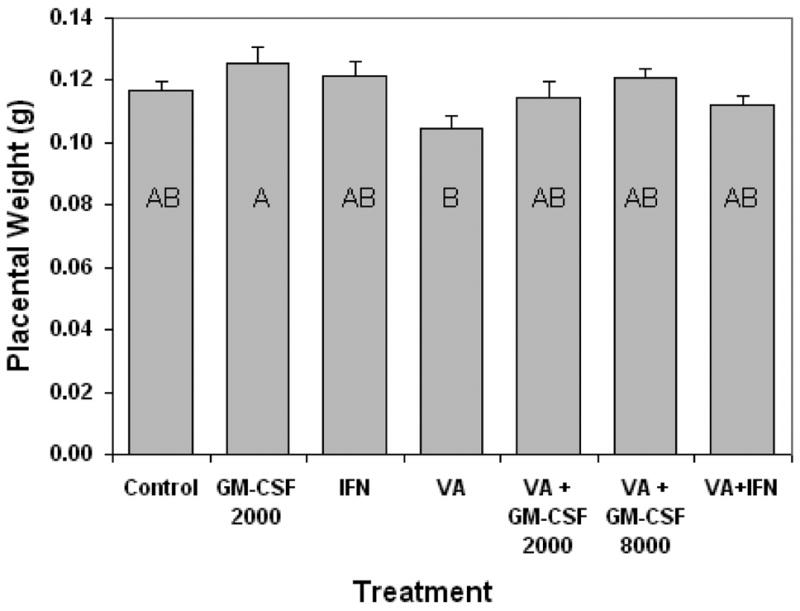
Alterations in placental weight (± SE) seen in gestational day 17 mouse embryos caused by maternal valproic acid exposure (500 mg/kg IP), and maternal immune stimulation. Dams were immune stimulated with either GM-CSF (at 2000 or 8000 units, IP injection), or IFN-γ (1000 units, IP injection). Bars with shared letters are not significantly different (p ≥ 0.05).
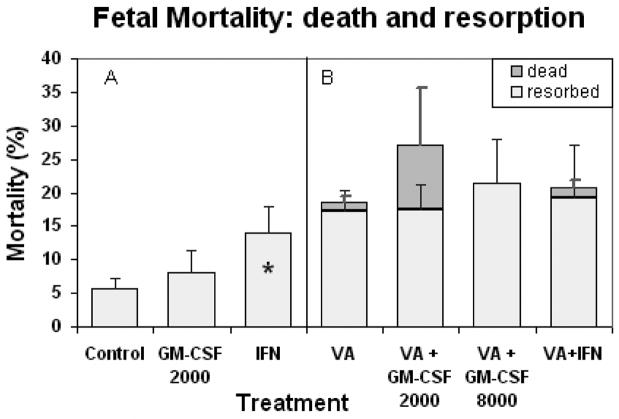
Fetal resorption was significantly increased in the IFNγ only treatment group above that seen in saline injected controls (Fig. 6A). Late gestational death was only observed in the groups exposed to VA (Fig 6B). Cumulative fetal death (resorption plus late gestational fetal death) was not significantly altered by maternal immune stimulation in any of these groups. Thus maternal immune stimulation with IFNγ, which caused increased early fetal resorption when administered alone, did not significantly increase over all fetal mortality in the presence of valproic acid.
Figure 6.
Cumulative fetal mortality per litter: early fetal death (resorptions) and dead but developed fetuses ± SE seen at gestational day 17 in dams exposed to valproic acid (500 mg/kg IP) and maternal immune stimulation. Panel A: Comparison of mortality in litters from saline control and immune stimulated dams. The IFNγ only group (*) was significantly different from control and GM-CSF only (p ≤ 0.05). Panel B: Comparison of mortality in litters of valproic acid treated plus immune stimulated dams. Dams were immune stimulated with either GM-CSF (at 2000 or 8000 units, IP injection), or IFN-γ (1000 units, IP injection). Groups were not significantly different.
DISCUSSION
The mechanism by which VA causes defective neurulation is unclear; although possible interference with folate metabolism may be one reason. Folic acid belongs to a group of B vitamins that influences both the rate of DNA synthesis and the synthesis of DNA nucleotides, and facilitates the formation of methionine from homocysteine (Da Costa and Sharon, 1980; van Poppel and van den Berg, 1997). Localized folate deficiencies in rapidly dividing tissues can result in ineffective DNA synthesis altering cell metabolism and replication. Folic acid is required for normal nervous system development and folic acid supplementation reduces the incidence of NTDs in fetuses (Fleming and Copp, 1998; Hishida and Nau, 1988; Wegner and Nau, 1991; Eskes et al., 1998). Additionally, mice lacking the genes for folate binding proteins that transport folic acid into cells exhibit NTDs (Tang and Finnell, 2003; Spiegelstein et al., 2003). Human studies indicate that folic acid supplementation may be protective against VA induced NTDs, but mouse models demonstrate strain specificity with some strains protected and others not (Dawson et al., 2006).
It has long been known that VA can disrupt the cell cycle and that cellular proliferation is essential for normal neural tube development. Numerous studies have demonstrated that alterations in normal proliferation rates of neuroectoderm cells can result in embryos with NTDs (Martin and Regan, 1991; Wlodarczyk et al., 1996; Craig et al., 2000; Bennett et al., 2000). Bennett et al. (2000), found that VA treated embryos demonstrated a uniform upregulation of transforming growth factors (TGFs) which affected downstream proliferative genes. The net result was a reduction in the number of cells moving into the S phase of the cell cycle and a decrease in the overall cell proliferation rate. Wlodarczyk et al. (1996) determined that VA altered the normal temporal pattern of gene expression to one consistent with drug induced inhibition of cell proliferation. This decreased cell proliferation delayed normal developmental events resulting in NTDs and reducing the overall growth of the embryo. Reduced fetal growth was noted by Al Deeb et al. (2000) in VA exposed fetuses as well.
Delayed fetal development and reduced fetal size are common sequelae of teratogenic exposures such as diabetes (Cederberg et al., 2003), ethyl carbamate (Sharova et al., 2000) and VA (Al Deeb et al., 2000; Padmanabhan and Shafiullah, 2003). Consistent with these previous studies, we observed decreased fetal size in the VA exposed fetuses. Maternal immune stimulation with GM-CSF or IFNγ did not protect the fetus from the VA induced growth impairment, even though NTDs were prevented. This phenomenon has been observed previously by others. Maternal immune stimulation reduced the incidence of ethyl carbamate induced cleft palate but did not prevent the fetal growth impairment (Sharova et al., 2000), and vitamin E reduced the incidence of VA induced malformations, but had no positive effect on fetal survival or growth (Al Deeb et al., 2000). Similarly, folic acid was able to reduce VA induced NTDs, but was not able to prevent fetal growth impairment (Padmanabhan and Shafiullah, 2003).
This disparity between the reduction of fetal malformations by various interventions and lack of reductions in fetal growth impairment and mortality may be explained by differential genetic contributions by the dam and fetus to VA teratogenicity. Beck (1999) used particular crosses of two inbred strains of mice with differing sensitivities to VA to separate the contributions of the dam and fetus to the teratogenicity and toxicity of VA. Reciprocal outcrosses, which simulate genetically identical fetuses developing in genetically different uterine environments, were used to determine the contribution of the dam genotype. Backcrosses of the F1 females to the pure line males, which simulate genetically different fetuses developing in genetically identical uterine environments, were used to determine the contribution of the conceptus. The sensitivity to VA was largely due to the maternal uterine environment controlled by the genetic make up of the mother. Fetal mortality caused by VA on the other hand, appeared to be in part a function of the conceptus (Beck, 1999). These results correlate well with Al Deeb et al.’s (2000) work with Vitamin E and what we observed with maternal immune stimulation (Fig.s 2, 3 and 6) suggesting an altered maternal uterine environment as the basis for protective intervention. In this case, uterine and placental derived cytokines and growth factors crossing the placenta and affecting the development of the fetus represent a maternally driven process.
The mechanisms by which maternal immune stimulation prevents developmental defects are unknown; however, there are indications that immunoregulatory cytokines of maternal or placental origin normalize dysregulated apoptosis or timing of cell proliferation in the fetus (Sharova et al., 2000; Punareewattana and Holladay, 2004; Hrubec et al., 2006a). The nervous system may be particularly responsive to cytokine signaling as neurons, microglia and astroglia produce and respond to cytokines. Another possible mechanism by which maternal immune stimulation may work is through improved placental function. Maternal immune stimulation improved placental morphology and reduced cleft palates in fetuses from dams exposed to urethane (Sharova et al., 2003). In other studies, maternal immune stimulation with IFNγ improved placental integrity and reduced fetal limb malformations in two strains of mice exposed to methylnitrosourea (Prater et al., 2004; Laudermilch et al., 2005). Both of these teratogens were cytotoxic to the placenta and caused specific observable lesions. Maternal immune stimulation was able to reduce the placental toxicity possibly by stimulating more normal placental growth. In the present study with VA, no obvious histopathological differences in the placentae were observed with any of the immune stimulant or VA treatment groups. Although VA is cytotoxic to the placenta in rats (Khera 1992), our findings concur with those of Emmanouil-Nikoloussi et al. (2004) who also found no placental pathology from VA exposure in mice. Detailed morphometric analysis was not conducted that may have identified alterations in specific placental compartments either with VA treatment or maternal immune stimulation; however, if these changes were present they were slight and not appreciable by diligent visual inspection. Maternal immune stimulation with an uncompromised placenta may have stimulated normal placental growth as evidenced by the placenta weights observed in litters from GM-CSF and IFNγ stimulated dams (Fig. 5). An imbalance in fetal/placental growth can create a situation where the placenta actually utilizes resources at the expense of the developing fetus resulting in relatively impaired or restricted fetal growth.
There have been numerous studies published demonstrating the effectiveness of maternal immune stimulation in preventing a wide variety of birth defects (reviewed by Hrubec et al., 2006a). Many immune stimulants and a variety of methods for stimulating the immune system have proved to be effective. Little published information exists on dose response for the different immune stimulants though. We have previously shown 8000 IU GM-CSF and 1000 IU IFNγ to be effective in reducing diabetes induced craniofacial, ocular and NTDs when administered prior to breeding; however, GM-CF at 8000 IU resulted in slightly reduced fetal size (Punareewattana and Holladay, 2004; Hrubec et al., 2006b). We wished to determine whether a lower dose of GM-CSF would remain protective against NTDs and abolish the reduction in fetal weight associated with higher GM-CSF dose. The present data demonstrating no protection from VA induced fetal malformation at 2000 IU of GM-CSF and significant protection at 8000 IU indicate that there is a threshold concentration below which alterations brought about by stimulation of the dam’s immune system are not sufficient to prevent fetal malformations.
These results indicate that VA induced NTDs can be reduced by non specific maternal immune activation. We are presently conducting studies to increase our understanding of the genetic mechanisms and cellular signals by which maternal immune modulation reduces NTDs. Clearly, this research is of importance to human health, as determining how maternal processes regulate fetal growth will improve our understanding of the causes of NTDs and other developmental defects. Research like this may possibly lead to a prevention or cure for many common birth defects.
Acknowledgments
The authors wish to thank Kimberly Toops for technical assistance with this study. This work was funded by the generous support of NIH, NCRR grant # K01RR16241-01.
LITERATURE CITED
- Al Deeb S, Al Moutaery K, Arshaduddin M, Tariq M. Vitamin E decreases valproic acid induced neural tube defects in mice. Neurosci Lett. 2000;292:179–82. doi: 10.1016/s0304-3940(00)01457-9. [DOI] [PubMed] [Google Scholar]
- Beck SL. Contributions of dam and conceptus to differences in sensitivity to valproic acid among C57 black and SWV mice. Reprod Toxicol. 1999;13:353–60. doi: 10.1016/s0890-6238(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Bennett GD, Wlodarczyk B, Calvin JA, Craig JC, Finnell RH. Valproic acid-induced alterations in growth and neurotrophic factor. Reprod Toxicol. 2000;14:1–11. doi: 10.1016/s0890-6238(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Cederberg J, Picard JJ, Eriksson UJ. Maternal diabetes in the rat impairs the formation of neural-crest derived cranial nerve ganglia in the offspring. Diabetologia. 2003:1245–1251. doi: 10.1007/s00125-003-1100-1. [DOI] [PubMed] [Google Scholar]
- Craig JC, Bennett GD, Miranda RC, Mackler SA, Finnell RH. Ribonucleotide reductase subunit R1: a gene conferring sensitivity to valproic acid induced neural tube defects in mice. Teratol. 2000;61:305–313. doi: 10.1002/(SICI)1096-9926(200004)61:4<305::AID-TERA10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Da Costa M, Sharon M. The synthesis of folate binding protein in lymphpcytes during transformation. Brit J Haematol. 1980;46:575–579. doi: 10.1111/j.1365-2141.1980.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Raymond AM, Winn LM. Folic acid and pantothenic acid protection against valproic acid-induced neural tube defects in CD-1 mice. Toxicol Appl Pharmacol. 2006;211:124–132. doi: 10.1016/j.taap.2005.07.008. [DOI] [PubMed] [Google Scholar]
- DeSesso JM, Scialli AR, Holson JF. Apparent lability of neural tube closure in laboratory animals and humans. Am J Med Gen. 1999;87:143–161. doi: 10.1002/(sici)1096-8628(19991119)87:2<143::aid-ajmg6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Durand P, Prost M, Blache D. Folic acid deficiency enhances oral contraceptive-induced platelet hyperactivity. Arterioscler Thromb Vasc Biol. 1997;17:1939–46. doi: 10.1161/01.atv.17.10.1939. [DOI] [PubMed] [Google Scholar]
- Ehlers K, Elmazar MM, Nau H. Methionine reduces the valproic acid induced spina bifida rate in mice without altering valproic acid kinetics. J Nutr. 1996;126:67–75. doi: 10.1093/jn/126.1.67. [DOI] [PubMed] [Google Scholar]
- Emmanouil-Nikoloussi EN, Foroglou NG, Kerameos-Foroglou CH, Thliveris JA. Effect of valproic acid on fetal and maternal organs in the mouse: a morphological study. Morphologie. 2004;88:41–45. doi: 10.1016/s1286-0115(04)97999-4. [DOI] [PubMed] [Google Scholar]
- Eskes TKAB, Mooij PNM, Steegers-Theunissen RPM, Lips JP, Pasker-de Jong PCM. Prepregnancy care and prevention of birth defects. J Perinat Med. 1992;20:253–265. doi: 10.1515/jpme.1992.20.4.253. [DOI] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Hishida R, Nau H. VPA induced neural tube defects in mice. 1. Altered metabolism of sulfur amino acids and glutathione. Teratog Carcinog Mutagen. 1998;18:49–61. [PubMed] [Google Scholar]
- Holladay SD, Sharova LV, Smith BJ, Gogal RM, Jr, Ward DL, Blaylock BL. Non-specific stimulation of the maternal immune system: I. Effects on teratogen-induced fetal malformations. Teratol. 2000;62:413–419. doi: 10.1002/1096-9926(200012)62:6<413::AID-TERA8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hrubec TC, Punareewattana K, Prater MR, Holladay SD. Reduction of teratogen induced birth defects in mice: role of maternal immune stimulation. Current Topics in Toxicology. 2006a In Press. [Google Scholar]
- Hrubec TC, Prater MR, Toops KA, Holladay SD. Reduction in diabetes induced craniofacial defects by maternal immune stimulation. Birth Defects Res B Dev Repro Toxicol. 2006b;77:1–9. doi: 10.1002/bdrb.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, Koren G. Valproic acid use in psychiatry: issues in treating women of reproductive age. J Psych Neurosci. 1998;23:223–228. [PMC free article] [PubMed] [Google Scholar]
- Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956;12:307–310. [Google Scholar]
- Laudermilch CL, Holladay SD, Sponenberg DP, Saunders GK, Ward DL, Prater MR. Placental improvement and reduced distal limb defects by maternal interferon-gamma injection in methylnitrosourea-exposed mice. Birth Defects Res A Clin Mol Teratol. 2005;73:597–604. doi: 10.1002/bdra.20176. [DOI] [PubMed] [Google Scholar]
- Nomura T, Hata S, Kusafuka T. Suppression of developmental anomalies by maternal macrophages in mice. J Exp Med. 1990;172:1325–1330. doi: 10.1084/jem.172.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R, Shafiullah MM. Amelioration of sodium valproate-induced neural tube defects in mouse fetuses by maternal folic acid supplementation during gestation. Congenit Anom. 2003;43:29–40. doi: 10.1111/j.1741-4520.2003.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Prater MR, Strahl ED, Zimmerman KL, Ward DL, Holladay SD. Reduced birth defects caused by maternal immune stimulation in methylnitrosourea-exposed mice: association with placental improvement. Birth Defects Res A Clin Mol Teratol. 2004;70:862–869. doi: 10.1002/bdra.20082. [DOI] [PubMed] [Google Scholar]
- Punareewattana K, Sharova LV, Li W, Ward DL, Holladay SD. Reduced birth defects caused by maternal immune stimulation may involve increased expression of growth promoting genes and cytokine GM-CSF in the spleen of diabetic ICR mice. Int Immunopharmacol. 2003;3:1639–1655. doi: 10.1016/S1567-5769(03)00200-5. [DOI] [PubMed] [Google Scholar]
- Punareewattana K, Holladay SD. Immunostimulation by complete Freund’s adjuvant, granulocyte macrophage colony-stimulating factor, or interferon-γ reduces severity of diabetic embryopathy in ICR mice. Birth Defects Res A Clin Mol Teratol. 2004;70:20–27. doi: 10.1002/bdra.10137. [DOI] [PubMed] [Google Scholar]
- Sharova L, Sura P, Smith BJ, Gogal RM, Jr, Sharov AA, Ward DL, Holladay SD. Nonspecific stimulation of the maternal immune system. II. Effects on gene expression in the fetus. Teratol. 2000;62:420–428. doi: 10.1002/1096-9926(200012)62:6<420::AID-TERA9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sharova LV, Gogal RM, Jr, Sharova AA, Crisman MV, Holladay SD. Immune stimulation in urethane-exposed pregnant mice increases expression of level of spleen leukocyte genes for TGFb3, GM-CSF and other cytokines that may play a role in reduced chemical-induced birth defects. Int Immunopharmacol. 2002;2:1477–1489. doi: 10.1016/s1567-5769(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Sharova LV, Sharova AA, Sura P, Gogal RM, Jr, Smith BJ, Holladay SD. Maternal immune stimulation reduces both placental morphologic damage and down-regulated placental growth factor and cell cycle gene expression caused by urethane: are these events related to reduced teratogenesis? Int Immunopharmacol. 2003;3:945–955. doi: 10.1016/S1567-5769(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O, Merriweather MY, Wicker NJ, Finnell RH. Valproate-induced neural tube defects in folate-binding protein-2 (Folbp2) knockout mice. Birth Defects Res A Clin Mol Teratol. 2003;67:974–978. doi: 10.1002/bdra.10128. [DOI] [PubMed] [Google Scholar]
- Tang LS, Finnell RH. Neural and orofacial defects in Folp1 knockout mice. Birth Defects Res A Clin Mol Teratol. 2003;67:209–218. doi: 10.1002/bdra.10045. [DOI] [PubMed] [Google Scholar]
- Torchinsky A, Toder V, Savion S, Shepshelovich J, Orenstein H, Fein A. Immunostimulation increases the resistance of mouse embryos to the teratogenic effect of diabetes mellitus. Diabetologia. 1997;40:635–640. doi: 10.1007/s001250050727. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Multiple Comparisons: 1948–1983. Chapman and Hall; New York: 1953. The problem with multiple comparisons. Unpublished manuscript in the collected works of John W. Tukey VIII; pp. 1–300. [Google Scholar]
- van Poppel G, van den Berg H. Vitamins and cancer. Cancer Let. 1997;114:195–202. doi: 10.1016/s0304-3835(97)04662-4. [DOI] [PubMed] [Google Scholar]
- Wagner C, Nau H. Diurnal variation of folate concentration in mouse embryo and plasma: the protective effect of folinic acid on valproic acid induced teratogenicity is time dependent. Repro Tox. 1991;5:465–471. doi: 10.1016/0890-6238(91)90017-a. [DOI] [PubMed] [Google Scholar]
- Walmod PS, Foley A, Berezin A, Ellerbeck U, Nau H, Bock E, Berezin V. Cell motility is inhibited by the antiepileptic compound, valproic acid and its teratogenic analogues. Cell Motil Cytoskeleton. 1998;40:220–237. doi: 10.1002/(SICI)1097-0169(1998)40:3<220::AID-CM2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk BC, Craig JC, Bennett GD, Calvin JA, Finnell RH. Valproic acid induced changes in gene expression during neurulation in a mouse model. Teratol. 1996;54:284–297. doi: 10.1002/(SICI)1096-9926(199612)54:6<284::AID-TERA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]