Abstract
Context: Hypoparathyroidism (HP) is characterized by low PTH levels, hypocalcemia, and hyperphosphatemia. Heterozygous mutations in pre-pro-PTH or the calcium-sensing receptor (CaSR) cause some forms of autosomal dominant HP (AD-HP). Furthermore, homozygous mutations in glial cells missing B (GCMB) have been implicated in autosomal recessive HP (AR-HP). In most other HP patients, however, the molecular defect remains undefined.
Objective: Our objectives were to determine the genetic defect in the affected members of two unrelated families with AD-HP and define the underlying disease mechanism.
Subjects: Several family members affected by AD-HP were investigated. The proband in family A had low calcium detected on routine blood testing, whereas the proband in family B had symptomatic hypocalcemia.
Methods: Mutational analysis of the genes encoding pre-pro-PTH, CaSR, and GCMB was performed using PCR-amplified genomic DNA of the probands and other available members of each family. The identified GCMB mutants were characterized by Western blot analysis and luciferase reporter assay using DF-1 fibroblasts.
Results: Two novel heterozygous mutations located in the last GCMB exon (c.1389delT and c.1399delC in families A and B, respectively) were identified that both lead to frame-shifts and replacement of the putative second transactivation domain within carboxyl-terminal region by unrelated amino acid sequence. The mutant GCMB proteins were well expressed, and both showed dose-dependent inhibition of the transactivation capacity of wild-type protein in luciferase reporter assays.
Conclusions: The dominant-negative effect observed in vitro for both GCMB mutations provides a plausible explanation for the impaired PTH secretion observed in the two unrelated families with AD-HP.
Two novel, heterozygous mutations in the parathyroid-specific transcription factor GCMB are identified in the affected members of two families with autosomal-dominant hypoparathyroidism. In vitro analyses are consistent with a dominant-negative effect of these mutations, representing a novel disease mechanism.
Hypoparathyroidism (HP) is characterized by levels of PTH insufficient to maintain normal serum calcium concentrations. Characteristic laboratory findings in patients affected by this disorder are hypocalcemia and hyperphosphatemia, low or normal 1,25-dihydroxyvitamin D levels, and often inappropriately normal urinary calcium excretion (1). Symptoms are related to hypocalcemia and can range from muscle cramps to tetany and seizures; they are more likely to occur when hypocalcemia develops rapidly or during times of acute illness. Cataracts, intracranial calcifications, and abnormal dentition can be signs of chronic HP.
Most cases of HP are sporadic, but familial forms with different modes of inheritance have been described, including syndromic (e.g. DiGeorge syndrome) and isolated (1,2) forms. Autosomal dominant (AD)-HP can be caused by activating mutations in the calcium-sensing receptor (CaSR) (3,4) or mutations in PTH that impair intracellular processing of the nascent protein (5,6,7). Furthermore, autosomal recessive forms of HP (AR-HP) have been shown to be caused by rare homozygous mutations in the genes encoding pre-pro-PTH (6,7) or glial cells missing B (GCMB, also referred to as GCM2) (8,9,10), respectively.
The transcription factor GCM, which belongs to a small family of key regulators of parathyroid gland development, was first described in the fruit fly as a master regulator of glial cell development (11,12). There are two mammalian orthologs, named Gcm1 and Gcm2 in the mouse and GCMA and GCMB in humans (13,14,15,16). GCMs comprise a sequence-specific DNA binding domain (the so-called GCM domain) that is located within the amino-terminal region and two putative transactivation (TA) domains that are both located within the carboxyl-terminal region. One of these TA domains follows immediately after the DNA binding domain, whereas the other one is located at the very carboxyl-terminal end of the protein. The carboxyl-terminal portion of GCMB (and Gcm2) furthermore contains a putative inhibitory domain that is located between the two TA domains and three PEST motifs that are likely to play a role in protein stability (17).
In the mouse, Gcm2 is exclusively expressed in parathyroid glands, and Gcm2-null animals lack parathyroid glands and consequently develop hypocalcemia and hyperphosphatemia (15,16,18). Mice heterozygous for Gcm2 ablation are phenotypically and biochemically normal. Because of the exclusive expression of Gcm2 in parathyroid glands and the absence of this organ in Gcm2-null mice, the human ortholog, GCMB, was a strong candidate for a gene potentially mutated in familial forms of HP. Indeed, three recent reports have implicated GCMB in the etiology of AR-HP (8,9,10). The proband of the first reported family with this disorder had hypocalcemic seizures and undetectable PTH at 5 wk of age, yet low but detectable levels of PTH later on. He was found to carry a homozygous intragenic microdeletion involving GCMB exons 1–4, which removes the entire DNA binding domain and the amino-terminal part of the first TA domain. Two other recent reports identified single homozygous missense mutations in the DNA binding domain of GCMB as other causes of AR-HP (9,10). One of these homozygous mutations causes an amino acid change from arginine (R) to leucine (L) at codon 47 (R47L), which reportedly leads to the disruption of GCMB binding to DNA (9). The affected members of another family were shown to carry a homozygous mutation causing a glycine (G) to serine (S) substitution at codon 63 (G63S). Although both amino acid substitutions are located within the DNA binding domain, the G63S mutation did not appear to alter DNA binding specificity, but instead reduced transactivation capacity to 5% of wild-type (10). As a consequence of either mutation, affected individuals had low but measurable levels of PTH, suggesting that the residual GCMB activity was sufficient to allow some limited parathyroid gland development and function. Heterozygous carriers of the microdeletion comprising GCMB exons 1–4 and of either point mutation were asymptomatic and had normal serum calcium and PTH levels (8,9,10).
We now describe two unrelated families with an autosomal dominant form of HP that is not caused by CaSR or PTH mutations. Instead, two novel heterozygous single-nucleotide deletions in GCMB were identified in the affected individuals of both families. Different from the previously reported molecular defects in this gene, both mutations lead to a shift in the open reading frame and thus replace the putative second transactivation domain located within the carboxyl-terminal region of GCMB with unrelated amino acid sequence. When tested in vitro, the mutant GCMB proteins exhibited dominant-negative properties, thereby providing a potential explanation for the autosomal dominant mode of inheritance.
Patients and Methods
Patients
Kindred A
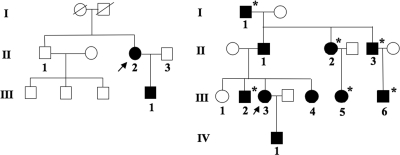
Family A was described previously in a report that excluded CaSR mutations in the affected members of this family (19). The index case in this kindred (II-2; Fig. 1, left panel) came to medical attention at the age of 28 yr because of low total serum calcium that was discovered during routine blood testing. Additional laboratory investigations established the diagnosis of HP with hypocalcemia, hyperphosphatemia, and an inappropriately low intact PTH of 10 pg/ml (normal, 10–65 pg/ml). In retrospect, she had experienced episodic carpal-pedal spasms and perioral tingling for the 5 yr preceding the diagnostic work-up. She was treated with oral calcium and calcitriol, which was titrated to achieve low-normal levels of serum calcium; she has remained asymptomatic on this therapy. After an otherwise unremarkable pregnancy, she gave birth to a healthy appearing son (III-1; Fig. 1, left panel). He had a hypocalcemic seizure on d 3 of life, at which time his serum calcium was low at 6.0 mg/dl with an inadequately normal PTH (22 pg/ml). Treatment with calcitriol (initially 0.25 μg twice daily) and oral calcium was initiated. He did well and is currently 19 yr old and being treated with calcium and calcitriol. The only sibling of II-2, the brother II-1, is healthy, as are his three sons. Both parents of the index case are deceased, and no laboratory information is available for them, but both appear to have been healthy.
Figure 1.
Pedigree of families A (left panel) and B (right panel) with AD-HP. The index cases are marked by an arrow. White symbols, unaffected; black symbols, affected individuals, whose DNA was sequenced; *, biochemically affected, but DNA was not obtained.
Kindred B
The index case in kindred B (III-3; Fig. 1, right panel) presented at age 35 with muscle cramps, tetany, and psychiatric symptoms. Laboratory evaluation revealed biochemical findings typical for HP, i.e. hypocalcemia, high-normal serum phosphate, and low serum PTH (Table 1). Since diagnosis, the patient has been treated with α-calcidol and calcium supplementation, and her clinical course has been uneventful; microcalcifications of the lenticular ganglia were noted at age 49.
Table 1.
Clinical manifestations and biochemical features of the proband (III-3) and other affected members in family B
| Patients of family B
|
Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 | II-1 | II-2 | II-3 | III-2 | III-3 | III-4 | III-5 | III-6a | IV-1a | ||
| Serum calcium (mmol/liter) | 1.50 | 1.62 | 1.77 | 1.65 | 2.10 | 1.95 | 1.90 | 1.80 | 1.90 | 1.80 | 2.10–2.55 |
| Serum phosphorus (mmol/liter) | 1.70 | 1.74 | 1.82 | 1.85 | 1.85 | 1.39 | 1.85 | 1.66 | 2.90 | 1.81 | 1.5–2.0 (children); 0.9–1.5 (adults) |
| Intact serum PTH (ng/liter) | <5 | 7 | 10 | 10 | 5 | 13–53 | |||||
| Urinary calcium (mmol/kg·24 h) | 0.0085 | 0.029 | 0.018 | ||||||||
The numbers correspond to individuals shown in Fig. 1, right panel. The blank spaces indicate test not performed.
Child.
Review of the family history revealed that nine additional members of kindred B in four different generations are being treated with vitamin D and calcium supplementation because of hypocalcemia, strongly suggesting an autosomal dominant form of HP (see Table 1). Among the 10 affected family members (see Fig. 1, right panel), three individuals (II-3, III-2, and III-6) had clinical manifestations of hypocalcemia before the age of 10, including seizures in two subjects (II-3 and III-6). Calcifications of the basal ganglia as a possible sign of chronic hypocalcemia were detected in individuals II-1 and III-3, and cataracts were noted in individual II-1. None of the affected family members had signs of Albright’s hereditary osteodystrophy or raised clinical suspicion for autoimmune hypoparathyroidism. All affected individuals furthermore have normal renal function and all are treated with α-calcidol and calcium supplementation, except for patient II-2, who is treated with calcidiol and calcium supplementation. The clinical course of all patients has been uneventful once treatment for hypoparathyroidism had been initiated.
Genomic DNA and sequencing
After informed consent was obtained, genomic DNA was isolated from peripheral leukocytes, as described (20). The study was approved by the institutional review boards of the Massachusetts General Hospital, Boston, and was in agreement with the French Ethical Committee recommendations. Peripheral blood for DNA extraction was obtained from the following individuals: kindred A, II-1, II-2, II-3, and III-1; kindred B, II-1, III-1, III-3, III-4, and IV-1. Intronic and, when required, exonic primers were used to amplify all coding exons and intron-exon junctions for the CaSR, PTH, and GCMB genes. The sequences of primers are available upon request. The PCR products were sequenced with the use of the BigDye Terminator Cycle Sequencing Ready Reaction kit on an ABI PRISM 3100 sequencer (Applied Biosystems, Foster City, CA).
For the index case of kindred A (II-2), all exons and intron-exon junctions of PTH and GCMB were amplified and sequenced. For her son (III-1), brother (II-1), and husband (II-3), only exon 5 of GCMB and corresponding intron-exon junctions were sequenced; analysis of the gene encoding CaSR, which had revealed no evidence for a mutation, was previously reported (19). For the index case of kindred B (III-3), all exons and intron-exon junctions of CaSR, PTH, and GCMB were amplified and sequenced. For her father (II-1) and son (IV-1), only exon 5 of the GCMB gene and corresponding intron-exon junctions were sequenced. Genomic DNA from 48 healthy Caucasians was used as controls.
Plasmids encoding wild-type and mutant GCMB
The plasmid encoding wild-type human GCMB was a gift from Drs. Rossana De Iiaco and Angela Giangrande, Strasbourg, France. The following four mutations were introduced by QuikChange (Stratagene, La Jolla, CA) into a pcDNA3.1-based plasmid encoding wild-type full-length human GCMB: GCMBmutA (c.1389delT identified in family A), GCMBmutB (c.1399delC identified in family B), and R47L and G63S, the two mutations identified previously in AR-HP (9,10). The presence of each nucleotide change and the absence of additional mutations were confirmed by nucleotide sequence analysis.
Functional analysis of wild-type and mutant forms of GCMB using luciferase reporter
The luciferase reporter plasmid 6xgbs luc with the consensus DNA binding sequence of GCM was a gift from Drs. Hashemolhosseini and Wegner, Erlangen, Germany. Six copies of the consensus GCM binding site 5′-ATGCGGGT-3′ were inserted into pTATAluc, which carried the firefly luciferase gene under the control of the β-globin minimal promoter (21). Chicken fibroblast DF-1 cells (American Type Culture Collection, Manassas, VA), previously reported to be suitable for GCMB luciferase assays (10), were transiently transfected in 24-well plates using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). A total of 300 ng/well of plasmid DNA was transfected that consisted of the following plasmids: 100 ng/well of the firefly luciferase reporter plasmid 6xgbs, 0.5 ng/well of plasmid encoding Renilla luciferase (pRL-TK Vector; Promega, Madison, WI) to allow normalization of the data (see below), and 200 ng of plasmids encoding wild-type and/or mutant GCMB and, when appropriate, empty vector pcDNA3.1 to keep the amount of transfected plasmid DNA constant. For baseline luciferase activity, empty vector (200 ng/well) was transfected. For wild-type GCMB activity, 100 ng/well of plasmid encoding wild-type GCMB and 100 ng/well empty vector, and for mutant GCMB activity, 100 ng/well of plasmid encoding mutant GCMB and 100 ng/well empty vector were transfected. For cotransfections of plasmids encoding wild-type and mutant GCMB, 100 ng/well of each plasmid were transfected. To ensure that equal amounts of DNA were transfected into each well, OD measurements and visualization of DNA on agarose gels were performed for each plasmid. For dose-response experiments, wild-type GCMB was used at 50 ng/well, and mutants at 12.5, 25, 50, and 100 ng/well, with empty vector added to ensure that equal amounts of DNA per well were transfected. Cells were harvested 48 h after transfection and assayed for luciferase activity using the Dual Luciferase Reporter Assay (Promega). This system uses the Renilla luciferase activity to normalize the data for transfection efficiencies. Four to 10 experiments were carried out in triplicate, and data are presented as mean ± sem of all experiments.
Generation of GCMB-specific antibodies
Peptides corresponding to the amino-terminal residues 111–130 (LKQQKKACPNCHSALELIPC) and the carboxyl-terminal residues 481–500 (CLSGLGSAVSYSDRVGPFFT) of human GCMB were chosen to produce the polyclonal antibodies N-GCMB and C-GCMB, respectively. Both peptides were synthesized at the Massachusetts General Hospital Biopolymer Core Facility with a cysteine residue at either the C terminus or the N terminus for subsequent conjugation to keyhole limpet hemocyanin to increase antigenicity. Immunization of rabbits and serum collection were performed at Cocalico Biologicals (Reamstown, PA). Antisera were evaluated using microtiter plates that were coated with the peptide that was used for immunizations. Rabbit IgG bound to the plate was detected with a horseradish peroxidase (HRP)-conjugated goat antirabbit antibody (Invitrogen), which showed readily detectable binding at a final dilution of 1:106 (data not shown).
Western blot analysis
DF-1 cells were transiently transfected in six-well plates with 1.0 μg plasmid per well using FuGENE 6. Cells were lysed 48 h after transfection with 100 μl SDS-PAGE samples buffer and, after shearing with an 18G needle, 5 μl protein sample per well was separated on an 8% acrylamide gel under reducing conditions. Transfer onto a polyvinylidene fluoride membrane and Western blot analysis were performed using standard procedure. Five percent nonfat milk in PBS with 0.05% Tween was used for blocking, GCMB antibodies were used at a final dilution of 1:10,000, and the secondary antibody HRP-conjugated goat antirabbit IgG antibody at 1:10,000. The blot was visualized with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer LAS; Boston, MA). For loading control, blots were reprobed with mouse antivinculin monoclonal antibodies (Sigma Chemical Co., St. Louis, MO) at 1:30,000 using HRP-conjugated goat antimouse IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:10,000 as secondary antibody. Before analysis with a different antibody, blots were stripped with Re-Blot Plus (Chemicon, Temecula, CA) according to the manufacturer.
Results
In both pedigrees, HP followed an autosomal dominant mode of inheritance (Fig. 1). The vertical transmission of the HP phenotype with apparently complete penetrance is well illustrated in pedigree B. In this family, HP was observed in four generations with each affected individual having an affected parent; males and females were equally affected, and several male-to-male transmissions had occurred (Fig. 1, right panel). A mutation in the gene encoding the CaSR, which was previously excluded for the index case in family A (named family H in the earlier report) (19), was also excluded for the index case in family B; for the index cases of both families, we furthermore excluded mutations in the exons encoding pre-pro-PTH by direct nucleotide sequence analysis of PCR-amplified DNA (data not shown). Because analysis of the two candidate genes, known at the time to cause AD-HP in some families (1,2,4), had failed to reveal any molecular abnormality, we postulated that the autosomal dominant form of HP in families A and B could be caused by mutations in the parathyroid-specific transcription factor GCMB.
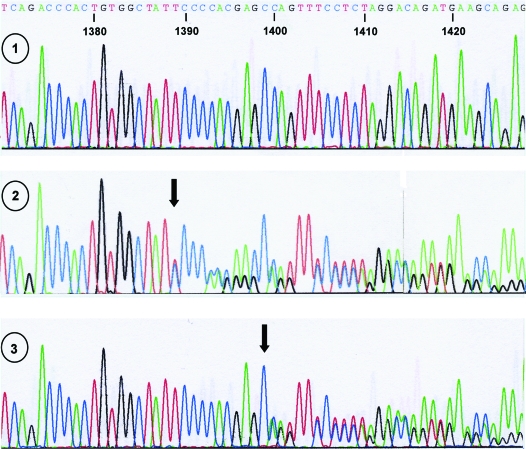
Indeed, direct nucleotide sequence analysis of all GCMB exons and adjacent intronic nucleotides in family A led to the identification of a heterozygous single-nucleotide deletion in exon 5, c.1389delT (named GCMBmutA in this report), in the affected male III-1 and his affected mother II-2 (Fig. 2); this deletion was not found in his father (II-3) and his maternal uncle (II-1), who has three healthy children (see Fig. 1, left panel). In family B, a heterozygous single-nucleotide deletion in exon 5, c.1399delC (named GCMBmutB), that is located 10 bases 3′ of the mutation observed in family A, was identified in the available affected individuals (II-1, III-3, III-4, and IV-1; the deletion was not found in the unaffected sister III-1; see Fig. 1, right panel, and Fig. 2). Neither mutation was found in the public databases, nor could it be identified by direct sequence analysis of genomic DNA from 48 healthy controls (data not shown).
Figure 2.
Nucleotide sequence analysis of GCMB using DNA from a healthy individual (wild-type, panel 1) and an affected member of families A (II-2; panel 2) and B (III-3; panel 3). A portion of the nucleotide sequence of exon 5 from each subject is shown. The arrow in panel 2 indicates the deletion of thymidine at position 1389 of the cDNA (c.1389delT), which causes a frame-shift after amino acid 464 (family A). The arrow in panel 3 indicates the deletion of cytosine at position 1399 (c.1399delC), which causes a frame-shift after amino acid 466 (family B).
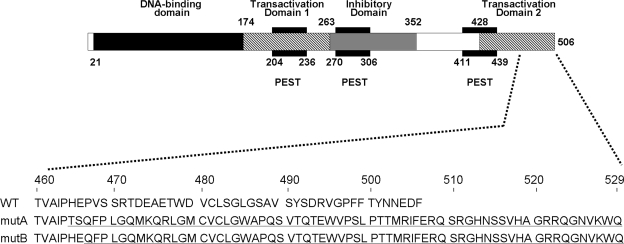
Both single-nucleotide deletions are located in a portion of exon 5 that encodes the carboxyl-terminal tail of GCMB containing the putative second TA domain. Both deletions lead to a shift in the open reading frame resulting in the loss of the last 42 and 40 amino acid residues, respectively (Fig. 3). More than half of the TA domain, which is predicted to comprise 78 amino acids, is therefore replaced by a novel carboxyl-terminal sequence of 65 and 63 amino acids, respectively, that follow proline 464 (mutation c.1389delT in family A) and glutamine 466 (mutation c.1399delC in family B). A search for homologies of this newly added stretch of amino acids using the BLAST search option of NCBI (http://www.ncbi.nlm.nih.gov/) or the SwissProt database (http://au.expasy.org/) did not reveal similarities to known mammalian proteins. Analysis by ExPASy of the deleted GCMB amino acid sequence predicted a site for protein kinase C phosphorylation, two sites for casein kinase II phosphorylation and two sites for N-myristoylation.
Figure 3.
Upper panel, Domain structure of human GCMB (adapted from Ref. 17); lower panel, carboxyl-terminal amino acid sequence of wild-type human GCMB (WT) and of the mutants identified in families A (mutA) and B (mutB). Both single-nucleotide deletions lead to a frameshift and translation of 65 novel amino acids after proline 464 and of 63 novel amino acids after glutamic acid 466, respectively. Novel amino acids are underlined. Numbering of amino acids is above.
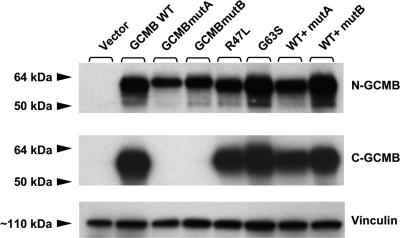
Western blot analysis using wild-type or mutant GCMB derived from lysates of transiently transfected chicken fibroblast DF-1 cells revealed no bands of the expected size, when using preimmune serum or GCMB antibodies that were neutralized with those peptides, which had been used for immunization (data not shown). In contrast, analysis of wild-type GCMB with the N-GCMB antibody revealed a protein band of about 60 kDa, which is the expected size; no immunoreactivity was observed in lysates from cells transfected with empty vector (Fig. 4, upper panel). Lysates from cells expressing GCMBmutA and GCMBmutB showed a protein band that was, as expected, slightly larger than that from cells expressing the wild-type protein. Protein levels of wild-type and mutant GCMB were similar, indicating that the identified mutations do not significantly impair expression. A protein band of the expected size was also observed in lysates from cells transfected with wild-type GCMB, when the blot was reprobed with the antibody C-GCMB that had been raised against a portion of the protein sequence that is replaced in mutA and mutB. However, no protein band was detected in lysates from cells transfected with mutant A or mutant B (Fig. 4, middle panel). Reprobing the Western blot with antivinculin antibodies indicated similar amounts of protein had been loaded.
Figure 4.
Western blot analysis using polyclonal antibodies against synthetic human GCMB fragments. Chicken DF-1 cells were transiently transfected with empty vector (lane 1), wild-type GCMB (lane 2), GCMBmutA (lane 3), GCMBmutB (lane 4), or GCMB carrying the mutation R47L (lane 5) or G63S (lane 6); furthermore, cells were cotransfected with equal amounts of plasmid encoding wild-type GCMB and GCMBmutA (lane 7) or wild-type GCMB and GCMBmutB (lane 8). Cells were lysed with SDS-PAGE sample buffer, and cellular proteins were separated on an 8% SDS-PAGE under reducing conditions (size marker, SeeBlue Plus2; Invitrogen). After transfer onto a polyvinylidene fluoride membrane and blocking with 5% nonfat milk, the GCMB antibody N-GCMB (upper panel) was added at a final dilution of 1:10,000 for 1 h at room temperature. The second antibody was HRP-conjugated goat antirabbit IgG, and the blot was visualized by chemiluminescence. After stripping, the blot was reprobed with antibody C-GCMB (middle panel) or with an antibody against vinculin to assess whether similar amounts of protein had been loaded (lower panel). Using antibody N-GCMB, protein bands of the expected sizes were detected in lysates from cells expressing wild-type GCMB and the different mutants, but not in lysates from cells transfected with empty vector. Antibody C-GCMB that is directed against an epitope within the portion of GCMB that is replaced with unrelated amino acid sequence in mutA and mutB also revealed the expected protein band in lysates from cells transfected with WT GCMB or the R47L or the G63S mutant, but not in lysates from cells transfected with GCMBmutA or GCMBmutB.
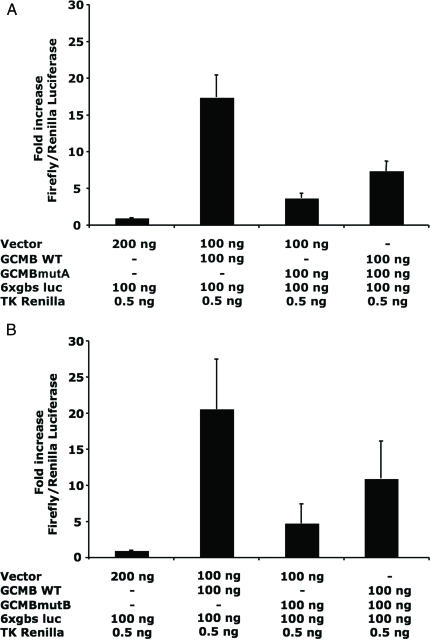
DF-1 cells transfected with plasmid encoding wild-type human GCMB (100 ng/well, together with empty vector and reporter plasmid, 100 ng/well each) showed an approximately 19-fold higher luciferase activity than cells transfected with empty vector alone (200 ng/well) (Fig. 5, A and B). Transfection of DF-1 cells with either GCMBmutA or GCMBmutB (100 ng/well, together with empty vector and reporter plasmid, 100 ng/well each) led to a luciferase activity that was about 4-fold higher than that observed in cells transfected with empty vector. DF-1 cells expressing two previously reported mutants that cause AR-HP, R47L, and G63S (9,10), showed 41 ± 4% and 38 ± 2%, respectively, of the activity observed with cells expressing wild-type GCMB. When either the R47L or the G63S mutant was cotransfected with plasmid encoding wild-type GCMB, no apparent reduction of wild-type GCMB activity was observed (93 ± 6 and 92 ± 5% of wild-type, respectively). However, when the plasmid encoding wild-type GCMB (100 ng/well) was cotransfected with plasmid encoding GCMB carrying the mutation found in family A (c.1389delT) (100 ng/well), or the mutation found in family B (c.1399delC) (100 ng/well), reporter activity was reduced to 42 ± 8 and 53 ± 10% of wild-type, respectively (Fig. 5). To determine whether the effect of the GCMB mutants is dose dependent, increasing amounts of plasmid DNA encoding either GCMBmutA or GCMBmutB (12.5–100 ng/well) were cotransfected with a constant amount of plasmid DNA encoding WT GCMB (50 ng/well). As indicated in Table 2, increased expression of either mutant was associated with a gradual decrease in reporter activity stimulated by wild-type GCMB.
Figure 5.
Luciferase reporter assay using chicken fibroblast DF-1 cells. Cells were cotransfected with plasmids encoding wild-type human GCMB (GCMB WT), combined with either the GCMBmutA mutant identified in family A (panel A), the GCMBmutB mutant identified in family B (panel B), or empty vector; amount of the different plasmids transfected per well is indicated below each graph; each well was furthermore cotransfected with 100 ng/well of 6xgbs luc reporter plasmid and with 0.5 ng/well of plasmid encoding Renilla that was used for normalization (see Patients and Methods for details). Results are shown as the means from 10 experiments for GCMBmutA and six experiments for GCMBmutB, each performed in triplicate wells; bars denote sem. Luciferase activity obtained with empty plasmid was defined as 1.
Table 2.
Luciferase reporter assay using chicken fibroblast DF-1 cells transfected with increasing amount of mutant GCMB
| Mutant A (ng plasmid/well) | % GCMB WT luciferase activity | Mutant B (ng plasmid/well) | % GCMB WT luciferase activity |
|---|---|---|---|
| 0 | 100 ± 5 | 0 | 100 ± 1 |
| 12.5 | 76 ± 21 | 12.5 | 84 ± 28 |
| 25 | 63 ± 6 | 25 | 62 ± 3 |
| 50 | 51 ± 8 | 50 | 52 ± 5 |
| 100 | 37 ± 4 | 100 | 38 ± 7 |
Cells were cotransfected with plasmids encoding wild-type human GCMB (GCMB WT, 50 ng/well) and either increasing concentrations of the c.1389delT mutant identified in family A (mutant A) or the c.1399delC mutant identified in family B (mutant B) (both 0–100 ng/well). Each well was additionally cotransfected with 100 ng/well of the 6xgbs luc reporter and 0.5 ng/well of plasmid encoding Renilla that was used for normalization (see Materials and Methods for details). Empty vector was added as needed to achieve a final DNA concentration of 250 ng/well. Results are shown as the mean ± sem of four independent experiments, each performed in triplicate wells; the response to GCMB WT was set as 100%.
Discussion
We have identified two unrelated families with an isolated AD-HP, as documented by the vertical transmission of the clinical and laboratory phenotype with apparently complete penetrance in both pedigrees. Mutations in the exons encoding pre-pro-PTH and CaSR, i.e. the two genes that have previously been implicated in AD-HP, were excluded. Because homozygous GCMB mutations had been implicated in a recessive form of the disease, we hypothesized that heterozygous, dominant-negative mutations in this transcription factor could be responsible for the disease in our families. Indeed, all affected members in both families for whom DNA was available were shown to carry distinct novel heterozygous GCMB mutations. Both heterozygous GCMB mutations are single-nucleotide deletions that reside within the last exon of GCMB (exon 5) and lead to a shift in the open reading frame, thereby replacing the putative second transactivation domain of this transcription factor with unrelated novel amino acid sequence (see Fig. 3) (17). The new termination codon resides in the last GCMB exon, and the mutant mRNA is therefore not expected to undergo nonsense-mediated decay. Consistent with this conclusion, both GCMB mutants were well expressed, as determined by Western blot analysis with N-GCMB, an antibody that recognizes an epitope within the amino-terminus of GCMB, but the mutants were not visualized with C-GCMB, an antibody that binds to an epitope within the carboxy-terminus of GCMB (see Fig. 4). Neither mutation was found in DNA from healthy controls.
To determine whether the identified heterozygous GCMB mutations cause AD-HP through a dominant-negative effect, we assessed the activity of both mutant proteins in DF-1 cells using a luciferase reporter assay. Consistent with previous findings (10), these cells revealed an increase in luciferase activity, which was approximately 19-fold. In contrast, cells expressing the mutant GCMB showed a reporter activity that was only approximately 22% of wild type, which is, however, higher than that observed with cells transfected with empty vector. This indicated that the mutant proteins continue to bind to target DNA and exhibit decreased, but not totally absent, transcriptional activity. However, cotransfection with equal amounts of wild-type and mutant GCMB vector led to a reduction of reporter activity by approximately 50% compared with cells cotransfected with empty vector and plasmid encoding wild-type GCMB alone. Consistent with the possibility that the mutant proteins compete with wild-type GCMB for DNA binding and thus transactivation, we furthermore observed a gradual decrease of reporter activity when transfecting cells with a constant amount of wild-type GCMB and increasing doses of either mutant (see Table 2). Similar to findings with wild-type fruitfly GCMa and either a mouse or a fruitfly mutant lacking the carboxyl-terminal second TA domain (22), our findings suggest direct competition between wild-type human GCMB and the mutants identified in humans with AD-HP. We were unable to show any dominant-negative effect for two previously identified homozygous GCMB mutations that change a single amino acid residue (9,10), which is consistent with the autosomal recessive nature of the disease in the families in which these mutations were identified. However, in contrast to the previously reported complete lack of transactivation by the R47L and the G63S mutant (9,10), we showed that both point mutations render the mutant GCMB partially active, which could be related to differences in the experimental approach for evaluating these mutant proteins.
Transcription factors often form dimers, and protein mutations that lead to a dominant-negative effect were shown to inhibit dimerization (23,24). However, dimerization does not appear to occur for GCM proteins (25), making it necessary to seek other explanations for the observed findings. The mutations identified in our families are in close vicinity to each other, and both lead to the replacement of approximately half of the carboxyl-terminal end of the putative second GCMB transactivation domain with 65 and 63 unrelated amino acid residues, respectively. This domain disruption is most likely responsible for the observed decrease in transcriptional activity of the mutant proteins. However, despite its decreased activity, the mutant GCMB proteins have readily detectable dominant-negative effects on the wild-type protein in vitro. The GCMB mutants appear to be as stable as the wild-type protein as shown by our Western blot analyses, and it therefore remains unknown how these mutant proteins impair activity of the wild-type protein and whether an interaction with cofactors is altered or disrupted. In this regard, it is interesting to recall that the carboxyl-terminal part of GCMB is a complex structure, which comprises, besides the TA domains, a putative inhibitory domain and three PEST motifs, one of which is located within in the TA domain 2 (17). PEST motifs could mark the protein for rapid degradation. However, especially the PEST motif located in the disrupted TA domain 2 may become less accessible in the mutant proteins, thereby possibly increasing stability of the mutant protein. Although metabolic labeling studies would be needed to formally assess stability of the mutant GCMB proteins, our Western blot data showed no obvious differences in the abundance of wild-type and mutant GCMB proteins, thus providing no evidence for increased stability of the mutant proteins.
In conclusion, inhibition of wild-type GCMB activity by the mutant proteins and the dominant mode of inheritance strongly suggest that the identified GCMB mutations exert a dominant-negative effect on the wild-type transcription factor, thus providing a reasonable explanation for the etiology of HP in our two families. It is conceivable that similar GCMB mutations exist in other HP patients, in whom PTH and CaSR mutations had been excluded. However, recent mutational analysis of DNA from several patients with familial or sporadic HP (26) revealed no evidence for GCMB mutations, making it likely that additional genes are required for normal parathyroid gland development and/or function and that mutations in these genes can also lead to HP. Furthermore, given that GCMB continues to be expressed in parathyroid tissue beyond embryonic development (27,28), it is plausible that this transcription factor has regulatory roles in parathyroid gland biology later in life and that the dominant-negative GCMB mutants identified in this report may facilitate the analysis of these presumed functions in adult parathyroid glands.
Acknowledgments
We thank all family members for their participation. We also thank Dr. Marie Demay and Dr. Henry Kronenberg for valuable advice.
Footnotes
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Disease (RO1-46718-10 to H.J. and K08-DK081669-01 to M.M.) and from Institut National de la Santé et de la Recherche Médicale and réseaux DHOS (to C.S. and B.G.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 26, 2008
Abbreviations: AD, autosomal dominant; AR, autosomal recessive; CaSR, calcium-sensing receptor; GCMB, glial cells missing B; HP, hypoparathyroidism; HRP, horseradish peroxidase; TA, transactivation.
References
- Thakker RV, Jüppner H 2006 Genetic disorders of calcium homeostasis caused by abnormal regulation of parathyroid hormone secretion or responsiveness. In: DeGroot LJ, Jameson JL, eds. Endocrinology, fifth edition, volume 2. Philadelphia: Elsevier Sanders; 1511–1531 [Google Scholar]
- Thakker RV 2001 Genetic developments in hypoparathyroidism. Lancet 357:974–976 [DOI] [PubMed] [Google Scholar]
- Pearce SH, Williamson C, Kifor O, Bai M, Coulthard MG, Davies M, Lewis-Barned N, McCredie D, Powell H, Kendall-Taylor P, Brown EM, Thakker RV 1996 A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med 335:1115–1122 [DOI] [PubMed] [Google Scholar]
- Thakker RV 2004 Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium 35:275–282 [DOI] [PubMed] [Google Scholar]
- Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM 1990 Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest 86:1084–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Thakker RV 1992 A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet 1:149–152 [DOI] [PubMed] [Google Scholar]
- Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S 1999 A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab 84:3792–3796 [DOI] [PubMed] [Google Scholar]
- Ding C, Buckingham B, Levine MA 2001 Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest 108:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumber L, Tufarelli C, Patel S, King P, Johnson CA, Maher ER, Trembath RC 2005 Identification of a novel mutation disrupting the DNA binding activity of GCM2 in autosomal recessive familial isolated hypoparathyroidism. J Med Genet 42:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomee C, Schubert SW, Parma J, Le PQ, Hashemolhosseini S, Wegner M, Abramowicz MJ 2005 GCMB mutation in familial isolated hypoparathyroidism with residual secretion of parathyroid hormone. J Clin Endocrinol Metab 90:2487–2492 [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y 1995 glial cells missing: a binary switch between neuronal and glial determination in drosophila. Cell 82:1025–1036 [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS 1995 glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82:1013–1023 [DOI] [PubMed] [Google Scholar]
- Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, Anderson DJ 1998 Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. Proc Natl Acad Sci USA 95:12364–12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y, Hosoya T, Poole AM, Hotta Y 1996 The gcm-motif: a novel DNA-binding motif conserved in Drosophila and mammals. Proc Natl Acad Sci USA 93:14912–14916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer M, Pirola B, Giglio S, Giangrande A 1999 GCMB, a second human homolog of the fly glide/gcm gene. Cytogenet Cell Genet 84:43–47 [DOI] [PubMed] [Google Scholar]
- Kanemura Y, Hiraga S, Arita N, Ohnishi T, Izumoto S, Mori K, Matsumura H, Yamasaki M, Fushiki S, Yoshimine T 1999 Isolation and expression analysis of a novel human homologue of the Drosophila glial cells missing (gcm) gene. FEBS Lett 442:151–156 [DOI] [PubMed] [Google Scholar]
- Tuerk EE, Schreiber J, Wegner M 2000 Protein stability and domain topology determine the transcriptional activity of the mammalian glial cells missing homolog, GCMb. J Biol Chem 275:4774–4782 [DOI] [PubMed] [Google Scholar]
- Günther T, Chen ZF, Kim J, Priemel M, Rueger JM, Amling M, Moseley JM, Martin TJ, Anderson DJ, Karsenty G 2000 Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature 406:199–203 [DOI] [PubMed] [Google Scholar]
- D'Souza-Li L, Yang B, Canaff L, Bai M, Hanley DA, Bastepe M, Salisbury SR, Brown EM, Cole DE, Hendy GN 2002 Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. J Clin Endocrinol Metab 87:1309–1318 [DOI] [PubMed] [Google Scholar]
- Schipani E, Weinstein LS, Bergwitz C, Iida-Klein A, Kong XF, Stuhrmann M, Kruse K, Whyte MP, Murray T, Schmidtke J, van Dop C, Brickman AS, Crawford JD, Potts Jr JT, Kronenberg HM, Abou-samra AB, Segre GV, Jüppner H 1995 Pseudohypoparathyroidism type Ib is not caused by mutations in the coding exons of the human parathyroid hormone (PTH)/PTH-related peptide receptor gene. J Clin Endocrinol Metab 80:1611–1621 [DOI] [PubMed] [Google Scholar]
- Schreiber J, Sock E, Wegner M 1997 The regulator of early gliogenesis glial cells missing is a transcription factor with a novel type of DNA-binding domain. Proc Natl Acad Sci USA 94:4739–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifegerste R, Schreiber J, Gulland S, Ludemann A, Wegner M 1999 mGCMa is a murine transcription factor that overrides cell fate decisions in Drosophila. Mech Dev 82:141–150 [DOI] [PubMed] [Google Scholar]
- Buettner R, Kannan P, Imhof A, Bauer R, Yim SO, Glockshuber R, Van Dyke MW, Tainsky MA 1993 An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol Cell Biol 13:4174–4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi LD, Von Seggern D, Tjian R 1993 Ectopic expression of wild- type or a dominant-negative mutant of transcription factor NTF-1 disrupts normal Drosophila development. Proc Natl Acad Sci USA 90:10563- 10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J, Enderich J, Wegner M 1998 Structural requirements for DNA binding of GCM proteins. Nucleic Acids Res 26:2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret A, Ding C, Kornfield SL, Levine MA 2008 Analysis of the GCM2 gene in isolated hypoparathyroidism: a molecular and biochemical study. J Clin Endocrinol Metab 93:1426–1432 [DOI] [PubMed] [Google Scholar]
- Correa P, Åkerström G, Westin G 2002 Underexpression of Gcm2, a master regulatory gene of parathyroid gland development, in adenomas of primary hyperparathyroidism. Clin Endocrinol (Oxf) 57:501–505 [DOI] [PubMed] [Google Scholar]
- Kebebew E, Peng M, Wong MG, Ginzinger D, Duh QY, Clark OH 2004 GCMB gene, a master regulator of parathyroid gland development, expression, and regulation in hyperparathyroidism. Surgery 136:1261–1266 [DOI] [PubMed] [Google Scholar]