Abstract
Context: Very few patients have been described with isolated 17,20-lyase deficiency who have had their mutations in P450c17 (17α-hydroxylase/17,20-lyase) proven by DNA sequencing and in vitro characterization of the mutations. Most patients with 17,20-lyase deficiency have mutations in the domain of P450c17 that interact with the electron-donating redox partner, P450 oxidoreductase (POR).
Objective: Our objective was to clarify the genetic and functional basis of isolated 17,20-lyase deficiency in familial cases who were previously reported as having 17,20-lyase deficiency.
Patients: Four undervirilized males of an extended Bedouin family were investigated. One of these has previously been reported to carry mutations in the CYP17A1 gene encoding P450c17 causing isolated 17,20-lyase deficiency.
Methods: Serum hormones were evaluated before and after stimulation with ACTH. Urinary steroid metabolites were profiled by gas chromatography-mass spectrometry. Exons 1 and 8 of CYP17A1 previously reported to harbor mutations in one of these patients and all 15 coding exons of POR were sequenced.
Results: Gas chromatography-mass spectrometry (GC-MS) urinary steroid profiling and serum steroid measurements showed combined deficiencies of 17,20-lyase and 21-hydroxylase. Sequencing of exons 1 and 8 of CYP17A1 in two different laboratories showed no mutations. Sequencing of POR showed that all four patients were homozygous for G539R, a previously studied mutation that retains 46% of normal capacity to support the 17α-hydroxylase activity but only 8% of the 17,20-lyase activity of P450c17.
Conclusion: POR deficiency can masquerade clinically as isolated 17,20-lyase deficiency.
Genetic and serum steroid levels in a patient with 17,20 lyase deficiency and three similarly affected family members reveals that homozygous G539R mutation in the P450 oxidoreductase gene appears to be responsible for this disorder.
Androgen biosynthesis requires microsomal P450c17, which converts pregnenolone to 17OH-pregnenolone (17α-hydroxylase activity) and then converts 17OH-pregnenolone to dehydroepiandrosterone (DHEA) (17,20-lyase activity). Both activities of P450c17 require electron donation from NADPH via P450 oxidoreductase (POR) (1). The 17,20-lyase activity of P450c17 is determined by three factors: a high molar ratio of POR to P450c17 (2,3), serine phosphorylation of P450c17 (4,5), and the action of cytochrome b5, which promotes the association of P450c17 with POR (6,7,8). Mutation of the CYP17A1 gene for P450c17 (9,10) causes severe 17α-hydroxylase deficiency, in which virtually no 17α-hydroxylated steroids or sex steroids are produced.
Genetically proven isolated 17,20-lyase deficiency was first described in two patients with point mutations in the P450c17 domain that interacts with POR; these mutations retained nearly normal 17α-hydroxylase activity but virtually no 17,20-lyase activity (11). Another patient with apparent isolated 17,20-lyase deficiency was reported as heterozygous for a frameshift mutation and F417C in P450c17 (12). The activity of the F417C mutant expressed in COS-1 cells and analyzed by immunoassay suggested that this mutant catalyzed 17α-hydroxylase activity but not 17,20-lyase activity (12). However, computational modeling of human P450c17 (13) indicated that F417C would cause a major structural disruption of P450c17, which should eliminate both activities. Expression of P450c17 F417C in a well-established yeast system and activity assays by more sensitive conversion of radiolabeled precursors demonstrated that F417C was devoid of activity (14). Thus, combination of F417C and a frameshift should ablate all P450c17 activities, yet the patient had an elevated 17OH-progesterone (17OHP) (15). Therefore, we have reinvestigated this patient and three similarly affected family members, demonstrating that the apparent isolated 17,20-lyase deficiency was caused by a homozygous mutation in POR, G539R.
Patients and Methods
Patients
The four 46,XY patients belong to a small Bedouin clan in Southern Israel (Fig. 1A); patient 2 was the subject of the initial report (12). All the patients were followed at the pediatric endocrine clinic of Soroka Medical Center. Endocrine evaluation of patients 1–3 in early childhood revealed subnormal testosterone response to human chorionic gonadotropin and a subnormal response of cortisol to ACTH. Basal plasma DHEA and androstenedione concentrations were low and unresponsive to ACTH; basal and post-ACTH 17OHP were elevated (15). The local ethics committee at Soroka Medical Center approved the study, and the parents gave informed consent.
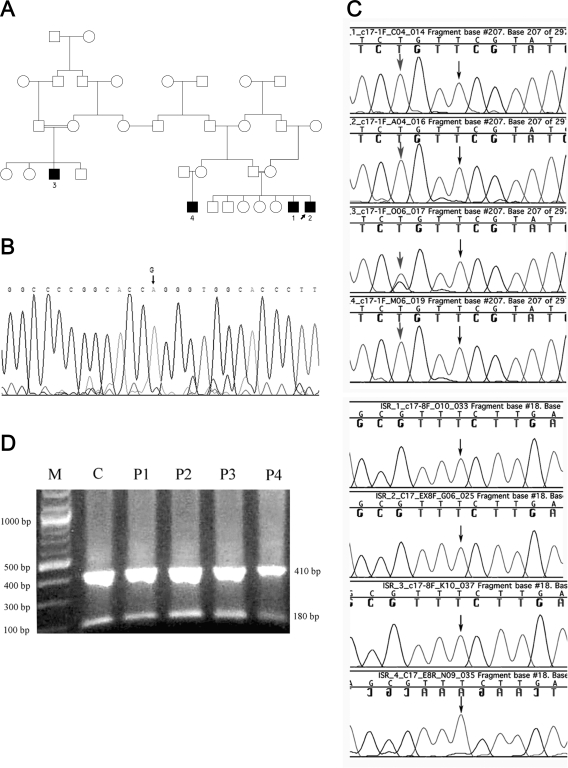
Figure 1.
Genetics of the affected kindred. A, Family tree showing the relationships among the four patients studied. The affected individuals are numbered as in the text. B, Sequence analysis of the POR gene; the mutation G→A at position 1697 in exon 12 causing the replacement of G539R is marked by an arrow on the sequence trace of patient 2. This mutation was identified in all four patients. C, Sequence analysis of CYP17A1. Upper panel (four tracings), Exon 1. Black arrows indicate that the previously reported T deletion at position 198 in CYP17A1 exon 1 was not presented in all four patients. Gray arrows indicate that patients 1, 2, and 4 are homozygous for the polymorphism 195 G→T, and patient 3 is heterozygous. Lower panel (four tracings), Exon 8. Black arrows indicate that the substitution 1235 T→G, reportedly responsible for the missense mutation F147C, was not present in all four patients. D, Digestion of CYP17A1 exon 8 PCR product with Hpy188III. M, Marker 100 bp (New England Biolabs); C, nlormal control; P1–P4: patients 1–4. The 591-bp PCR product of CYP17A1 exon 8 was cut into fragments of 181 and 410 bp in all four patients and in the normal control, confirming the presence of the wild-type sequence.
Methods
Methods have been published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
Results
Clinical and hormonal findings
Patient 1 was born to healthy first-degree cousins after a normal-term pregnancy and delivery. Micropenis, inguinal testes, and an underdeveloped scrotum were noted at birth; physical examination was otherwise normal. Evaluation on d 1 showed low testosterone [52 ng/dl (1.8 nmol/liter); normal up to 345 ng/dl (12 nmol/liter)]. He is now 21 yr old, is +1.42 sd in height, and is Tanner 5 with a 7-cm penis. Patient 2, the younger brother of patient 1, was born after a normal pregnancy and uneventful delivery. He had micropenis, chordee, scrotal hypospadias, and small testes (volume 0.5 ml) in a bifid scrotum; physical examination was otherwise normal. Low concentration of testosterone [84 ng/dl (2.9 nmol/liter)] and abnormally high levels of 17OHP [11.88 μg/liter (36nmol/liter); normal, 0.66–2.5 μg/liter (2–7.5 nmol/liter)] were found 48 h after birth. Cystoscopy and uretherography showed normal internal male genital anatomy. He is now 14.6 yr old, −1.0 sd in height, and Tanner 2 with a 3-cm penis.
Patient 3 was evaluated at 10 months for micropenis but otherwise normal external genitalia. His parents are cousins who were followed for maternal infertility. He is now 16.5 yr old, +0.89 sd in height, and Tanner 5 with an 8-cm penis.
Patient 4 was born after normal pregnancy and uneventful delivery and evaluated on d 1 for micropenis with chordee and undescended testes; testosterone was low [20 ng/dl (0.7 nmol/liter)].
All four patients were 46,XY. None of the mothers became virilized during their pregnancies. Patient 2 underwent hypospadias repair and left orchiectomy for recurrent testicular torsion. Patient 4 underwent genitoplasty with urethral reconstruction and scrotal transposition. Linear growth of the patients was within the target range. The onset of puberty (testicular volume ≥4 cm3) was normal in patients 1 and 3, but inadequate virilization required testosterone replacement in both. Patient 2 retained one dysfunctional testis and a small penis. The patients consistently had normal blood pressure and serum electrolytes. No skeletal malformation, dysmorphism, or limited limb movements were found, and x-rays showed no radioulnar or radiohumeral synostosis. Serum cortisol responded poorly to ACTH administration. DHEA sulfate (DHEA-S) levels were low. Basal and ACTH-stimulated 17OHP levels were elevated. Testosterone increased during puberty but failed to reach normal adult male levels without supplementation (Table 1). Gonadotropins were in the normal pubertal range at onset of puberty. However, FSH levels rose steadily afterward in patients 1 and 2. Patient 1 had high FSH (36.5 mIU/liter) and LH (14 mIU/liter), and serum testosterone was low [150 ng/dl (5.25 nmol/liter)] at 21 yr of age. In patient 2, serum FSH was 19 mIU/ml, LH was 2.4 mIU/ml, and testosterone was 100 ng/dl (3.5 nmol/liter) at 14.5 yr of age.
Table 1.
Hormone levels
| Patient 1 (16 yr)
|
Patient 2 (14.5 yr)
|
Patient 3 (15 yr)
|
||||
|---|---|---|---|---|---|---|
| Basal | pACTH | Basal | pACTH | Basal | pACTH | |
| Cortisol (μg/dl) | 7 | 7.6 | 8.15 | 10.6 | 7.9 | 8.16 |
| 17OHP (μg/liter ) | 7.73 (<2) | 16.35 | 5.59 (<2) | 19.35 | 13.80 (<2) | 15.50 |
| DHEA-S (μg/dl) | 95 (80−560) | 72 (80−560) | 90 (80−560) | |||
| Testosterone (ng/dl) | 100 (245−1200) | 110 (245−1200) | 200 (245−1200) | |||
| FSH (mIU/ml) | 4.8 | 19 | 8 | |||
| LH (mIU/ml) | 6.6 | 2.4 | 11 | |||
| ACTH (pmol/liter) | 15 (1−10) | 8.3 (1−10) | ||||
Age-appropriate normal ranges are given in parentheses for the patients. These values were obtained when the patients were not receiving testosterone replacement therapy. The columns pACTH designate steroid hormone values in response to 0.25 mg of ACTH. Conversion factors for SI units are as follows: to convert cortisol to nmol/liter, multiply by 27.6; to convert DHEA-S to μmol/liter, multiply by 0.0271; to convert 17OHP to nmol/liter, multiply by 3.03; and to convert testosterone to nmol/liter, multiply by 0.0347.
Gas chromatography-mass spectrometry analysis of urinary steroids
Gas chromatography-mass spectrometry profiles of urinary steroids in patients 1 and 3 were similar and typical for POR deficiency. The pattern of urinary C19 and C21 steroids revealed elevated markers of 17-hydroxylase deficiency, such as the pregnenolone metabolite 5-pregnenediol and the corticosterone metabolites allo-tetrahydrocorticosterone and tetrahydro-11-dehydrocorticosterone. Elevations of pregnanetriol, a metabolite of 17OHP, and pregnanetriolone, a metabolite of 21-deoxycortisol, provided signs of 21-hydroxylase deficiency. The ratios of urinary key steroid metabolites (Table 2) showed impaired activities of 1) the global 17-hydroxylase/lyase system; 2) 17-hydroxylase, particularly in the Δ5-pathway; 3) 17,20-lyase, particularly in the Δ5-pathway; and 4) 21-hydroxylase. The ratio androsterone/etiocholanolone did not indicate an active backdoor pathway to C19-steroids via 5α-pregnane-3α,17α-diol-20-one.
Table 2.
Characteristics of the urinary steroid metabolites in patients 1 and 3: Diagnostic ratios of metabolites
| Patient 1 | P95 | Patient 3 | P95 | |
|---|---|---|---|---|
| Age (yr) | 19 | 15.5 | ||
| Global 17-hydroxylase/lyase activity: Bs/(An+Et) | 1.8 | 0.3 | 11.0 | 0.3 |
| 17-hydroxylase-activity | ||||
| Δ5-pathway: P5D/P5T-17α | 1.5 | 0.4 | 1.4 | 0.3 |
| Δ4-pathway: PD/PT | 0.0 | 0.1 | 0.2 | 0.1 |
| Global: Bs/Fs | 1.5 | 0.2 | 1.8 | 0.1 |
| 17,20-lyase-activity | ||||
| Δ5-pathway: P5T-17α/A5–3β,17β | 22.6 | 1.6 | 36.2 | 4.8 |
| Δ4-pathway: PT/(An+Et) | 1.7 | 0.2 | 4.4 | 0.2 |
| Global: Fs/(An+Et) | 1.2 | 2.2 | 6.2 | 2.0 |
| 21-hydroxylase-activity: 11-O-PT/a-Cl | 0.8 | 0.0 | 2.1 | 0.0 |
| Backdoor pathway: An/Et | 0.8 | 1.3 | 1.7 | 1.6 |
Abbreviations and trivial and systematic names of steroids are as follows: An, Androsterone, 5α-androstane-3α-ol-17-one; Et, etiocholanolone, 5β-androstane-3α-ol-17-one; P5D, pregnenediol, 5-pregnene-3β,20α-diol; P5T-17α, pregnenetriol-17α,5-pregnene-3β,17α,20α-triol; PD, pregnanediol, 5β-pregnane-3α,20α-diol; PT, pregnanetriol, 5β-pregnane-3α,17α,20α-triol; A5–3β,17β, androstenediol-17β, 5-androstene-3β,17β-diol; 11-O-PT, pregnanetriolone, 5β-pregnane-3α,17α,20α-triol-11-one; a-CL, α-cortolone, 5β-pregnane-3α,17α,20α,21-tetrol-11-one; THA, tetrahydro-11-dehydrocorticosterone, 5β-pregnane-3α,21-diol-11,20-dione; THB, tetrahydrocorticosterone, 5β-pregnane-3α,11β,21-triol-20-one; aTHB, allo-tetrahydrocorticosterone, 5α-pregnane-3α,11β,21-triol-20-one; THF, tetrahydrocortisol, 5β-pregnane-3α,11β,17α,21-tetrol-20-one; aTHF, allo-tetrahydrocortisol, 5α-pregnane-3α,11β,17α,21-tetrol-20-one; THE, tetrahydrocortisone, 5β-pregnane-3α,17α,21-triol-11,20-dione; Bs, Sum of metabolites of corticosterone (THB+aTHB+THA); Fs, sum of metabolites of cortisol (THF+aTHF+THE); P95, 95th percentile of reference values (19,20).
Genetic studies
Sequencing the entire coding region of POR in all four patients revealed a change of G→A at position 1697 (GenBank sequence NM_000941), causing missense mutation G539R (Fig. 1B). Based on the crystallographic structure of rat POR and computational modeling of human POR, the mutated glycine is located in the NADPH binding site of POR and is phylogenetically highly conserved from yeast to mammals (16). Because mutations of two genes (POR and CYP17A1 as reported previously in patient 2) would be most unlikely, we examined the two exons (1 and 8) of CYP17A1 where mutations had been reported previously (12). No mutation was found in exon 1 or exon 8 in all four patients.
To provide an independent confirmation of these sequencing results, the relevant regions of POR and CYP17A1 were sequenced with different primers in a second laboratory. The sequencing of POR exon 12 confirmed the homozygous POR missense mutation G539R in all four patients and confirmed that CYP17A1 exon 1 and exon 8 did not carry mutations (Fig. 1C). The putative F417C mutation in CYP17A1 was reported to arise from a T→G mutation, changing TCTTGA to GCTTGA (12), which would destroy the TCNNGA site recognized by restriction endonuclease Hpy188III. As a third, independent test we digested PCR-amplified patient DNA with Hpy188III, which cut the PCR product of CYP17A1 exon 8 from the control and all four patients into the predicted fragments of 181 and 410 bp, whereas mutation of the Hyp188III site would yield a product of 591 bp (Fig. 1D). Thus, sequencing in different laboratories using different primers and restriction fragment length analysis confirms that none of the patients carried the CYP17A1 F417C mutation.
Discussion
Patient 2 was the subject of previous clinical (15) and genetic (12) reports. Those reports diagnosed this patient as having isolated 17,20-lyase deficiency, despite an ACTH test showing hyperresponsiveness of 17OHP and subnormal responsiveness of cortisol. A genetic study reported compound heterozygosity for two CYP17A1 mutations, a frameshift reportedly inherited from the mother and the missense mutation F417C reportedly inherited from the father (12). Immunoassays of steroids from the culture medium of cells transfected with a vector expressing the F417C mutant were interpreted as showing the mutant retained 17α-hydroxylase activity but lacked 17,20-lyase activity. However, rigorous studies of the F417C mutant showed that this mutant was devoid of 17α-hydroxylase and 17,20-lyase activities (14). We have now resequenced exons 1 and 8 of CYP17A1 where the mutations were reported, and we found no mutations. This sequencing was done in two different laboratories using different primers, and the normality of the site thought to harbor the mutation at F417 was confirmed further by its sensitivity to digestion with restriction endonuclease Hpy188III. These results are consistent with the presence of relatively preserved 17α-hydroxylase activity, as evidenced by the elevated 17OHP values. Furthermore, we have found that all four of our patients are homozygous for the POR missense mutation G539R. The sequencing identifying this mutation was also done in two laboratories using different primers, so one can be confident with these results.
The diagnosis of POR deficiency was initially missed in these patients because the disorder was not characterized until 2004 (17) and because of the borderline nature of serum hormone values and the lack of the dysmorphic features of Antley-Bixler syndrome. The POR mutation G539R has previously been reported in a compound heterozygous Irish patient who carried a null frameshift mutation on the other allele (16). That patient had the Antley-Bixler phenotype, whereas our homozygous G539R patients did not. It is not clear whether this phenotypic difference is due to the Irish patient having a more severe POR defect due to the POR frameshift mutation on the other allele or whether the Irish patient’s phenotype was attributable to his heterozygosity for a mutation in fibroblast growth factor receptor 1 that had been reported in a single case of trigonocephaly (16).
Compared with normal POR, the G539R mutant supported 46% of the 17α-hydroxylase activity but only 8% of the 17,20-lyase activity of human P450c17 (16). Thus, the G539R POR mutant would be predicted to support a level of 17α-hydroxylase activity similar to that seen in heterozygotes for null mutations of CYP17A1 but would be predicted to support minimal 17,20-lyase activity. The prediction for the net effect on P450c17 would be that POR mutant G539R would cause isolated 17,20-lyase deficiency, as was initially diagnosed in the index case in this clan (15). This impairment in androgen production was manifested in the undervirilization seen in our patients at birth and was further manifested at puberty. Although our three adolescent-aged patients entered puberty, its progression was slow and incomplete, associated with low testosterone values. Two patients also had high FSH values, indicating associated Sertoli cell failure. None of our patients has had adrenal insufficiency, but adrenal crisis has been reported in some cases of POR deficiency (18). Thus, we recommend that POR-deficient patients receive hydrocortisone replacement during severe physiological stress.
Supplementary Material
Footnotes
This work was supported by Ben Gurion University Grant 80138401 to R.P and E.H. and by National Institutes of Health Grants R01-HD41958 and R01-GM073020 to W.L.M.
Disclosure Statement: The authors declare that they have nothing to disclose.
First Published Online June 17, 2008
Abbreviations: DHEA, Dehydroepiandrosterone; DHEA-S, DHEA sulfate; 17OHP, 17OH-progesterone; POR, P450 oxidoreductase.
References
- Miller WL 2005 Regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- Yanagibashi K, Hall PF 1986 Role of electron transport in the regulation of the lyase activity of C-21 side-chain cleavage P450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- Lin D, Black SM, Nagahama Y, Miller WL 1993 Steroid 17α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- Zhang L, Rodriguez H, Ohno S, Miller WL 1995 Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AV, Mellon SH, Miller WL 2003 Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Miller WL 1999 P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- Pandey AV, Miller WL 2005 Regulation of 17,20-lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- Chung B, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shivley JE, Miller WL 1987 Cytochrome P450c17 (steroid 17α-hydroxylase/17,20-lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA 84:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J, Miller WL 1987 Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20-lyase): similarity to the gene for P450c21. DNA 6:439–448 [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Mendonça BB, Miller WL 1997 The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Leiberman E, Zachmann M 1997 A single amino acid substitution in the putative redox partner-binding site of P450c17 as cause of isolated 17,20-lyase deficiency. J Clin Endocrinol Metab 82:3807–3812 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Miller WL 1999 Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): insights into reaction mechanisms and effects of mutations. Mol Endocrinol 13:1169–1182 [DOI] [PubMed] [Google Scholar]
- Gupta MK, Geller DH, Auchus RJ 2001 Pitfalls in characterizing P450c17 mutations associated with isolated 17,20-lyase deficiency. J Clin Endocrinol Metab 86:4416–4423 [DOI] [PubMed] [Google Scholar]
- Leiberman E, Hershkovitz E, Lauber-Biason A, Phillip M, Zachmann M 1997 Subnormal cortisol response to adrenocorticotropin in isolated partial 17,20-lyase activity. J Pediatr Endocrinol Metab 10:387–390 [DOI] [PubMed] [Google Scholar]
- Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, van Vliet G, Sack J, Flück CE, Miller WL 2005 Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonca BB, Fujieda K, Miller WL 2004 Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, Nakai H, Soneda S, Tachibana K, Matsuo N, Sato S, Homma K, Nishimura G, Hasegawa T, Ogata T 2005 Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab 90:414–426 [DOI] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA 2005 Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 [DOI] [PubMed] [Google Scholar]
- Wudy SA, Hartmann MF, Remer T 2007 Sexual dimorphism in cortisol secretion starts after age 10 in healthy children: urinary cortisol metabolite excretion rates during growth. Am J Physiol Endocrinol Metab 293:E970–E976 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.