Abstract
Context: Hypophosphatasia (HPP) is a heritable metabolic disorder of the skeleton that includes variable expressivity conditioned by gene dosage effect and the variety of mutations in the tissue nonspecific alkaline phosphatase (TNSALP) gene. Patient age when skeletal problems first manifest generally predicts the clinical course, with perinatal HPP causing bone disease in utero with postnatal lethality.
Objective: Our objective was to identify TNSALP mutations and characterize the inheritance pattern of a family with clinically variable HPP with one child manifesting in utero with long bone deformity but showing spontaneous prenatal and postnatal improvement.
Design: TNSALP enzyme and substrate analysis and TNSALP mutation analysis were performed on all family members.
Patients: A boy with HPP showing long bone deformity that spontaneously improved in utero and after birth is described. His older brother has the childhood form of HPP without findings until after infancy. His parents and twin sister are clinically unaffected.
Results: Both boys are compound heterozygotes for the same missense mutations in TNSALP, documenting autosomal recessive inheritance for their HPP. The parents each carry one defective allele.
Conclusions: The patient is an autosomal recessive case of HPP with prenatal long bone deformity but with spontaneous prenatal and postnatal improvement. Thus, prenatal detection by sonography of bowing of long bones from HPP, even with autosomal recessive inheritance, does not necessarily predict lethality but can represent variable expressivity or the effects of modifiers on the TNSALP defect(s).
Prenatal detection of long bone bowing in hypophosphatasia, even with autosomal recessive inheritance, does not predict death, but represents variable expression or modifier effects on defects in the tissue nonspecific alkaline phosphatase (TNSALP) gene.
The gene that encodes the “tissue nonspecific” isoenzyme of alkaline phosphatase (TNSALP) is located on the short arm of chromosome 1 (1,2), and its protein product (TNSALP) is essential for skeletal mineralization (3). Hypophosphatasia (HPP) is a rare, heritable, metabolic disorder of the skeleton and dentition caused by decreased activity of TNSALP due to loss-of-function mutation(s) within TNSALP (4). Diminished TNSALP activity endogenously leads to extracellular accumulation of several phosphocompound substrates for TNSALP, including phosphoethanolamine, inorganic pyrophosphate, and pyridoxal-5′-phosphate (PLP) (5,6,7,8). Inorganic pyrophosphate is an inhibitor of mineralization, and skeletal excesses explain the rickets or osteomalacia that characterizes this “inborn error of metabolism” (3,9). Nevertheless, the expressivity of HPP is extremely broad ranging (4). Clinically, four principal forms have been described showing severity correlated with earlier presentation of skeletal disease: perinatal HPP, infantile HPP, childhood HPP, and adult HPP (4,10,11). Odonto-HPP, the fifth major clinical form, consists of dental manifestations without skeletal disease (4,10).
Genotype-phenotype correlations have been described for HPP (7,8), yet management and counseling for HPP remain based upon the clinical nosology. The perinatal form of HPP, which can be detected in utero by sonography, has been considered nearly always lethal (4,12,13). However, there have been reports of HPP detected prenatally by sonography due to long bone deformity but demonstrating considerable, spontaneous, postnatal improvement (14,15,16,17,18,19). Interestingly, several of these families manifested autosomal dominant transmission patterns for HPP [the genetic basis was reported for only two of the probands (15)], whereas others were compound heterozygous Japanese patients with F310L mutations in exon 9 of the TNSALP gene (17,18,19). Pauli et al. (14) proposed that this “bent, but not broken,” phenotype in utero be considered a sixth form of HPP.
This report describes a boy with prenatal presentation of HPP manifesting as long bone deformity detected by sonography who had spontaneous in utero as well as postnatal improvement, yet due to autosomal recessive inheritance of two TNSALP missense mutations, neither of which was the F310L mutation (now called p.Phe327Leu).
Patients and Methods
Patient
Sib no. 1 presented in utero at 18-wk gestation with sharp angulation of the midshaft of the right humerus, radius, and ulna detected by sonography performed for twin gestation (Fig. 1). Dichorionic, diamniotic twin gestation was previously documented at 14 wk by sonography performed for the establishment of gestational age in a mother taking clomiphene. At 18 wk, all major long bones were subjectively of slightly narrow diameter and poorly ossified. The right humerus, radius, and ulna were sharply angulated and 2.5–4 sd values below the mean for normal length, and the right arm was held in a fixed, bent position. The long bones on the left side otherwise appeared normal. His femurs were 1.5 sd values below the mean for normal length. At 22 wk, sonography showed improved growth of the right humerus, radius, ulna, and femurs, although the angulations in the upper and lower segments of the right arm persisted, and the right femur appeared curved. At 26-wk gestation, there was a mildly shortened and angulated right radius (Fig. 1C), ulna, and humerus, with note of progressive improvement of the angulations. Movement of the right shoulder was observed.
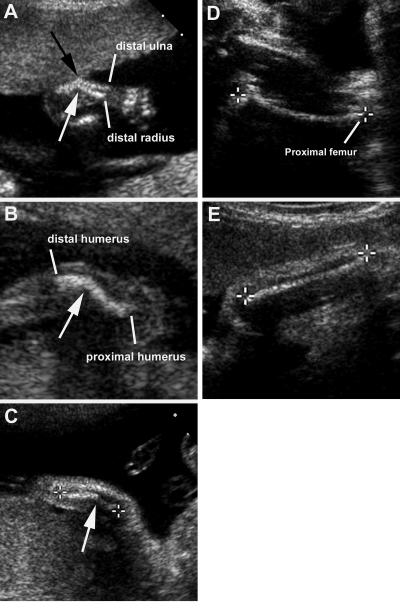
Figure 1.
Sonographic images of sib no. 1. A, Sharp mid-diaphyseal angulation of his right radius (white arrow) and ulna (black arrow) at 18-wk gestation. B, Sharp, mid-diaphyseal angulation (arrow) of his right humerus at 20-wk gestation. C, Improvement in angulation (arrow) of his right radius at 26-wk gestation. Calipers mark the ends of the radius. D, Mildly curved left femur at 34-wk gestation. Calipers mark the ends of the femur. E, Improvement over time of angulation of his right radius at 34-wk gestation. Calipers mark the ends of the radius.
Nevertheless, at 34 wk, sonography continued to show that all of his major long bones were subjectively thin with somewhat irregular contours. His femora were mildly curved with lengths 2 sd values below the control mean (Fig. 1D). His upper extremity long bones were 2–3 sd values below mean lengths for gestation, with mild residual angulation of his right humerus, radius (Fig. 1E), and ulna that had improved over time in utero. His skull and spine were normal throughout gestation. Amniotic fluid levels had been unremarkable, showing no polyhydramnios common in perinatal HPP (4).
Sib no. 1 was a dizygotic twin, and his twin sister (sib no. 2) showed no sonographic abnormalities throughout the pregnancy and was born without apparent skeletal deformities. Furthermore, she demonstrated no clinical or biochemical evidence of HPP. Her serum alkaline phosphatase (ALP) activity soon after birth was 145 IU/liter (normal 110–300). However, lethality for sib no. 1 was a concern given his prenatal findings and a diagnosis of childhood HPP for his older brother.
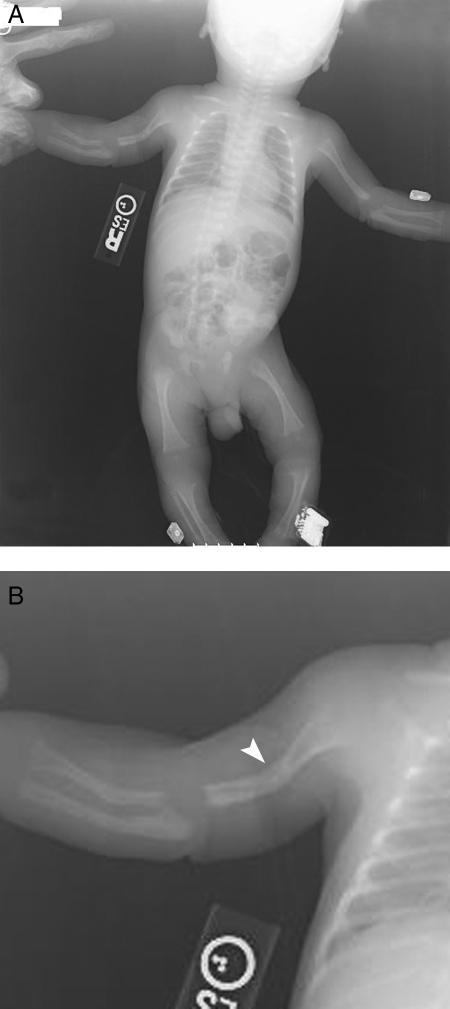
At birth, sib no. 1’s APGAR scores were nine at 1 min and nine at 5 min. There was bowing of his right arm with dimpling of his skin near the apex of the bowing, but his physical examination was otherwise unremarkable. Length and head circumference were both at the 50th centile, but his weight was at the 10th centile. Radiographs showed bowing of his right humerus, radius, and ulna with callus formation suggestive of healing fractures (Fig. 2A, B). Metaphyses were osteopenic and irregular, but the growth plates were unremarkable. There was also mild bowing and irregularity of his left femur, and mild bowing of his left tibia (Fig. 2A). Radiographs of his skull were reported as normal. Serum ALP activity soon after birth was less than 20 IU/liter, and he was diagnosed with HPP.
Figure 2.
A, Radiographic images of sib no. 1 at birth showing angulation of the right humerus, radius, and ulna, and mild bowing of the left femur. B, Inset of A detailing the angulation and callus formation of the right humerus (arrowhead).
He was evaluated next at 6 months of age with persistent bowing of his right arm. Radiographs showed healed fractures of the midshaft of his right humerus, radius, and ulna with posttraumatic deformity, but there was no interval history of trauma.
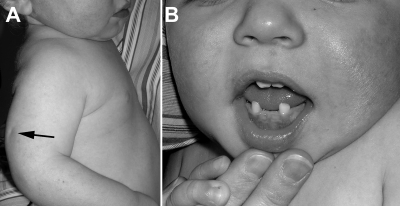
At age 17 months, he was evaluated for premature loss of two deciduous teeth starting at age 16 months (Fig. 3B). He had mild bowing of his right arm where the previous in utero humerus bowing was noted, but bowing and range of motion had improved spontaneously since the perinatal evaluation (Fig. 3A). Extensive skeletal radiographs were not taken because his postnatal clinical deformities were minimal. Other clinical findings included short stature (<3rd centile) with relative macrocephaly (75th centile) and genu valgum. His weight was at the 10th centile.
Figure 3.
Photographs of sib no. 1 at 17 months of age. A, Bowing of the right upper arm with posterior dimpling (arrow). B, Premature loss of teeth (loss of bottom two central incisors).
Otherwise, his clinical course, up to the last evaluation at age 17 months, was unremarkable.
Family history
His 4-yr-old brother has HPP (sib no. 3). His fetal sonography at 20-wk gestation was reported as normal, but the images were not available for review. He was first evaluated at age 19 months for the premature loss of three teeth starting at age 18 months. He was reevaluated at 2.5 yr with mild delays in gross motor skills. No bowing of his extremities was noted. He too had short stature (5th centile) with macrocephaly (98th centile) and a scaphocephalic skull with a high forehead (Fig. 4). Radiographs, first taken at age 18 months, showed flared ribs and radiolucencies of his humeri that projected from the growth plates into the metaphyses characteristic of HPP (4).
Figure 4.
The older brother (sib no. 3). A, Frontal view showing premature loss of teeth at age 2.5 yr. B, Lateral view at age 2.5 yr showing macrocephaly, with a scaphocephalic contour. C, Radiograph at age 18 months showing radiolucencies (delineated by arrows) of the left proximal humerus projecting from the growth plate into the metaphysis.
Both parents were tall [mother = 175 cm (95th centile); father = 188 cm (95th centile)] and had no physical features of HPP, with no known premature loss of deciduous teeth.
TNSALP mutation analysis
Patient, parent, and sib blood leukocyte DNA was obtained together with blood and urine for routine biochemical studies after informed written consent approved by the Human Studies Committee, Washington University School of Medicine, St. Louis, MO.
Genomic DNA was extracted using the PUREGENE DNA Purification Kit (Gentra Systems, Minneapolis, MN). For both affected brothers, all coding exons (nos. 2–12) and adjacent mRNA splice junctions of TNSALP were amplified by PCR and sequenced in both directions using previously described methods and primers (20). The DNA sequence was examined visually and using VectorNTI AlignX software (Invitrogen Corp., Carlsbad, CA). Only TNSALP exons 6, 8, and 12 were sequenced for both parents and the apparently healthy twin sister.
TNSALP activity and substrate analyses
Serum total and bone-specific ALP (BALP) activities were measured at the Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, and compared with age-matched control values established at that facility (21). Plasma PLP levels were quantitated using published HPLC techniques (22,23) in the Department of Chemistry, Indiana-Purdue University, and compared with values from the same control specimens (21).
Results
TNSALP activity and substrate analyses
A summary of the family’s biochemical findings is found in Table 1.
Table 1.
Biochemical studies indicating HPP
| Test | Patient (sib no. 1) | Father | Mother | Sister (sib no. 2) | Brother (sib no. 3) | Normal rangea | ||
|---|---|---|---|---|---|---|---|---|
| Age (yr)b | 3 | 29 | 28 | 4 | 2.5 | 6 | Pediatric | Adult |
| Serum | ||||||||
| ALP (IU/liter) | 47 | 55 | 32 | 308 | 36 | 63 | 133–347 | 30–114 |
| BALP (IU/liter)c | 29 | 21 | 11 | 144 | 30 | 36 | 47–181 | 3–38 |
| Plasma | ||||||||
| PLP (nmol/liter) | 3505 | 191 | 258 | 117 | 1332 | 4034 | 5–107 | 5–107 |
Normal ranges (±2 sd mean) were constructed from data from fasting blood specimens obtained from 20 children ages 4.6–12.9 yr with ad libitum diets and assayed at Shriners Hospital for Children, in 1997. The range for plasma PLP concentration is appropriate for both children and adults (18).
Age when samples for biochemical studies were obtained.
Metra Biosystems, Inc., Mountain View, CA.
At birth, sib no. 1 had serum total ALP activity (measured at the University of Utah) that was remarkably low (i.e. <20 IU/liter). His serum ALP (measured at the Shriners Hospital for Children) was higher at 47 IU/liter at 3 yr of age with a BALP of 29 IU/liter (normal 47–181). At that time his plasma PLP concentration was markedly elevated at 3505 nmol/liter (normal 5–107) (Table 1).
Sib no. 1’s older affected brother (sib no. 3) had a serum total ALP activity assayed at the University of Utah that was low at 33 IU/liter at 17 months of age. At 2.5 yr of age, his serum total ALP and BALP activity (measured at the Shriners Hospital for Children) was low at 36 and 30 IU/liter, respectively. At 6 yr of age, his serum ALP and BALP activity was 63 and 36 IU/liter, respectively. His plasma PLP concentration was markedly elevated at 1332 nmol/liter at 2.5 yr of age and 4034 nmol/liter at 6 yr of age (Table 1).
Sib no. 1’s unaffected twin sister (sib no. 2) had normal serum total ALP activity soon after birth (145 IU/liter). At 4 yr of age, serum total ALP activity of 308 IU/liter and BALP of 144 IU/liter were normal, and plasma PLP concentration of 117 nmol/liter was minimally elevated (Table 1).
The mother’s serum total ALP and BALP activity were normal (32 and 11 IU/liter, respectively). The father’s serum total ALP and BALP activity were normal (55 and 21 IU/liter, respectively). However, both parents had elevated plasma PLP levels (mother = 258 nmol/liter; father = 191 nmol/liter) (Table 1) (4).
TNSALP gene analysis
All coding exons and adjacent mRNA splice sites of TNSALP were sequenced for both affected brothers. Both boys were compound heterozygotes for loss-of-function TNSALP defects, revealing missense mutations in both exons 6 and 8 [exon 6 (c.526G>A, p.Ala176Thr); exon 8 (c.814C>T, p.Arg272Cys)]. Therefore, autosomal recessive inheritance accounted for their HPP. The mother carried the exon 8 mutation (c.814C>T, p.Arg272Cys), which is rare; it has not been detected in a cohort of approximately 150 HPP probands (unpublished data), but it has been reported with another TNSALP mutation once in an individual described as having perinatal HPP (24). The father carried the exon 6 mutation (c.526G>A, p.Ala176Thr), which is very common in a HPP patient cohort (unpublished data), and has been reported elsewhere (25).
The apparently healthy twin sister had neither TNSALP mutation.
Discussion
Sib no. 1 had prenatal manifestations of HPP with long bone deformity, yet there was spontaneous prenatal and postnatal improvement. His biochemical markers of HPP, which included hypophosphatasemia (low serum ALP activity) and an elevated plasma PLP level (4), were entirely consistent with HPP. In general, the magnitude of these biochemical disturbances correlates with the clinical severity of HPP (4). Soon after birth, his serum ALP activity was remarkably low (i.e. <20 IU/liter), falling within the range seen with perinatal HPP, but at 3 yr of age, the value was 47 IU/liter and seemed more in keeping with childhood HPP. The explanation for his apparent increase in serum total ALP activity measured in separate laboratories is unknown but was of interest because it accompanied his clinical and radiographic improvement. Nevertheless, at 3 yr of age, his plasma PLP concentration (3505 nm) was consistent with levels encountered in the perinatal and infantile forms of HPP (4).
The biochemical markers of HPP quantitated in sib no. 1’s family members generally reflected what was revealed by TNSALP mutation analysis. The clinically unaffected twin (sib no. 2) had normal serum total ALP and BALP activities and minimally elevated plasma PLP levels and harbored no TNSALP mutation. The mother’s serum total ALP activity of 32 IU/liter is at the bottom of the normal range, and lies between values that have been encountered in adult controls and those with adult HPP. The father’s serum ALP is within the range of adult controls (4). Both parents had elevated plasma PLP levels. The mother had taken just one prenatal vitamin tablet 2 wk before the PLP measurement. Therefore, this was unlikely to have increased her plasma PLP level. These biochemical findings are consistent with both parents carrying a TNSALP mutation (Table 1). Sib no. 1’s older affected brother’s serum total ALP activity was also low at 33 IU/liter at 17 months of age, and was higher at 63 IU/liter at 6 yr of age, and now within the range encountered in childhood HPP (4). At 6 yr of age, the older brother also had a distinctly elevated plasma PLP concentration (4034 nm), in keeping with levels observed in perinatal and infantile HPP. However, differences in the clinical and biochemical findings between sib nos. 1 and 3 occurred despite the fact that both were compound heterozygotes for the same two missense mutations within TNSALP.
Interfamilial variability of HPP is wide ranging, and largely explained by the patterns of inheritance and the variety of causal TNSALP mutations (4). Yet, most publications describe little intrafamilial variability for HPP and indicate that affected sibs are phenotypically similar. Occasionally, unexplained variations have been noted among affected sibs (26,27,28,29). There are three published reports of sibs with HPP in which one died perinatally, and the other had HPP with later onset (27,28,29). Of interest, for two of these reports, the more severely affected case was one of twins, with the other twin being unaffected (27,28). The third such case presented with oligohydramnios (29). Although in the family reported herein the brother diagnosed prenatally (sib no. 1) did not have a poor postnatal outcome, he seemed more severely affected than his older brother (sib no. 3), and was also a twin to an unaffected sister (sib no. 2). Perhaps twin gestation can modify HPP expression in utero, but how an unaffected twin could cause more severe HPP during gestation is an enigma. It may be that fetal crowding, either from twin gestation or in other cases from oligohydramnios, could be an explanation. However, upon serial sonography a force acting on the upper-right side of sib no. 1, where the majority of his deformities were located, could not readily be identified. In addition, oligohydramnios was not present.
Sib no. 1’s clinical course resembled what has been detailed in a few cases previously with skeletal manifestations of HPP detected in utero by sonography yet with spontaneous postnatal improvement (14,15,16,17,18,19). Several of these cases inherited HPP due to an autosomal dominant transmission pattern. The genetic etiology explored by TNSALP mutation analysis in the cases with autosomal dominant transmission patterns has been reported for two cases: the child described from the second family of Moore et al. (15) and the child reported by Comstock et al. (16). The child reported by Moore et al. (15) had a c.1133A>T, p.Asp378Val mutation, and the child reported by Comstock et al. (16) had a c.1144G>A, p.Val382Ile sequence variant. The autosomal dominant transmission patterns suggest possible genetic heterogeneity. However, as described before, two of the four previously reported families were found to have heterozygous mutations in the TNSALP gene (15,16), suggesting that a heterozygous mutation in the TNSALP gene alone or in combination with other genetic or environmental modifiers could result in the described phenotype. In addition, several Japanese cases, which were compound heterozygotes, share an identical c.979T>C, p.Phe327Leu mutation in the TNSALP gene (17,18,19). Nevertheless, the two brothers with HPP (sib nos. 1 and 3) inherited HPP as an autosomal recessive trait, as shown by TNSALP mutation analysis, but without the p.Phe327Leu mutation. The fact that both parents appeared clinically unaffected by HPP gives further evidence to an autosomal recessive trait for in utero long bone deformities with spontaneous postnatal improvement in HPP. Notably, sib no. 1 displayed prenatal improvement.
Sib no. 1 represents a detailed report of an autosomal recessive case of this relatively benign clinical form of HPP. He carries two mutations of TNSALP (without a p.Phe327Leu mutation) manifesting in utero with deformity of long bones, yet with spontaneous prenatal and postnatal improvement. Thus, accumulating experience shows that prenatal or perinatal skeletal manifestations of HPP, even with autosomal recessive inheritance, are not uniformly lethal and can represent the variable expressivity or the effects of modifiers on the underlying TNSALP allelic defect(s).
Acknowledgments
We thank the study family for their participation. Dr. Xiafang Zhang (Washington University School of Medicine, St. Louis, MO) performed the tissue nonspecific alkaline phosphatase gene analyses. Angelia English (Shriners Hospital for Children, St. Louis, MO) provided expert secretarial help. Dr. Aaron Stevenson contributed an overview of radiographs and technical support. We thank Heather Hanson and Bronte Clifford from the University of Utah Clinical Genetics Research Program for helping collect samples and coordinating clinical evaluations.
Footnotes
Address reprint requests to: Michael P. Whyte, M.D., Shriners Hospital For Children, 2001 South Lindbergh Boulevard, St. Louis, Missouri 63131. E-mail: Mwhyte@shrinenet.org.
This work was supported in part by Grant no. M01-RR00064 from the National Center for Research Resources, research Grant no. K23 NS052500 from the National Institute of Neurological Disorders and Stroke, the Children’s Health Research Center and Clinical Genetics Research Program at the University of Utah, Shriners Hospitals for Children, The Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund, The Hypophosphatasia Research Fund, and The Barnes-Jewish Hospital Foundation.
Presented in part at The 2006 David W. Smith Workshop on Malformations and Morphogenesis (Stevenson et al. Proc Greenwood Genet Center 2007;26:153–154 abstract), and the 30th Annual Meeting of the American Society for Bone and Mineral Research, Montreal, Canada, September 12–16, 2008 (J Bone Miner Res, in press).
Disclosure Statement: Dr. Whyte is a consultant for Enobia Pharma, Montreal, Canada.
First Published Online June 17, 2008
Abbreviations: ALP, Alkaline phosphatase; BALP, bone-specific alkaline phosphatase; HPP, hypophosphatasia; PLP, pyridoxal-5′-phosphate; TNSALP, tissue nonspecific alkaline phosphatase.
References
- Smith M, Weiss MJ, Griffin CA, Murray JC, Buetow KH, Emanuel BS, Henthorn PS, Harris H 1988 Regional assignment of the gene for human liver/bone/kidney alkaline phosphatase to chromosome 1p36.1-p34. Genomics 2:139–143 [DOI] [PubMed] [Google Scholar]
- Greenberg CR, Evans JA, McKendry-Smith S, Redekopp S, Haworth JC, Mulivor R, Chodirker BN 1990 Infantile hypophosphatasia: localization within chromosome region 1p36.1–34 and prenatal diagnosis using linked DNA markers. Am J Hum Genet 46:286–292 [PMC free article] [PubMed] [Google Scholar]
- Whyte MP 1994 Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev 15:439–461 [DOI] [PubMed] [Google Scholar]
- Whyte MP 2001 Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 5313–5329 [Google Scholar]
- McCance RA, Morrison AB, Dent CE 1955 The excretion of phosphoethanolamine and hypophosphatasia. Lancet 268:131 [DOI] [PubMed] [Google Scholar]
- Whyte MP, Mahuren JD, Vrabel LA, Coburn SP 1985 Markedly increased circulating pyridoxal-5′-phosphate levels in hypophosphatasia. Alkaline phosphatase acts in vitamin B6 metabolism. J Clin Invest 76:752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn PS, Raducha M, Feddle KN, Lafferty MA, Whyte MP 1992 Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci USA 89:9924–9928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurutuza L, Muller F, Gibrat JF, Taillandier A, Simon-Buoy B, Serre JL, Mornet E 1999 Correlations of genotype and phenotype in hypophosphatasia. Hum Mol Genet 8:1039–1046 [DOI] [PubMed] [Google Scholar]
- Millan JL 2006 Mammalian alkaline phosphatases: from biology to applications in medicine and biotechnology. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Fraser D 1957 Hypophosphatasia. Am J Med 22:730–746 [DOI] [PubMed] [Google Scholar]
- Fallon MD, Teitelbaum SL, Weinstein RS, Goldfischer S, Brown DM, Whyte MP 1984 Hypophosphatasia: clinicopathologic comparison of the infantile, childhood, and adult forms. Medicine (Baltimore) 63:12–24 [PubMed] [Google Scholar]
- Silver MM, Vilos GA, Milne KJ 1988 Pulmonary hypoplasia in neonatal hypophosphatasia. Pediatr Pathol 8:483–493 [DOI] [PubMed] [Google Scholar]
- Gehring B, Mornet E, Plath H, Hansmann M, Bartmann P, Brenner RE 1999 Perinatal hypophosphatasia: diagnosis and detection of heterozygote carries within the family. Clin Genet 56:313–317 [DOI] [PubMed] [Google Scholar]
- Pauli RM, Modaff P, Sipes SL, Whyte MP 1999 Mild hypophosphatasia mimicking severe osteogenesis imperfecta in utero: bent but not broken. Am J Med Genet 86:434–438 [DOI] [PubMed] [Google Scholar]
- Moore CA, Curry CJ, Henthorn PS, Smith JA, Smith JC, O'Lague P, Coburn SP, Weaver DD, Whyte MP 1999 Mild autosomal dominant hypophosphatasia: in utero presentation in two families. Am J Med Genet 86:410–415 [DOI] [PubMed] [Google Scholar]
- Comstock C, Bronsteen R, Lee W, Vettraino I 2005 Mild hypophosphatasia in utero: bent bones in a family with dental disease. J Ultrasound Med 24:707–709 [DOI] [PubMed] [Google Scholar]
- Michigami T, Uchihashi T, Suzuki A, Tachikawa K, Nakajima S, Ozono K 2005 Common mutations F310L and T1559del in the tissue-nonspecific alkaline phosphatase gene are related to distinct phenotypes in Japanese patients with hypophosphatasia. Eur J Pediatr 164:277–282 [DOI] [PubMed] [Google Scholar]
- Cai G, Michigami T, Yamamoto T, Yasui N, Satomura K, Yamagata M, Shima M, Nakajima S, Mushiake S, Okada S, Ozono K 1998 Analysis of localization of mutated tissue-nonspecific alkaline phosphatase proteins associated with neonatal hypophosphatasia using green fluorescent protein chimeras. J Clin Endocrinol Metab 83:3936–3942 [DOI] [PubMed] [Google Scholar]
- Ozono K, Yamagata M, Michigami T, Nakajima S, Sakai N, Cai G, Satomura K, Yasui N, Okada S, Nakayama M 1996 Identification of novel missense mutations (Phe310Leu and Gly439Arg) in a neonatal case of hypophosphatasia. J Clin Endocrinol Metab 81:4458–4461 [DOI] [PubMed] [Google Scholar]
- Mumm S, Jones J, Finnegan P, Henthorn PS, Podgornik MN, Whyte MP 2002 Denaturing gradient gel electrophoresis analysis of the tissue nonspecific alkaline phosphatase isoenzyme gene in hypophosphatasia. Mol Genet Metab 75:143–153 [DOI] [PubMed] [Google Scholar]
- Whyte MP, Kurtzberg J, McAlister WH, Mumm S, Podgornik MN, Coburn SP, Ryan LM, Miller CR, Gottesman GS, Smith AK, Douville J, Waters-Pick B, Armstrong RD, Martin PL 2003 Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res 18:624–636 [DOI] [PubMed] [Google Scholar]
- Coburn SP, Mahuren JD 1983 A versatile cation-exchange procedure of measuring the seven major forms of vitamin B6 in biological samples. Anal Biochem 129:310–317 [DOI] [PubMed] [Google Scholar]
- Ericson KL, Mahuren JD, Zubovic YM, Coburn SP 2005 Use of chlorite to improve HPLC detection of pyridoxal 5′-phosphate. J Chromatogr B Analyt Technol Biomed Life Sci 823:218–220 [DOI] [PubMed] [Google Scholar]
- Spentchian M, Brun-Heath I, Taillandier A, Fauvert D, Serre JL, Simon-Bouy B, Carvalho F, Grochova I, Mehta SG, Müller G, Oberstein SL, Ogur G, Sharif S, Mornet E 2006 Characterization of missense mutations and large deletions in the ALPL gene by sequencing and quantitative multiplex PCR of short fragments. Genet Test 10:252–257 [DOI] [PubMed] [Google Scholar]
- Taillandier A, Cozien E, Muller F, Merrien Y, Bonnin E, Fribourg C, Simon-Bouy B, Serre JL, Bieth E, Brenner R, Cordier MP, De Bie S, Fellmann F, Freisinger P, Hesse V, Hennekam RC, Josifova D, Kerzin-Storrar L, Leporrier N, Zabot MT, Mornet E 2000 Fifteen new mutations (-195C>T, L-12X, 298–2A>G, T117N, A159T, R229S, 997+2T>A, E274X, A331T, H364R, D389G, 1256delC, R433H, N461I, C472S) in the tissue-nonspecific alkaline phosphatase (TNSALP) gene in patients with hypophosphatasia. Hum Mutat 15:293 [DOI] [PubMed] [Google Scholar]
- Whyte MP, Essmyer K, Geimer M, Mumm S 2006 Homozygosity for TNSALP mutation 1348C>T (Arg433Cys) causes infantile hypophosphatasia manifesting transient disease correction and variably lethal outcome in a kindred of black ancestry. J Pediatr 148:753–758 [DOI] [PubMed] [Google Scholar]
- Macfarlane JD, Kroon HM, van der Harten JJ 1992 Phenotypically dissimilar hypophosphatasia in two sibships. Am J Med Genet 42:117–121 [DOI] [PubMed] [Google Scholar]
- Macpherson RI, Kroeker M, Houston CS 1972 Hypophosphatasia. J Can Assoc Radiol 23:16–26 [PubMed] [Google Scholar]
- Wladimiroff JW, Niermeijer MF, Van der Harten JJ, Stewart PA, Versteegh FG, Blom W, Huijmans JG 1985 Early prenatal diagnosis of congenital hypophosphatasia: case report. Prenat Diagn 5:47–52 [DOI] [PubMed] [Google Scholar]