Abstract
Context: Epidemiological data suggest a genetic susceptibility to thyroid antibody (TAb) production.
Objective: The objective of the study was to identify genetic loci that are linked with TAb production.
Design: The design of the study was a whole genome linkage study in families with clustering of thyroid autoimmunity.
Settings: The study took place at an academic medical center.
Participants: Participants included 102 multigenerational families (540 individuals) multiplex for autoimmune thyroid disease (AITD) and TAb production.
Main Outcome Measures: We computed two-point logarithm of odds (LOD) scores and multipoint heterogeneity LOD scores for 400 microsatellite markers spanning the entire human genome at an average distance of 10 cm (∼10 Mb).
Results: Three loci showed evidence for linkage with TAb production: 1) 2q locus, which gave a maximum multipoint heterogeneity LOD score (HLOD) of 2.8 and contained the CTLA-4 gene, previously reported to be linked and associated with clinical AITD; (2) 6p locus (HLOD 2.5), which was the same AITD-1 locus found to be linked with clinical AITD; and (3) 8q locus (HLOD 2.2), which contained the thyroglobulin gene, also previously reported to be linked and associated with AITD. All loci that were linked to TAb were also linked to AITD, suggesting that TAb and AITD share the same genetic predisposition.
Conclusions: We conclude that: 1) some of the genes/loci predisposing to TAb and AITD are shared, whereas distinct genes/loci also exist; (2) the presence of TAb in relatives of AITD patients may be associated with increased risk for the development of clinical AITD; and (3) further studies are needed to determine the predictive value of TAb levels for the development of clinical AITD in relatives of patients with familial AITD.
A whole genome linkage study in 102 multiplex families for thyroid autoimmunity finds three loci associated with thyroid antibody production, on 2q (CTLA-4), 6p (AITD-1), and 8q (thyroglobulin). These loci are also linked to clinical autoimmune thyroid disease (AITD), suggesting that some of the loci/genes predisposing to TAb are shared with AITD. Thus, TAb production is a risk factor for clinical AITD.
The autoimmune thyroid diseases (AITD) include two distinct but related disorders, Graves’ disease (GD) and Hashimoto’s thyroiditis (HT). In both diseases thyroid-reactive T cells are formed and infiltrate the thyroid gland. In GD, thyroid-infiltrating T cells activate B cells to produce TSH receptor antibodies, which stimulate the thyroid and cause clinical hyperthyroidism (reviewed in Ref. 1). In contrast HT is characterized by Th1 switching of the thyroid infiltrating T cells, which induce apoptosis of thyroid follicular cells and clinical hypothyroidism (reviewed in Ref. 2). Both disorders are common with a prevalence in the United States of approximately 1% (3,4). One of the hallmarks of AITD is the production of thyroid autoantibodies (TAb), comprising antibodies to thyroglobulin (Tg) and thyroid peroxidase (TPO; the microsomal antigen). The production of TAb often precedes the development of clinical AITD, and TAb have been widely used to show the population most at risk for the development of AITD. For example, in females who were positive for TAb and who had abnormal TSH, the annual risk of developing hypothyroidism was 2–4% (5). However, it is still unclear whether the etiology of TAb production is identical with the etiology of clinical AITD, and some patients develop TAb without progressing to clinical AITD.
The etiology of the AITD is strongly influenced by genetic factors (6). Indeed, there is solid epidemiologic evidence for a genetic susceptibility to AITD including family studies demonstrating familial clustering of AITD (7,8), giving a sibling risk ratio of 16.9 (9) and a significantly higher concordance rate in monozygotic twins when compared with dizygotic twins (10,11). Genetic susceptibility to the production of thyroid antibodies was first suggested by Hall and Stanbury (12). Their studies of first-degree relatives of probands with AITD indicated proportions of affected relatives similar to the theoretical expectation for dominant inheritance (13). More recent family studies have shown a strong familial component in TAb susceptibility with up to 50% of the siblings of AITD patients being TAb positive (8,14,15,16,17), far higher than the TAb population prevalence of 6–11% (4,18). Moreover, segregation analyses in families with TAb have shown vertical transmission of TAb, suggesting a dominant inheritance component to TAb transmission (19,20,21). Recently, Jaume et al. (22) found evidence for the genetic transmission of TPO antibody fingerprints, suggesting that autoantibody recognition of the TPO antigen was genetically transmitted. However, it is not known whether the TAb susceptibility genes are distinct from those predisposing to clinical AITD or whether the genetic susceptibility to TAb and AITD is caused by the same genes. If the latter is true, then TAb represents a genetic marker for AITD predisposition. Most studies have focused on the genetics of clinical AITD (i.e. GD and HT) (reviewed in Ref. 23). Whereas we previously reported the identification of a major susceptibility locus for TAb at the CTLA-4 gene region on chromosome 2q33, using a cohort of 56 AITD families (24), we have now nearly doubled the data set to 102 multiplex multigenerational families (540 individuals) and performed a complete genome scan for TAb production using this expanded data set.
Subjects and Methods
Ascertainment of the study families
The project was approved by the institutional review board. One hundred two families (540 individuals) were analyzed in the current study. These families have been previously described in detail (25). All families were multiplex for AITD and TAb (more than one affected) and/or multigenerational. Families were ascertained through a patient with AITD, who confirmed having at least one other first-degree relative with AITD. On the average, our families had 5.3 members.
Clinical assessment
GD was diagnosed by the following: 1) documented clinical and biochemical hyperthyroidism requiring treatment, 2) a diffuse goiter, 3) presence of TSH receptor antibodies, and/or (4) diffusely increase I-131 uptake in the thyroid gland. Hashimoto’s thyroiditis was diagnosed by documented clinical and biochemical hypothyroidism requiring thyroid hormone replacement and presence of autoantibodies to TPO and Tg. The TAb phenotype used in all analyses (see below) included, in addition to family members with clinical AITD, any relatives that had TPO and/or Tg antibodies above the cutoff for positivity in our assay.
Measurement of TAb levels
Anti-Tg and anti-TPO antibodies were measured by specific RIA (Kronus, Boise, ID). The sensitivity of the assay, defined as the lowest nonzero calibrator, is 0.3 Kronus U/ml for Tg antibody (equal to 3.0 World Health Organization IU/ml), and 0.3 Kronus U/ml for TPO antibody (equal to 1.5 World Health Organization IU/ml). Therefore, any individual with Tg and/or TPO antibody levels of 0.3 Kronus U/ml or greater was considered affected. For all subjects, phenotype was determined with the clinician blinded to the individual’s genotype.
Affectedness status
Individuals were considered affected if they had either clinical AITD (i.e. either GD or HT) and/or positive TAb. Family members were considered unaffected in the analysis if they had no clinical AITD and negative TAb tests. Individuals who did not have TAb levels measured were considered unknown in the analysis.
Microsatellite marker genotyping
DNA was extracted from whole blood using the Puregene kit (Gentra Systems, Minneapolis, MN). We used the existing genotypes generated in our whole genome linkage studies in clinical AITD (25). The genotyping was performed using the Applied Biosystems (Foster City, CA) microsatellite panels (version 2.0, a total of 400 markers), as previously reported (26). Briefly, PCRs were performed in a 15:ul reaction volumes containing 50 ng of genomic DNA; 5 pmol of each primer (one of which was fluorescent labeled); PCR buffer containing 50 mm KCl, and 10 mm Tris HCl (pH 8.3); 1.5 mm MgCl2; 200 μm of each dATP, dGTP, dTTP, dCTP; and 1 U of AmpliTaq DNA polymerase (PerkinElmer, Applied Biosystems). Reaction mixtures were heated to 94 C for 7 min and then cycled 30 times as follows: 30 sec at 94 C, 30 sec at 55 C, and 30 sec at 72 C. The PCR products were diluted 1:20 in double-distilled H2O and pooled. Two microliters of the pooled products were mixed with 0.5 μl of the internal size standard and 10 μl of deionized formamide, denatured, and separated using an ABI 310 genetic analyzer (Applied Biosystems, Foster City, CA). Allele calling was performed using the Genotyper 2.0 software (Applied Biosystems). The marker data were then automatically exported to our database (Oracle database) in which they were integrated with the already existing phenotype information and prepared for linkage analysis.
Logarithm of odds (LOD) score analysis
Linkage analysis was performed using maximum likelihood-based (LOD score) methods of linkage analysis.
Two-point linkage analysis
Two-point LOD scores for the different markers studied were computed using LIPED software (27) assuming both dominant and recessive models. Population data and our own previous analyses suggest that a penetrance of 30% is appropriate (11,26), but the actual value of the assumed penetrance does not have a major effect on the final LOD score (28,29). Based on the guidelines suggested by Lander and Kruglyak (30), we considered a LOD score of 1.9 or greater (P ≤ 1.7 × 10−3) as suggestive evidence for linkage and a LOD score of 3.3 or greater (P ≤ 4.9 × 10−5) as evidence for significant linkage, after maximizing the LOD score with respect to dominant and recessive models (31). If a LOD score was suggestive of linkage, we then tested at a higher penetrance (80%) because our families were ascertained for multiple affected members and therefore were likely to have a higher penetrance of disease than in the general population. Thus, we were not simultaneously estimating evidence for linkage and the penetrance (Table 1).
Table 1.
Characteristics of the study data set
| Number | Percent | |
|---|---|---|
| Families | 102 | 100 |
| GD families | 34 | 33 |
| HT families | 30 | 30 |
| Mixed families (GD+HT) | 38 | 37 |
| Individuals | 540 | 100 |
| Affected Individuals | 250 | 46.3 (of total individuals) |
| Females | 208 | 83 (of affected) |
| Males | 42 | 17 (of affected) |
| Healthy relatives with TAb | 78 | 35 (of healthy relatives with TAb measured) |
| Females | 47 | 60 (of TAb+) |
| Males | 31 | 40 (of TAb+) |
| Healthy relatives with no TAb | 145 | 65 (of healthy relatives with TAb measured) |
| Females | 61 | 42 (of unaffected) |
| Males | 84 | 58 (of unaffected) |
| Healthy relatives, TAb not measured | 67 | 23 (of healthy relatives) |
| Females | 30 | 45 (of relatives with TAb not measured) |
| Males | 37 | 55 (of relatives with TAb not measured) |
Multipoint linkage analysis
We did a multipoint linkage analysis, assuming homogeneity and heterogeneity, for the six chromosomes that showed evidence for linkage on the two-point LOD score analysis (chromosomes 2, 6, 7, 8, 13, 15). The marker positions on chromosomes and their distances were obtained from Genethon chromosomal genetic maps (http://www.genethon.fr). The order of the markers and recombination fractions were verified in our data set. Multipoint LOD scores assuming homogeneity and heterogeneity LOD scores (HLODs) were computed by the GeneHunter program (32) using all the markers on each chromosome.
Results
Characteristics of the study sample
Table 1 shows the clinical characteristics of the 102 families studied. Of the 540 family members, 250 (46.3%) had clinical AITD (i.e. GD or HT) and 290 were clinically and biochemically euthyroid. Of the 290 euthyroid relatives, Tg and TPO antibodies were measured in 223 (77%). Of the 223 relatives in whom TAb were measured, 78 (35%) had positive Tg and/or TPO antibodies, similar to the prevalence reported in previous studies (8,33). These TAb-positive individuals were considered affected in our LOD score analysis in addition to the family members who had clinical AITD.
Whole genome screening for TAb-specific genes
Two-point LOD score analysis
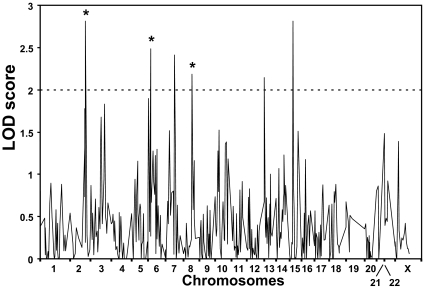
For this analysis, individuals with positive TAb were considered affected whether or not they had clinical AITD. Figure 1 shows the results of whole genome screening for TAb loci. Six loci on chromosomes 2q, 6p, 7q, 8q, 13q, and 15q showed evidence for linkage with (two point) LOD scores greater than 2.0. However, the LOD score peaks at the 7q, 13q, and 15q loci were narrow, essentially consisting of one positive marker, suggesting that they were spurious peaks (on multipoint these genomic areas did not support linkage; see below). The 2q, 6p, and 8q loci had broad peaks of positive LOD scores at multiple marker loci, suggesting consistent linkage evidence. At the 2q locus, the maximum two-point LOD score was 3.0 for marker D2S325 (208.5 cm), assuming a dominant model with 80% penetrance (Fig. 1 and Table 2). This locus contained the CTLA-4 gene, previously reported to be linked and associated with clinical AITD (34,35,36,37). At the 6p locus, the maximum two-point LOD score was 2.5 for marker D6S276 (36.6 cm), assuming a dominant model with 30% penetrance (Fig. 1 and Table 2). This locus was the same AITD-1 locus found to be linked with clinical AITD (25). This locus is located about 8 Mb telomeric to the major histocompatibility complex (MHC) class II locus. The maximum two-point LOD score at the 8q locus was 2.4 for marker D8S1784 (110.9 cm), assuming a recessive model of inheritance and 30% penetrance (Fig. 1 and Table 2). This locus was close to the Tg gene, also previously reported to be linked and associated with AITD (38,39,40,41,42). In summary, all three TAb loci are identical with the loci we and others have found to be linked with clinical AITD.
Figure 1.
Whole genome analysis for loci linked with both GD and HT (AITD). The x-axis shows the relative marker positions on each chromosome, and the y-axis shows the LOD score obtained for each marker on every chromosome. Six loci on chromosomes 2q, 6p, 7q, 8q, 13q, and 15q showed evidence for linkage with LOD scores greater than 2.0. However, the LOD score peaks at the 7q, 13q, and 15q loci were narrow, and on multipoint analysis gave nonsignificant LOD scores (Table 2), suggesting that they were false-positive peaks. In contrast, the 2q, 6p, and 8q loci (marked by asterisk) consisted of broad peaks of positive LOD scores and remained significant in the multipoint analysis (Table 2) and therefore are likely to harbor TAb susceptibility genes.
Table 2.
Two-point and multipoint LOD scores at loci showing two-point LOD scores greater than 2
| Marker name | Chromosomal location | 2-point LOD* | Mode of inheritance | Penetrance, % | a | Multipoint HLOD* | b |
|---|---|---|---|---|---|---|---|
| D2S325 | 2q | 3 | Dominant | 80 | 0.1 | 2.8 | 0.23 |
| D6S276 | 6p | 2.5 | Dominant | 30 | 0.2 | 2.0 | 0.28 |
| D7S515 | 7q | 2.4 | Recessive | 80 | 0.2 | 0.1 | 0.3 |
| D8S1784 | 8q | 2.4 | Recessive | 30 | 0.2 | 2.8 | 0.40 |
| D13S217 | 13q | 2.1 | Dominant | 30 | 0.2 | 0.3 | 0.1 |
| D15S978 | 15q | 2.8 | Dominant | 30 | 0.2 | 0 | 0 |
, Recombination fraction;
, a statistic that estimates the proportion of linked families. Significant HLOD scores are shown in bold.
Multipoint analysis
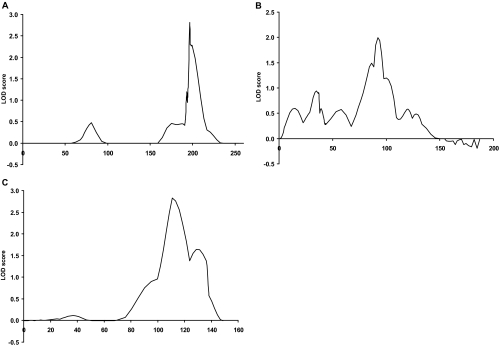
Multipoint analyses using Genehunter was performed for the six loci showing two-point LOD scores greater than 2.0, on chromosomes 2, 6, 7, 8, 13, and 15. (Table 2 and Fig. 2). Assuming linkage homogeneity, the maximum multipoint LOD scores were negative at all six loci. In addition, the 7q, 13q, and 15q loci showed no evidence of linkage when also assuming heterogeneity (Table 2). In contrast, the 2q, 6p, and 8q loci showed positive evidence for linkage on multipoint analysis, with HLOD scores of 2.0 or greater. The maximum HLOD were 2.8 for the 2q locus, 2.0 for the 6p locus, and 2.8 for the 8q locus (Fig. 2 and Table 2). Thus, whereas we cannot test whether there is statistically significant evidence for heterogeneity in the multipoint analysis, the fact that the homogeneity multipoint scores were negative, whereas the HLODs at all these loci were strongly positive, suggests the existence of heterogeneity at these linked loci, i.e. that subsets of our families were linked with TAb at each of these loci, as we have previously shown for AITD (43). Indeed, the subset analysis supported the notion that heterogeneity existed in our data set.
Figure 2.
Multipoint analysis for chromosomes 2 (A), 6 (B), and 8 (C). The x-axis shows the relative marker position in centimorgans, and the y-axis shows the multipoint HLOD score. The maximum multipoint HLOD scores were 2.8 for the 2q locus, 2.0 for the 6p locus, and 2.8 for the 8q locus.
Resolving heterogeneity by subset analysis
LOD score analysis in subsets
Our multipoint analysis suggested heterogeneity at all three loci that were linked with TAb. This implied that certain subsets of our data set were linked with each specific locus. Subset analysis is one of the most powerful tools for resolving heterogeneity, as we have previously shown for AITD (43). We hypothesized that the three loci were specific for certain subsets of our families and that susceptibility would be caused by only one of the three genes in a given subset. The problem is: how do we determine the subsets? We did the following: 1) analyzed all the families for each of the three loci; 2) for each locus, we excluded the families that showed positive evidence at that locus; 3) we then reanalyzed the remaining families (the ones not linked to the originally chosen locus) at each of the other two loci. Thus, the families were grouped according to which families gave negative LOD scores for a given locus, and then those families were tested at a different locus. Using this analysis, no subset of families was identified that was linked with the CTLA-4 locus on chromosome 2q. However, the chromosome 6p locus showed a marked increase in the two-point LOD score, from 2.5 to 4.2, when analyzed for the subset of families that were not linked with 8q (Table 3). Moreover, the reverse analysis showed a marked increase in the two-point LOD score at 8q, from 2.4 to 3.3, when analyzed for linkage in the subset of families that were not linked with the 6p locus (Table 3). This suggested that families that are linked with 8q are not linked with 6p and that these two loci represent independent influences on TAb susceptibility.
Table 3.
Comparison of the maximum two-point LOD scores obtained for subsets of the data set using the PST
| Locus | Marker | Subset | Zmax1 | Zmax2 | ZmaxT | χ2 | P value |
|---|---|---|---|---|---|---|---|
| 6p | D6S276 | Families not linked with 8q | 4.2 | 0 | 2.5 | 7.8 | 5.1×10−3 |
| 8q | D8S1784 | Families not linked with 6p | 3.3 | 0.23 | 2.4 | 5.2 | 0.02 |
Zmax1, Maximum LOD score obtained for the subset; Zmax2, maximum LOD score obtained for the inverse subset (ie the remainder of the families not included in the specified subset); ZmaxT, maximum LOD score obtained in the entire data set of families.
Predivided-sample test (PST)
To examine whether the increase in the LOD scores at the 6p and 8q loci was statistically significant, we applied the PST (43,44,45,46). In this test the data set is divided into different groups based on predefined clinical, demographic, or other criteria. The criterion we used for dividing the sample was the presence of negative LOD scores at locus A; we then tested that group for being homogenously linked at locus B. This approach is valid because the two loci are physically unrelated to each other because they are on different chromosomes. The LOD scores obtained in each subset were compared with the LOD scores obtained in its inverse subset (i.e. the remainder of the families not included in the subset). Statistically significant differences in the LOD scores between the subset and the inverse subset suggest that the two groups are genetically different from one another at the tested locus (44,45). Applying the PST demonstrated that the increase in the LOD score at the 6p and 8q loci in the subsets of families we tested were statistically significant (Table 3).
Comparison of the LOD scores obtained for TAb and AITD
Our previous whole genome scan identified three loci that were linked with clinical AITD, on 6p, Tg, and 10q (25). Therefore, we compared the maximum LOD scores (MLS) obtained for AITD with those obtained for TAb (Table 4). The MLS at 10q was markedly higher for AITD than for TAb, suggesting that this is an AITD-specific locus. In contrast, the MLS at the CTLA-4 was markedly higher for TAb than for AITD, suggesting that, in our data set, CTLA-4 contributed mostly to the production of TAb (Table 4). The Tg and 6p loci had high MLSs for both AITD and TAb, suggesting that they contributed to both TAb and AITD.
Table 4.
Comparison of the MLSs at loci that were linked with AITD and/or TAb
| Locus | Marker | MLS for AITD | MLS for TAb |
|---|---|---|---|
| 2q | D2S325 | 0.5 | 3.0 |
| 6p | D2S276 | 2.1 | 2.3 |
| 8q | D8S1784 | 3.8 | 2.8 |
| 10q | D10S537 | 3.6 | 0.3 |
Discussion
Autoantibodies to Tg and TPO are present in the majority of patients with clinical AITD and in 30–50% of clinically euthyroid family members (18,47,48) (for a review see Ref. 6). A variety of explanations for the production of TAb have been proposed (reviewed in Ref. 49), including: 1) anti-Tg and anti-TPO are early markers of AITD, which precede and facilitate the development of clinical disease; 2) TAb production is an epiphenomenon secondary to the release of thyroid antigens by thyrocytes undergoing either stimulation or apoptosis induced by the autoimmune response to the thyroid; and 3) anti-TAb represent a separate, but related, phenotype from GD and HT with some overlap. Indeed, previous studies demonstrating a specific genetic predisposition to the production of TAb with a dominant Mendelian pattern of inheritance supported the third hypothesis, namely that the TAb trait has a different genetic susceptibility than the GD/HT trait itself (19,20). In the current study, we dissected the genetic predisposition to the development of TAb by a whole genome linkage study in families in which both clinical AITD and TAb cluster. Our study, supporting both shared and distinct loci for TAb production and clinical AITD, suggests a more complex interaction between the genetic risk for TAb and AITD. On the one hand, some susceptibility genes (e.g. Tg) predispose to both the development of TAb and AITD and contribute to the shared genetic susceptibility. On the other hand, we cannot rule out that genes that are specific for TAb or clinical disease exist. Indeed, our data suggest that the 10q locus is specific for clinical disease (Table 4). Moreover, analysis of our data using the posterior probability of linkage method also suggested the existence of unique TAb genes (50). This notion is further supported by the identification, by us and others, of genes that are specific for certain AITD phenotypes, such as the CD40 gene, which is associated with high antibody level GD (51).
Our study suggests that individuals who come from families that are multiplex for AITD and who have positive TAb are at increased risk for developing clinical AITD. However, it should be emphasized that this conclusion applies only to individuals who are positive for TAb and have relatives with AITD, and it cannot be extrapolated to individuals who are positive for TAb but have no family history of AITD.
Previous studies performed by us and others have suggested that CTLA-4 is a major TAb and AITD susceptibility gene (24,35,37,52). In the present study, the CTLA-4 locus was confirmed as a major locus for TAb. The observation that the LOD score for AITD was low suggests an effect on antibody production. Because CTLA-4 was shown to be linked and associated with all autoimmune thyroiditis phenotypes (GD, HT, and TAb), it must play a role in the etiology of thyroid autoimmunity in general and is not a disease-specific gene (53). Indeed, CTLA-4 has been shown to be associated with many other autoimmune diseases including systemic lupus erythematosus (54), type 1 diabetes (55,56), myasthenia gravis (57), and Addison’s disease (58).
In addition to CTLA-4, two additional TAb loci were identified on 6p (AITD-1) and 8q (the Tg locus). The 8q locus contained the Tg gene, also previously reported to be linked and associated with AITD (25,39,40,41). Even though the LOD score at the Tg locus was lower for TAb than for AITD, it was still significant for TAb (2.8, Table 2). Thus, the Tg gene may be a general autoimmune thyroiditis gene and may not be specific to a specific AITD phenotype. Indeed, there is abundant evidence that Tg plays a key role in the etiology of AITD (49). Immunization with Tg induces autoimmune thyroiditis in experimental animals (59,60,61), and even in spontaneous models of autoimmune thyroiditis, the hallmark of disease is the production of antibodies to Tg (62). The Tg gene may predispose to TAb and AITD in a number of ways (for example: 1) sequence changes in Tg may change its antigenicity making it more immunogenic; 2) sequence changes in Tg may change its interaction with human leucocyte antigen molecules; and 3) sequence changes in or near the Tg gene may alter its expression), and this may lead to reduced immune tolerance to the Tg molecule. Recent data from our laboratory suggested that certain amino acid variants of Tg interact with human leucocyte antigen class II molecules, resulting in a significantly increased risk for AITD (42).
The AITD-1 locus is located about 8 Mb telomeric to the MHC class II locus. However, whereas the peak LOD score for AITD-1 was at marker D6S276, the LOD scores obtained at markers within the MHC class II locus (TNF microsatellite and D6S273) were low positive (1.0 and 0.5, respectively). Therefore, we cannot exclude the possibility that the AITD-1 locus represents the MHC class II genes, which are known to be associated with AITD, albeit the MHC class II region did not show linkage with GD and HT in previous studies in clinical AITD (63). Fine mapping of the AITD-1 locus may demonstrate if it indeed represents the MHC class II region.
Interestingly, the positive LOD scores at all three loci (CTLA-4, AITD-1, and Tg) were obtained assuming heterogeneity. Whereas this does not prove heterogeneity, it does suggest that our data set consists of subsets that are linked with different loci. The method we used to resolve heterogeneity is by subsetting the data set into predefined subgroups and reanalyzing these subgroups separately, as we have successfully done in AITD (43). Indeed, our subset analysis using the PST, whereas not enabling us to identify subsets based on phenotype, strongly suggested that the 6p and 8q loci represent independent influences on TAb susceptibility. These findings support the existence of heterogeneity within the TAb and AITD phenotypes (i.e. different AITD subtypes are influenced by distinct genes) and the fact that the heterogeneity can be resolved by careful analysis.
In summary, our study identified three loci (CTLA-4, Tg, and AITD-1) that conferred susceptibility to both thyroid antibody production and clinical disease, suggesting that at least some of the genetic susceptibility to TAb and AITD is shared. We conclude that the presence of TAb in relatives of AITD patients is a marker of genetic susceptibility to AITD and may be associated with increased risk for the development of clinical AITD. Further studies are needed to determine the predictive value of TAb levels for the development of clinical AITD in relatives of patients with familial AITD.
Acknowledgments
First, we thank Dr. Veronica Vieland for helpful advice and discussions. We also thank all the AITD families who graciously agreed to participate in the study. Family enrollment was achieved through the collaboration of an International Consortium for the Genetics of Autoimmune Thyroid Disease. Members of the consortium included Drs. Luca Chiovato and Aldo Pinchera (Pisa, Italy); Sandra McLachlan (Los Angeles, CA); Bernard Rees Smith (Cardiff, Wales, UK); Fred Clark and Eric Young (Newcastle upon Tyne, UK); Meir Berezin (Tel-Hashomer, Israel); George Carayanniotis (Newfoundland, Canada); and Rhoda Cobin (New York, NY).
Footnotes
This work was supported in part by Grants DK61659, DK067555, and DK073681 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to Y.T.), Grants DK31775, NS27941, and MH48858 from National Institutes of Health (to D.A.G.), and Grant DK052464 from the NIDDK and the David Owen Segal Endowment (to T.F.D.).
Disclosure Statement: Y.B., D.A.G., E.J., E.C., and Y.T. have nothing to declare. T.F.D. received consulting fees from Kronus Inc. and lecture fees from Abbott Labs.
First Published Online June 17, 2008
Abbreviations: AITD, Autoimmune thyroid disease; GD, Graves’ disease; HLOD, heterogeneity LOD score; HT, Hashimoto’s thyroiditis; LOD, logarithm of odds; MHC, major histocompatibility complex; MLS, maximum LOD scores; PST, predivided-sample test; TAb, thyroid antibody; Tg, thyroglobulin; TPO, thyroid peroxidase.
References
- Davies TF 2000 Graves’ diseases: pathogenesis. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. Philadelphia: Lippincott Williams & Wilkins; 518–530 [Google Scholar]
- Weetman AP 2000 Chronic autoimmune thyroiditis. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid. Philadelphia: Lippincott Williams & Wilkins; 721–732 [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM 1997 Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243 [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET 1995 The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Davies TF 2003 Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev 24: 694–717 [DOI] [PubMed] [Google Scholar]
- Mather BA, Roberts DF, Scanlon MF, Mukhtar ED, Davies TF, Smith BR, Hall R 1980 HLA antigens and thyroid autoantibodies in patients with Graves’ disease and their first degree relatives. Clin-Endocrinol (Oxf) 12:155–163 [DOI] [PubMed] [Google Scholar]
- Burek CL, Hoffman WH, Rose NR 1982 The presence of thyroid autoantibodies in children and adolescents with AITD and in their siblings and parents. Clin Immunol Immunopathol 25:395–404 [DOI] [PubMed] [Google Scholar]
- Villanueva R, Greenberg DA, Davies TF, Tomer Y 2003 Sibling recurrence risk in autoimmune thyroid disease. Thyroid 13:761–764 [DOI] [PubMed] [Google Scholar]
- Brix TH, Kyvik KO, Christensen K, Hegedus L 2001 Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab 86:930–934 [DOI] [PubMed] [Google Scholar]
- Brix TH, Kyvik KO, Hegedus L 2000 A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab 85:536–539 [DOI] [PubMed] [Google Scholar]
- Hall R, Stanbury JB 1967 Familial studies of autoimmune thyroiditis. Clin Exp Immunol 2:719–725 [PMC free article] [PubMed] [Google Scholar]
- Hall R, Dingle PR, Roberts DF 1972 Thyroid antibodies: a study of first degree relatives. Clin Genet 3:319–324 [DOI] [PubMed] [Google Scholar]
- Chopra IJ, Solomon DH, Chopra U, Yodhihara E, Tersaki PL, Smith F 1977 Abnormalities in thyroid function in relatives of patients with Graves’ disease and Hashimoto’s thyroiditis: lack of correlation with inheritance of HLA-B8. J Clin Endocrinol Metab 45:45–54 [DOI] [PubMed] [Google Scholar]
- Volpe R 1985 Autoimmune thyroid disease. In: Volpe R, ed. Autoimmunity and endocrine disease. New York: Marcel Dekker; 109–285 [Google Scholar]
- Tamai H, Ohsako N, Takeno K, Fukino O, Takahashi H, Kuma K, Kumagai LF, Nagataki S 1980 Changes in thyroid function in euthyroid subjects with family history of Graves’ disease: a followup study of 69 patients. J Clin Endocrinol Metab 51:1123–1128 [DOI] [PubMed] [Google Scholar]
- Tamai H, Kumagai LF, Nagataki S 1986 Immunogenetics of Graves’ disease. In: McGregor AM, ed. Immunology of endocrine diseases. Lancaster, UK: MTP Press; 123–141 [Google Scholar]
- Sapin R, d'Herbomez M, Gasser F, Meyer L, Schlienger JL 2003 Increased sensitivity of a new assay for anti-thyroglobulin antibody detection in patients with autoimmune thyroid disease. Clin Biochem 36:611–616 [DOI] [PubMed] [Google Scholar]
- Pauls DL, Zakarija M, McKenzie JM, Egeland JA 1993 Complex segregation analysis of antibodies to thyroid peroxidase in Old Order Amish families. Am J Med Genet 47:375–379 [DOI] [PubMed] [Google Scholar]
- Phillips D, McLachlan S, Stephenson A, Roberts D, Moffitt S, McDonald D, Ad'Hiah A, Stratton A, Young E, Clark F 1990 Autosomal dominant transmission of autoantibodies to thyroglobulin and thyroid peroxidase. J Clin Endocrinol Metab 70:742–746 [DOI] [PubMed] [Google Scholar]
- Phillips DI, Shields DC, Dugoujon JM, Prentice L, McGuffin P, Ree SB 1993 Complex segregation analysis of thyroid autoantibodies: are they inherited as an autosomal dominant trait? Hum Hered 43:141–146 [DOI] [PubMed] [Google Scholar]
- Jaume JC, Guo J, Pauls DL, Zakarija M, McKenzie JM, Egeland JA, Burek CL, Rose NR, Hoffman WH, Rapoport B, McLachlan SM 1999 Evidence for genetic transmission of thyroid peroxidase autoantibody epitopic “fingerprints.” J Clin Endocrinol Metab 84:1424–1431 [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Tomer Y 2007 The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun 28:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y, Greenberg DA, Barbesino G, Concepcion ES, Davies TF 2001 CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab 86:1687–1693 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF 2003 Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet 73:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y, Barbesino G, Greenberg DA, Concepcion ES, Davies TF 1999 Mapping the major susceptibility loci for familial Graves’ and Hashimoto’s diseases: evidence for genetic heterogeneity and gene interactions. J Clin Endocrinol Metab 84:4656–4664 [DOI] [PubMed] [Google Scholar]
- Ott J 1976 A computer program for linkage analysis of general human pedigrees. Am J Hum Genet 28:528–529 [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Abreu P, Hodge SE 1998 The power to detect linkage in complex disease by means of simple LOD-score analyses. Am J Hum Genet 63:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA 1989 Inferring mode of inheritance by comparison of LOD scores. Am J Med Genet 34:480–486 [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L 1995 Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Hodge SE, Abreu PC, Greenberg DA 1997 Magnitude of type I error when single-locus linkage analysis is maximized over models: a simulation study. Am J Hum Genet 60:217–227 [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES 1996 Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Aho K, Gordin A, Sievers K, Takala J 1983 Thyroid autoimmunity in siblings: a population study. Acta Endocrinol Suppl 251:11–15 [PubMed] [Google Scholar]
- Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ 1995 CTLA-4 gene polymorphism associated with Graves’ disease in a Caucasian population. J Clin Endocrinol Metab 80:41–45 [DOI] [PubMed] [Google Scholar]
- Ban Y, Davies TF, Greenberg DA, Kissin A, Marder B, Murphy B, Concepcion ES, Villanueva RB, Barbesino G, Ling V, Tomer Y 2003 Analysis of the CTLA-4, CD28 and inducible costimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun 4:586–593 [DOI] [PubMed] [Google Scholar]
- Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, Large DM, Toft AD, McCarthy MI, Kendall-Taylor P, Pearce SH 1999 The cytotoxic T lymphocyte antigen-4 is a major Graves’ disease locus. Hum Mol Genet 8: 1195–1199 [DOI] [PubMed] [Google Scholar]
- Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Rønningen KS, Guja C, Ionescu-Tîrgovişte C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC 2003 Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423:506–511 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Greenberg DA, Concepcion E, Ban Y, Davies TF 2002 Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab 87:404–407 [DOI] [PubMed] [Google Scholar]
- Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y 2003 Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA 100:15119–15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JE, Heward JM, Carr-Smith J, Daykin J, Franklyn JA, Gough SCL 2003 Association of a rare thyroglobulin gene microsatellite variant with autoimmune thyroid disease. J Clin Endocrinol Metab 88:5039–5042 [DOI] [PubMed] [Google Scholar]
- Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y 2004 Association of a thyroglobulin gene polymorphism with Hashimoto’s thyroiditis in the Japanese population. Clin Endocrinol (Oxf) 61:263–268 [DOI] [PubMed] [Google Scholar]
- Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, Villanueva R, Tomer Y 2006 Possible interaction between HLA-DRβ1 and thyroglobulin variants in Graves’ disease. Thyroid 16:351–355 [DOI] [PubMed] [Google Scholar]
- Tomer Y, Menconi F, Davies TF, Barbesino G, Rocchi R, Pinchera A, Concepcion E, Greenberg DA 2007 Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J Autoimmun 29:69–77 [DOI] [PubMed] [Google Scholar]
- Morton NE 1956 The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet 8:80–96 [PMC free article] [PubMed] [Google Scholar]
- Ott J 1999 Analysis of human genetic linkage. 3rd ed. Baltimore: Johns Hopkins University Press [Google Scholar]
- Hodge SE, Anderson CE, Neiswanger K, Sparkes RS, Rimoin DL 1983 The search for heterogeneity in insulin dependent diabetes mellitus (IDDM): linkage studies, two-locus models, and genetic heterogeneity. Am J Hum Genet 35:1139–1155 [PMC free article] [PubMed] [Google Scholar]
- Ericsson UB, Christensen SB, Thorell J 1985 A high prevalence of thyroglobulin autoantibodies in adults with and without thyroid disease as measured with a sensitive solid-phase immunosorbent radioassay. Clin Immunol Immunopathol 37:154–162 [DOI] [PubMed] [Google Scholar]
- Guilhem I, Massart C, Poirier JY, Maugendre D 2006 Differential evolution of thyroid peroxidase and thyrotropin receptor antibodies in Graves’ disease: thyroid peroxidase antibody activity reverts to pretreatment level after carbimazole withdrawal. Thyroid 16:1041–1045 [DOI] [PubMed] [Google Scholar]
- Tomer Y 1997 Anti-thyroglobulin autoantibodies in autoimmune thyroid diseases: cross-reactive or pathogenic? Clin Immunol Immunopathol 82:3–11 [DOI] [PubMed] [Google Scholar]
- Vieland VJ, Huang Y, Bartlett C, Davies TF, Tomer Y 2008 A multilocus model of the genetic architecture of autoimmune tyroid disorder, with clinical implications. Am J Hum Genet 82:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, Ho K, Tomer Y 2007 A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun 8:205–214 [DOI] [PubMed] [Google Scholar]
- Kavvoura FK, Akamizu T, Awata T, Ban Y, Chistiakov DA, Frydecka I, Ghaderi A, Gough SC, Hiromatsu Y, Ploski R, Wang PW, Ban Y, Bednarczuk T, Chistiakova EI, Chojm M, Heward JM, Hiratani H, Juo SH, Karabon L, Katayama S, Kurihara S, Liu RT, Miyake I, Omrani GH, Pawlak E, Taniyama M, Tozaki T, Ioannidis JP 2007 Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab 92:3162–3170 [DOI] [PubMed] [Google Scholar]
- Tomer Y 2001 Unraveling the genetic susceptibility to autoimmune thyroid diseases: CTLA-4 takes the stage. Thyroid 11:167–169 [DOI] [PubMed] [Google Scholar]
- Ahmed S, Ihara K, Kanemitsu S, Nakashima H, Otsuka T, Tsuzaka K, Takeuchi T, Hara T 2001 Association of CTLA-4 but not CD28 gene polymorphisms with systemic lupus erythematosus in the Japanese population. Rheumatology (Oxford) 40:662–667 [DOI] [PubMed] [Google Scholar]
- Donner H, Rau H, Walfish PG, Braun J, Siegmund T, Finke R, Herwig J, Usadel KH, Badenhoop K 1997 CTLA4 alanine-17 confers genetic susceptibility to Graves’ disease and to type 1 diabetes mellitus. J Clin Endocrinol Metab 82:143–146 [DOI] [PubMed] [Google Scholar]
- Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y 2005 Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab 90: 4904–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Liu L, Noren K, Xia SQ, Trifunovic J, Pirskanen R, Lefvert AK 1998 Genetic association of Ctla-4 to myasthenia gravis with thymoma. J Neuroimmunol 88:192–198 [DOI] [PubMed] [Google Scholar]
- Vaidya B, Imrie H, Geatch DR, Perros P, Ball SG, Baylis PH, Carr D, Hurel SJ, James RA, Kelly WF, Kemp EH, Young ET, Weetman AP, Kendall-Taylor P, Pearce SH 2000 Association analysis of the cytotoxic T lymphocyte antigen-4 (CTLA-4) and autoimmune regulator-1 (AIRE-1) genes in sporadic autoimmune Addison’s disease. J Clin Endocrinol Metab 85: 688–691 [DOI] [PubMed] [Google Scholar]
- Vladutiu AO, Rose NR 1971 Autoimmune murine thyroiditis: relation to histocompatibility (H-2) type. Science 174:1137–1139 [DOI] [PubMed] [Google Scholar]
- Beisel K, David CS, Giraldo AA, Kong Y-CM, Rose NR 1982 Regulation of experimental autoimmune thyroiditis: mapping of susceptibility to the I-A subregion of the mouse H-2. Immunogenetics 15:427–430 [DOI] [PubMed] [Google Scholar]
- Kong YM, David CS, Lomo LC, Fuller BE, Motte RW, Giraldo AA 1997 Role of mouse and human class II transgenes in susceptibility to and protection against mouse autoimmune thyroiditis. Immunogenetics 46:312–317 [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H, Sharp GC, Medling B, Tang H 1999 Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun 12:157–165 [DOI] [PubMed] [Google Scholar]
- Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y 2002 The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf) 57:81–88 [DOI] [PubMed] [Google Scholar]