Over 15 years of follow-up, mild primary hyperparathyroidism patients’ biochemical and densitometric features were generally stable for the first 8–10 years in those who did not undergo surgery. Thereafter, a progressive reduction in bone density was seen at the hip and distal radius, providing further insight into the natural history of mild primary hyperparathyroidism.
Abstract
Context: Primary hyperparathyroidism (PHPT) often presents without classical symptoms such as overt skeletal disease or nephrolithiasis. We previously reported that calciotropic indices and bone mineral density (BMD) are stable in untreated patients for up to a decade, whereas after parathyroidectomy, normalization of biochemistries and increases in BMD ensue.
Objective: The objective of the study was to provide additional insights in patients with and without surgery for up to 15 yr.
Design: The study had an observational design.
Setting: The setting was a referral center.
Patients: Patients included 116 patients (25 men, 91 women); 99 (85%) were asymptomatic.
Intervention: Fifty-nine patients (51%) underwent parathyroidectomy and 57 patients were followed up without surgery.
Main Outcome Measure: BMD was measured.
Results: Lumbar spine BMD remained stable for 15 yr. However, BMD started to fall at cortical sites even before 10 yr, ultimately decreasing by 10 ± 3% (mean ± sem; P < 0.05) at the femoral neck, and 35 ± 5%; P < 0.05 at the distal radius, in the few patients observed for 15 yr. Thirty-seven percent of asymptomatic patients showed disease progression (one or more new guidelines for surgery) at any time point over the 15 yr. Meeting surgical criteria at baseline did not predict who would have progressive disease. BMD increases in patients who underwent surgery were sustained for the entire 15 yr.
Conclusions: Parathyroidectomy led to normalization of biochemical indices and sustained increases in BMD. Without surgery, PHPT progressed in one third of individuals over 15 yr; meeting surgical criteria at the outset did not predict this progression. Cortical bone density decreased in the majority of subjects with additional observation time points and long-term follow-up. These results raise questions regarding how long patients with PHPT should be followed up without intervention.
Introduction of the multichannel screening test in the early 1970s signaled a marked change in the incidence and in the clinical profile of primary hyperparathyroidism (1,2). The most common presentation, namely classical symptoms and signs of skeletal and renal involvement, was replaced by one in which patients were discovered incidentally during a routine medical evaluation. Secure clinical recommendations for management of this asymptomatic disorder required access to a cohort of patients who could be followed long term, both with regard to those who would undergo surgery or be managed without intervention (3).
In 1984 we initiated a prospective study to define the natural history, pathophysiology, densitometric, and other skeletal abnormalities of primary hyperparathyroidism (PHPT) in the multichannel screening era (3). In terms of that natural history component of this study, we previously reported on this cohort at the conclusion of the first 10 yr of observation. In this report, mixed model analysis (see Statistical analysis below) allows a more statistically efficient attempt at detecting systematic patterns than the methods we previously used. This has permitted an analysis of all the data, which now includes more time points within the 10 yr and extends to 15 yr of prospective longitudinal follow-up. The longer follow-up period has led to new observations that have implications for decision-making and for management of this disorder.
Patients and Methods
Patients
The study, which began in 1984, enrolled 116 subjects with PHPT who had at least one follow-up bone mineral density test (Fig. 1). The protocol was approved by the Institutional Review Board of Columbia University Medical Center, and all patients gave written informed consent.
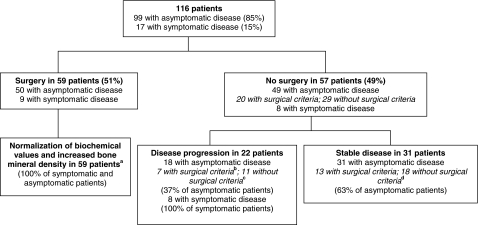
Figure 1.
Baseline characteristics and outcomes in 116 patients with PHPT. All patients with symptomatic disease had kidney stones. In patients with disease progression, either an indication for surgery, according to National Institutes of Health Consensus Conference guidelines (4,25) developed, or an overt complication of hyperparathyroidism (such as kidney stones or fracture) occurred during follow-up. a, Fifteen patients remaining at 15 yr; b, three patients remaining at 15 yr; c, one patient remaining at 15 yr; d, two patients remaining at 15 yr.
The decision to recommend parathyroidectomy was based on guidelines adopted by the 1990 National Institutes of Health Consensus Conference on the Management of Asymptomatic PHPT (4). Criteria for surgery included complications of PHPT (osteitis fibrosa cystica, nephrolithiasis (documented by a review of the medical records and/or by a discrete event), classic neuromuscular symptoms or parathyroid crisis) or one or more of the following criteria (4): serum calcium concentration greater than 12 mg/dl (3 mmol/liter), 24-h urinary calcium excretion greater than 400 mg (10 mmol), bone density Z score at the distal one third radius less than −2, an unexplained reduction in creatinine clearance, or age younger than 50 yr. Although these guidelines were used as the basis for recommendations for or against surgery, some patients chose to have surgery, even though they did not meet any guidelines, whereas others who met criteria refused intervention. All patients included in the surgical group were operated on within a year of study entry. As previously reported (5) calcium intake at presentation was often low (mean ∼600 mg/d). Intake of no more than 1000 mg (without calcium supplementation) was generally recommended for patients who did not undergo surgery; compliance with this recommendation was not specifically monitored.
Study protocol
All patients underwent biochemical studies at baseline and every 4 months if they were followed up with no intervention, or every 6 months if they had parathyroid surgery Serum calcium, phosphorus, and alkaline phosphatase activity were measured by automated techniques (Technicon Instruments, Tarrytown, NY). Serum PTH was measured by immunoradiometric assay (interassay coefficient of variation, 5.6%) (6), urinary calcium by atomic absorption spectrophotometry, and serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D as previously described (interassay coefficients of variation, 9.6 and 9.8%, respectively) (7).
Bone mineral density (BMD) of the lumbar spine, femoral neck, and distal third of the nondominant radius was measured at baseline and yearly thereafter. The short-term in vivo precision error (root mean square sd) was 0.026 g/cm2 for L1-L4, 0.032 g/cm2 for the total hip, 0.041 g/cm2 for the femoral neck, and 0.033 g/cm2 for the forearm. The data on bone density are reported both as absolute measurements and as Z scores (8).
During the first 5 yr of the study, densitometric data were obtained using single- and dual-photon absorptiometry techniques (SP2 and DP3; Lunar Radiation Corp., Madison, WI), whereas data for virtually the entire past 20 yr were obtained using dual-energy x-ray absorptiometry (DXA). Twenty-six subjects who did not undergo parathyroidectomy switched densitometers (mean time from study entry was 3 yr; range 1–6 yr). Of the 11 patients followed up beyond 10 yr, eight switched densitometers (mean time of switch from study entry was 4 yr). When this transition occurred, the different machines were cross-calibrated by scanning 60 people on both machines within a few weeks. Subjects were selected to represent the range of bone densities that represented our cohort. Regression modeling was used to create the predictive equations that allowed interconversion of the data to the newer instrumentation. Data obtained on the cohorts followed for 20 yr with the DXA technology were essentially identical with the data that spanned the transition to DXA, giving assurance that an artifact of measurement was not introduced into the monitoring process.
Quality control included reading phantoms daily that were analyzed by Shewhart criteria for process stability (9,10).
Statistical analysis
Independent t tests were used to compare baseline characteristics of the medical and surgical groups. All data were transformed to percent change from baseline before analysis and descriptive statistics examined for distributional characteristics. No measure exceeded a range of 3 orders of magnitude, and all mean and median percent changes from baseline were within 30% of each other on all measures. Whereas our 10-yr report used ANOVA for comparison among surgical and nonsurgical cohorts, and paired t tests to measure changes over time, a more precise tool was chosen for this report. Linear mixed model analysis for repeated measures was used to estimate within-subject changes in BMD and biochemical parameters. Identical models with a fixed effect of time (annual years of follow-up), random effects of subject, time and error, and an empirically determined block diagonal autoregressive [1] covariance structure were created for each bone measurement site and biochemical parameter (11). Estimated within-subject change in outcome between specific time points was taken from the differences between model estimated means for each time, after adjustment of the degrees of freedom, to test the statistical significance of the comparison (12). Adjustments for multiple comparisons were not used because the mixed model adjusted degrees of freedom for the calculation of the confidence interval for the difference. All models were run under restricted maximum likelihood.
Separate models were performed for medically managed subjects from the time of enrollment, with and without patients who were treated with antiresorptive medications and with and without patients who completed the 15 yr of the study and for surgical subjects starting with their last available presurgical evaluation. The proportion of subjects meeting surgical criteria at baseline that went on to have surgery was assessed with Fisher’s exact test. Data are presented as mean ± sem.
Results
Baseline characteristics
The cohort of 116 patients with PHPT included 66 postmenopausal women (56%), 25 premenopausal women (22%), and 25 men (22%). Although 17 patients (15%) had a history of kidney stones, none had osteitis fibrosa cystica or classical neuromuscular symptoms. Patients were followed up according to their treatment category (with or without surgery) and according to the presence or absence of PHPT symptoms (Fig. 1 and Table 1). Patients who underwent parathyroidectomy (n = 59) had higher serum calcium and PTH concentrations than those (n = 57) who did not undergo surgery (Table 1). Patients who underwent surgery also had significantly lower BMD Z-scores at the lumbar spine and femoral neck but not at the radius. In both groups, a typical pattern of skeletal involvement was seen with greater reductions at the distal one third radius (cortical site) than the lumbar spine (cancellous site). Urinary calcium excretion, alkaline phosphatase activity, and vitamin D concentrations were similar between the surgical and nonsurgical groups.
Table 1.
Baseline characteristics of patients with primary hyperparathyroidism
| Characteristic | No surgery (n = 57) | Surgery (n = 59) | Normal range | P value |
|---|---|---|---|---|
| Age (yr) | 57 ± 2 | 55 ± 2 | NS | |
| Sex (n) | NS | |||
| Postmenopausal | 32 | 34 | ||
| Premenopausal | 13 | 12 | ||
| men | 12 | 13 | ||
| Kidney stones (n) | 8 | 9 | NS | |
| Serum calcium (mg/dl) | 10.5 ± 0.1 | 10.8 ± 0.1 | 8.4–10.2 | 0.01 |
| Serum PTH (pg/ml) | 116 ± 7 | 144 ± 13 | 10–65 | 0.05 |
| Urinary calcium (mg/g creatinine) | 236 ± 17 | 262 ± 17 | <300 | NS |
| Serum total alkaline phosphatase (U/liter) | 98 ± 6 | 98 ± 6 | <100 | NS |
| Serum 25-hydroxyvitamin D (ng/ml) | 21 ± 1 | 21 ± 1 | 9–52 | NS |
| Serum 1,25-dihydroxyvitamin D (pg/ml) | 57 ± 2 | 58 ± 3 | 15–60 | NS |
| BMD Z score lumbar spine | −0.03 ± 0.2 | −0.80 ± 0.2 | 0.02 | |
| BMD Z score femoral neck | −0.63 ± 0.1 | −1.22 ± 0.1 | 0.002 | |
| BMD Z score distal one third radius | −0.98 ± 0.2 | −1.30 ± 0.2 | NS |
Values are shown as means ± sem. To convert values for serum calcium to millimoles per liter, multiply by 0.25; to convert values for PTH to picomoles per liter, divide by 9.5; to convert values for urinary calcium to millimoles per liter, multiply by 0.025; to convert values for 25-hydroxyvitamin D to nanomol/liter, multiply by 2.496; to convert values for 1,25-dihydroxyvitamin D to picomoles per liter, multiply by 2.6. NS, Non-significant.
Clinical course over 15 yr: no parathyroid surgery
Fifty-seven subjects with PHPT were followed without intervention for up to 15 yr. The vast majority (n = 49; 86%) were asymptomatic. During this period of time, 11 patients died. Four of the 11 subjects who died had increased risk factors for cardiovascular disease at study entry, such as diabetes mellitus and hypertension. The causes of death included myocardial infarction (n = 2), complications of diabetes mellitus (n = 2), congestive heart failure (n = 1), stroke (n = 1), aortic aneurysm rupture (n = 1), gall bladder cancer (n = 1), and unknown causes (n = 3). The subjects who died did not have higher serum calcium levels at baseline (10.3 ± 0.1 mg/dl) than the remainder of the asymptomatic medical subjects but did have higher initial PTH levels (161 ± 25 vs. 107 ± 8 pg/ml, P < 0.05). Eight subjects were unable to comply with regular study visits and five were eventually lost to follow-up. None of the demographic variables available to predict duration of follow (age at study enrollment, sex, and race) achieved statistical significance. Nearly half of the nonintervention group (20 of 57) ultimately had successful surgery. Of these, one was symptomatic at baseline but had initially refused surgery, six met surgical criteria but deferred intervention initially, six had disease progression, and seven no longer wished to be followed up without intervention.
Biochemical changes
Of the 49 asymptomatic subjects, 29 (59%) did not meet surgical criteria at baseline, whereas 20 (41%) did meet criteria but refused intervention. There were no significant changes in PTH, total alkaline phosphatase activity, or urinary calcium excretion (Table 2) among the asymptomatic patients who did not have surgery, for as long as 15 yr of follow-up. However, in the subjects who were followed up conservatively for up to 15 yr, serum calcium concentrations rose to levels, beginning at yr 13, that were slightly but significantly higher than their individual baseline values. In the 11 patients who completed 10 yr of follow-up, neither the serum calcium nor the PTH changed significantly over yr 0, 5, and 10 (serum calcium: 10.5 ± 0.1, 10.7 ± 0.1, 10.8 ± 0.2 mg/dl; PTH: 109 ± 13, 102 ± 13, 110 ± 14 pg/ml). In the six patients who completed 15 yr of follow-up, the serum calcium changed significantly only at yr 15, compared with baseline (yr 0, 5, 10, and 15 were 10.6 ± 0.2, 10.7 ± 0.2, 11.1 ± 0.3, and 11.1 ± 0.2 mg/dl, respectively). PTH did not change significantly (yr 0, 5, 10, and 15 were 98 ± 12, 81 ± 12, 74 ± 14, and 89 ± 12 pg/ml, respectively). Longitudinal biochemical profiles were similar among those asymptomatic subjects who met or did not meet surgical criteria.
Table 2.
Biochemical changes in asymptomatic patients followed up without parathyroidectomy (n = 49)
| Variable | Baseline (n = 49) | Yr 5 (n = 25) | Yr 10 (n = 11) | Yr 13 (n = 9) | Yr 15 (n = 6) |
|---|---|---|---|---|---|
| Serum calcium (mg/dl) | 10.5 ± 0.1 | 10.7 ± 0.1 | 10.8 ± 0.2 | 11.0 ± 0.2a | 11.1 ± 0.2a |
| PTH (pg/ml) | 122 ± 10 | 119 ± 12 | 123 ± 14 | 124 ± 16 | 121 ± 18 |
| Serum creatinine (mg/dl) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.1 |
| Urinary calcium (mg/dl) | 238 ± 19 | 215 ± 23 | 185 ± 32 | 247 ± 36 | 202 ± 36 |
| Serum 25-(OH) vitamin D (ng/ml) | 21 ± 1 | 22 ± 2 | 22 ± 3 | 21 ± 3 | 19 ± 4 |
| Serum 1,25-(OH)2 vitamin D (pg/ml) | 56 ± 2 | 58 ± 3 | 54 ± 5 | 40 ± 5a | 48 ± 7 |
Values are shown as means ± sem. Pair-wise comparisons are shown at each time point with respect to the retained subjects’ baseline values. To convert values for serum calcium to millimoles per liter, multiply by 0.25; to convert values for PTH to picomoles per liter, divide by 9.5; to convert values for serum creatinine to micromoles per liter, multiply by 76.26; to convert values for urinary calcium to millimoles per liter, multiply by 0.025; to convert values for 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496; to convert values for 1,25-dihydroxyvitamin D to picomoles per liter, multiply by 2.6. 25-(OH) vitamin D, 25-hydroxyvitamin D; 1,25-(OH)2 vitamin D, 1,25-dihydroxyvitamin D.
P < 0.01 for the comparison with the individual baseline values for these groups.
Densitometric changes
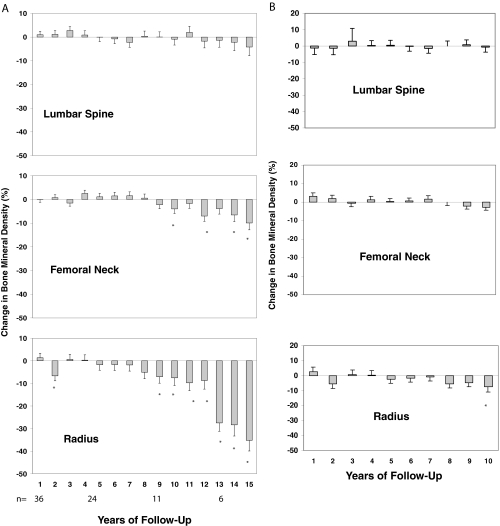
BMD did not change at any measurement site during the first 8 yr of follow-up (Fig. 2) in the 49 asymptomatic patients. The lumbar spine BMD was stable for the entire 15 yr of follow-up, in both the overall cohort and the 28 postmenopausal women. However, even before the 10-yr time point, the femoral neck, a site containing a substantial amount of cortical bone, and the distal one third radius, a site containing a predominance of cortical bone, showed significant reductions in BMD. Overall, 29 of the 49 asymptomatic patients (59%) had more than a 10% decline in BMD at one or more sites over the 15-yr period. Eleven of the 29 subjects were patients who had been previously found to have declined within 10 yr (3); eight other patients, with the availability of additional time points, were now found to also have declined within 10 yr, whereas 10 other patients declined after 10 yr of observation.
Figure 2.
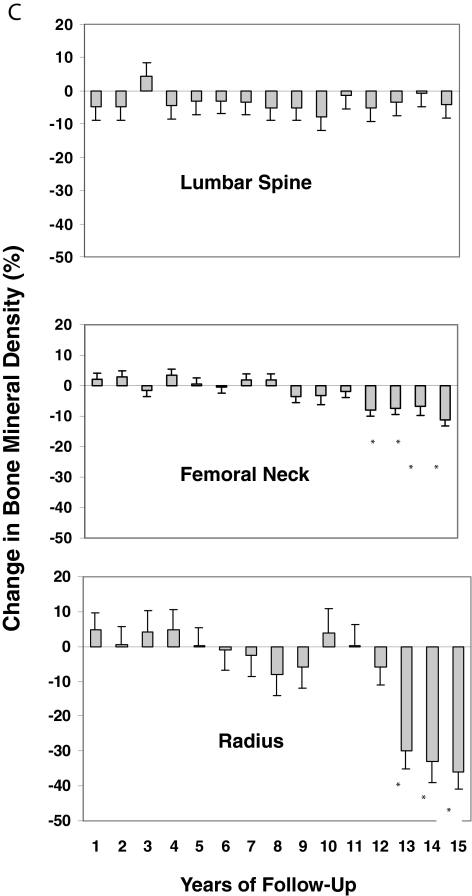
A, Mean (± sem) changes in BMD at three sites in patients with PHPT. Data shown are cumulative percent changes from baseline at each site in subjects who did not undergo parathyroidectomy after 1–15 yr of follow-up. *, P < 0.05, compared with baseline. B, Mean (± sem). Changes in BMD at three sites in patients with PHPT are shown only in subjects who completed 10 yr of follow-up. Eleven subjects were followed up for a total of 10 yr. C, Mean (± sem). Changes in BMD at three sites in patients with PHPT are shown only in subjects who completed 15 years of follow-up. Six subjects were followed up for a total of 15 yr.
Of the 11 patients followed beyond 10 yr, eight switched densitometers early in the study (mean time of switch from study entry was 4 yr). In that subgroup of eight patients, before the switch, BMD increased on average 4% at the lumbar spine and decreased 2% at the femoral neck and 1% at the distal radius. After the switch (using the first BMD after the switch as a new baseline), there were further declines in average BMD of 1% at the lumbar spine, 2% at the femoral neck, and 10% at the distal radius.
The six asymptomatic subjects who completed the 15-yr period of monitoring did not differ from the rest of the asymptomatic subjects (n = 43) in terms of age, baseline biochemistries (serum calcium, PTH, urinary calcium excretion, total alkaline phosphatase, 25-hydroxyvitamin D, or 1,25-dihydroxyvitamin D), or baseline Z-scores. However, even before year 10, BMD at the distal one third radius site showed accelerated losses. Linear mixed-model analyses for repeated measures were performed, comparing BMD changes in the first 10 yr of study among those followed up for less than 10 yr, and those followed up for a longer period. At the lumbar spine or femoral neck, the rates of change between these groups did not differ (P = 0.85, lumbar spine; P = 0.09, femoral neck). However, after yr 10, BMD declined significantly at the distal one third radius (P < 0.001). The slope of BMD loss therefore differed at the distal radius (there was an accelerated rate of loss after 10 yr) but not at the femoral neck (there was the same rate of loss continuing from before 10 yr).
Thirteen asymptomatic subjects who were followed up without surgery received antiresorptive therapy at some time point in the study (off protocol). The median duration of antiresorptive drug use was 4 yr (range 1–11 yr), whereas the median observation time in the remaining 36 asymptomatic subjects was 5 yr (range 1–15 yr). However, linear mixed-model analyses for repeated measures, comparing BMD changes in those who did and did not receive antiresorptive therapy, showed no differences in terms of the densitometric measurements over the 15 yr at any site.
Disease progression
Most of the 49 asymptomatic patients did not develop densitometric or other surgical guidelines. However, 18 patients (∼37%) did develop new surgical criteria at any time point over the 15 yr of observation. Meeting surgical criteria at study baseline did not predict who would have progressive disease. Of the 20 asymptomatic subjects who met surgical criteria at baseline (5 yr, n = 10; 10 yr, n = 5; 15 yr, n = 3), seven had disease progression (35%); likewise, of the 29 asymptomatic subjects who did not meet surgical criteria at baseline (5 yr, n = 15; 10 yr, n = 12; 15 yr, n = 3), 11 had disease progression (38%), a similar proportion. Changes in bone density over time were also similar among the asymptomatic subjects, regardless of whether they met surgical criteria at baseline.
All eight of the patients who had nephrolithiasis, and were therefore symptomatic, but refused surgery, showed progression by at least one criterion: six had recurrent kidney stones, one sustained a fracture, and one developed marked hypercalcemia. Despite these further complications, only one of these patients agreed to undergo parathyroidectomy.
Clinical course over 15 yr in subjects who underwent parathyroidectomy
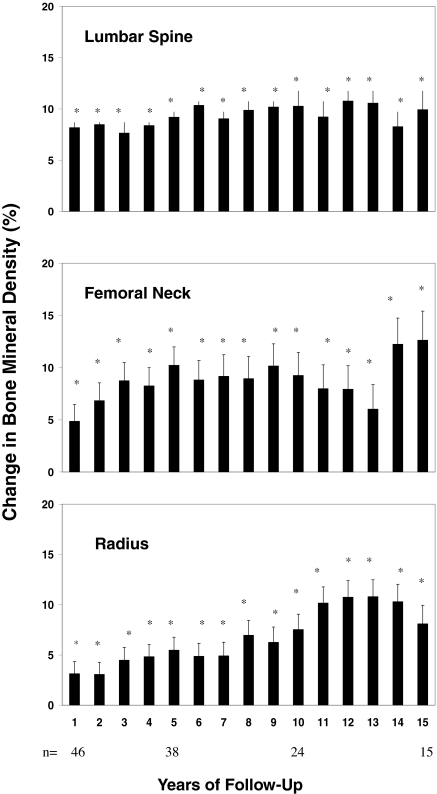
All 59 patients had successful surgery as evidenced by normal serum calcium and PTH values postoperatively. Only nine of the 59 subjects (15%) who underwent surgery were symptomatic with kidney stones. At 15 yr, serum calcium was 9.8 ± 0.2 mg/dl (2.5 ± 0.1 mmol/liter), PTH 34 ± 21 pg/ml (3.6 ± 2 pmol/liter), and urinary calcium excretion 138 ± 36 mg/dl (3.5 ± 0.9 mmol/liter), all significantly lower (P < 0.01) in comparison with the individual subjects’ baseline values and all well within normal limits. Postoperative increases in BMD were sustained with BMD remaining significantly above baseline for the entire 15 yr of follow-up at all three skeletal sites (Fig. 3). Pre- and postmenopausal women and men, and those with and without symptoms, all had similar increases in BMD. Assessment of postoperative changes in BMD in those subjects who were initially followed up without intervention, but ultimately underwent parathyroidectomy (n = 20), demonstrated similar increases in BMD to those who had parathyroidectomy at the outset (at yr 1, 5, and 10, the lumbar spine increased 9, 6, and 12%; the femoral neck 1, 7, and 10%; the distal radius 4, 8, and 7%). There were no recurrent kidney stones in any of the nine symptomatic patients with kidney stones who underwent parathyroid surgery.
Figure 3.
Mean (± sem) changes in BMD at three sites in patients with PHPT after parathyroidectomy. Data shown are cumulative percent changes from surgery at each site after 1–15 yr of follow-up. *, P < 0.05, compared with baseline.
Discussion
This report, the longest prospective follow-up of mild PHPT ever conducted, provides data on the long-term natural history of the treated and untreated disorder. In our previous report, covering the first 10 yr, we showed that most asymptomatic patients who did not have surgery did well, although 25% did show evidence of progressive disease (3). Average serum levels of calcium and PTH did not change and hypercalciuria, if present, did not worsen. Average BMD as measured by DXA was stable. Among the 25% who did show evidence for progression, worsening hypercalcemia, hypercalciuria, and reductions in BMD were the most common complications. In the earlier report, we also described patients who underwent successful surgery. In addition to normalization of serum calcium and PTH levels, BMD improved significantly at the lumbar spine and hip regions (3). The current report, which extends findings on this same cohort to 15 yr, provides new information that may well influence treatment decisions in this disease.
In those who did not undergo surgery, lumbar spine BMD continued to be relatively stable during yr 10–15. Although progressive osteoarthritic changes at this site could have contributed to the stable lumbar spine findings, it is also consistent with our prior observations that the lumbar spine is a site that is relatively protected from bone loss in PHPT. Thus, despite histomorphometric evidence for increased bone turnover in PHPT (13), cancellous bone appears to be relatively well preserved, even after many years. Potential mechanisms for preservation of cancellous bone mass are suggested by quantitative histomorphometry of bone biopsies from patients with PHPT (13,14,15). Although activation frequency is increased, resorption pits are relatively shallow such that increased osteoblast activity is likely to ensure reasonably well-balanced remodeling units. As a result, the expected age-associated loss of trabecular plates is not regularly seen in PHPT (16).
In contrast to the stability at the lumbar spine, declines in BMD became apparent before yr 10 at the femoral neck and distal one third radius sites in the few remaining patients who were followed up without surgery. The detection of statistically significant declines in cortical bone density before yr 10 is a result of both the additional observation points in the time line as well as the more powerful statistical mixed model approach, in that variance attributable to within-subject covariance among repeated measures no longer inflates the error term (3).
This observation suggests that the catabolic effects of PTH at the femoral neck and distal radius, which contain more cortical bone than the lumbar spine, might begin to emerge with prolonged exposure to PTH excess. Cortical bone is more vulnerable than cancellous bone to the catabolic effects of PTH (17). The possibility that cortical bone density is eventually compromised in PHPT has been suggested by some (18,19) but not all studies (20,21). Why so much time is required before these changes manifest themselves may relate to the low turnover rate of cortical bone, requiring years of exposure to demonstrate the negative effect. Alternatively, the process by which excess PTH leads to resorption of cortical bone may be compensated initially but not sustained over time.
The declines in cortical bone density were observed regardless of whether the patients taking antiresorptive therapy were excluded. Including those on antiresorptive therapy might have potentially masked an even greater bone loss than what was observed. However, the important point is that use of antiresorptives did not alter the natural history of the eventual cortical reductions after 10 yr or the prolonged maintenance of bone density at the lumbar spine.
Concepts of bone loss in mild PHPT may need to be re-examined in light of these new observations. It is currently hypothesized (22,23) that cortical BMD is lost early in the disease before hypercalcemia brings the disorder to clinical attention. Although some patients may have had PHPT for years before diagnosis, for most patients, as demonstrated in this study, BMD remains stable for several years after coming to medical attention. The new steady state may be sustained for an even longer period at the cancellous lumbar spine but may not be at sites in which cortical bone is more prominent. The loss of bone mass in the hip and distal one third radius suggests that cortical bone might not remain stable over time.
Over the 15-yr follow-up period, 37% of asymptomatic patients showed evidence for disease progression at some time point, a substantial increase over the 25% progression during the first 10 yr. Meeting surgical criteria at study entry did not predict who would have worsening disease. In asymptomatic patients who did not undergo parathyroidectomy, there were no reliable predictors of bone loss, the development of symptomatic complications, or other surgical criteria. This point, along with the eventual decline in cortical bone density, highlights the importance of regular monitoring in asymptomatic patients with PHPT who do not undergo parathyroid surgery.
Meeting surgical guidelines at the time of diagnosis did not predict a worse outcome in asymptomatic patients who were followed up without surgery, raising questions as to whether the guidelines for surgery in asymptomatic patients with PHPT are sufficient to support appropriate decision making (24). Since this study began, surgical criteria were revised after a National Institutes of Health-sponsored workshop on asymptomatic PHPT in 2002 (25). Surgical recommendations were expanded to include a serum calcium concentration greater than 1.0 mg/dl (0.25 mmol/liter) above normal or a marked reduction in BMD at any site (T score below −2.5). Using these new more stringent criteria would, in all likelihood, have uncovered evidence for even greater disease progression. In addition, a larger group of less severely affected individuals would have met surgical criteria at baseline.
Fifteen years after successful parathyroid surgery, patients sustained their postoperative increases in BMD both at cancellous and cortical sites despite the expectation of age-related bone loss. Most likely, the early increase in cancellous BMD results from mineralization of the expanded remodeling space that is characteristic of PHPT (26,27). Although the increase in BMD at the more highly cortical distal one third radius was not as great as that at cancellous sites, these data suggest that cortical bone loss in patients with PHPT is at least partially reversible. This new observation contrasts with data from previous reports (19,28) and is most likely explained by the longer period of observation.
A limitation of this study is the small number of asymptomatic participants who remained in the nonsurgical group at the conclusion of the 15 yr of observation, a byproduct of this type of very long-term natural history study. Nevertheless, the six asymptomatic subjects who completed the 15-yr period of monitoring did not differ from the rest of the asymptomatic subjects in terms of age, baseline biochemistries, or baseline BMD Z-scores. An additional limitation was the change in densitometers in some of the patients in the early years of the study. However, the contrast between the increase in BMD in the patients who underwent parathyroidectomy (many of whom were also studied before and after change in equipment) and the lack of change in patients who did not argues against any widespread systematic error.
A further limitation is that the study was not randomized or controlled. The patients who underwent surgery were different at the outset from those who were managed conservatively. Despite this point, the presence within each group (surgical and nonsurgical) of patients who did or did not meet criteria for surgery has allowed us to gain some insights that would not have been possible with a more rigorous experimental design. Moreover, it is noteworthy that data from short-term studies in which identically matched PHPT subjects were randomized to a surgical or nonsurgical group are virtually indistinguishable from the findings we currently present (29,30). Like the randomized trial of Rao et al. (30), many of our medical subjects eventually elected to undergo parathyroidectomy. Most recently, randomized 1- (31) and 2-yr (32) trials of surgery have also corroborated our early densitometric observations. These studies, together with ours, seem to raise the question as to whether long-term follow-up without intervention is the best option in PHPT.
The clinical significance of densitometric changes in PHPT needs to be considered along with other indices of bone strength (33). For example, the decreases in cortical BMD in the group followed up medically might be due to increased cortical diameter, which itself might counteract the apparent BMD reduction and thus reduce fracture risk by virtue of size-related improvements in biomechanical indices (34). It is also unclear whether postoperative improvements in cortical BMD are associated with improved bone strength. Assessment of bone geometry, microarchitecture, and material properties of bone (33,35,36), as well as fractures per se, is necessary for a complete description of fracture risk in PHPT (37,38).
Whether mortality is increased in mild PHPT, as it clearly is in more severe disease, is controversial (39,40). In our study, the number of deaths in the surgical group is unknown because many subjects were lost to follow-up, but 11 of the nonsurgical subjects (19%) died over the 15 yr of follow-up. The observations that the majority of these deaths were related to cardiovascular causes, and that baseline PTH levels in these subjects were significantly higher, is consistent with the observation that more severe PHPT and/or higher sustained PTH levels might be associated with increased cardiovascular mortality. Nevertheless, it is important to reiterate that this study was not powered to allow us to comment on this issue. Only epidemiological studies can address the issue of mortality in PHPT and whether there is a difference between those who undergo parathyroid surgery and those who do not.
These results indicate that there is evidence of progressive disease in individuals with mild PHPT, particularly with regard to declines in cortical bone density. Although the study was limited by its observational design and the small number of patients who completed 15 yr of observation, these new data raise the question as to whether conservative, nonsurgical management of PHPT, among those deemed not to be surgical candidates, should be limited to a decade of follow-up. Decisions about further conservative management depend on continued densitometric stability. These results also emphasize the need for continued long-term surveillance of biochemical indices and bone densitometry in all patients with mild disease who do not undergo parathyroid surgery.
Footnotes
This work was supported by Grant 32333 from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. S.J.S. is supported in part by Grant K24 DK074457 from the National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases.
Disclosure Statement: M.R.R., J.P.B., D.J.M., T.J., E.Sh., E.Si., J.U., and S.J.S. have nothing to declare.
First Published Online June 10, 2008
For editorial see page 3302
Abbreviations: BMD, Bone mineral density; DXA, dual-energy x-ray absorptiometry; PHPT, primary hyperparathyroidism.
References
- Albright F, Reifenstein EC 1948 The parathyroid glands and metabolic bone disease. Baltimore: Williams & Wilkins Co. [Google Scholar]
- Heath 3rd H, Hodgson SF, Kennedy MA 1980 Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N Engl J Med 302:189–193 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP 1999 A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 [DOI] [PubMed] [Google Scholar]
- NIH conference 1991 Diagnosis and management of asymptomatic primary hyperparathyroidism: consensus development conference statement. Ann Intern Med 114:593–597 [DOI] [PubMed] [Google Scholar]
- Locker FG, Silverberg SJ, Bilezikian JP 1997 Optimal dietary calcium intake in primary hyperparathyroidism. Am J Med 102:543–550 [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts Jr JT, Segre GV 1987 Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 33:1364–1367 [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Bilezikian JP 1989 Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4:283–291 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1990 Interpretation of bone densitometry measurements: disadvantages of a percentage scale and a discussion of some alternatives. J Bone Miner Res 5:537–540 [DOI] [PubMed] [Google Scholar]
- Fang Z, Smith JA, Kleppinger A, Reisine ST, Emerson D, Kulldorff M 2002 Retrospective evaluation and adjustment of dual energy X-ray absorptiometry measurements for bone mineral density research studies. J Clin Densitom 5:421–433 [DOI] [PubMed] [Google Scholar]
- Faulkner KG, McClung MR 1995 Quality control of DXA instruments in multicenter trials. Osteoporos Int 5:218–227 [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger S 1986 Longitudinal data analysis using generalized linear models. Biometrika 73:13–22 [Google Scholar]
- Kenward MG, Roger JH 1997 Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997 [PubMed] [Google Scholar]
- Eriksen EF, Mosekilde L, Melsen F 1986 Trabecular bone remodeling and balance in primary hyperparathyroidism. Bone 7:213–221 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP 1999 On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 84:1562–1566 [DOI] [PubMed] [Google Scholar]
- Parisien M, Mellish RW, Silverberg SJ, Shane E, Lindsay R, Bilezikian JP, Dempster DW 1992 Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res 7:913–919 [DOI] [PubMed] [Google Scholar]
- Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW 1990 The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab 70:930–938 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1986 Accelerated cortical loss: primary and secondary hyperparathyroidism. In: Uhthoff H, Stahl E, eds. Current concepts of bone fragility. Berlin: Springer-Verlag; 279–85 [Google Scholar]
- Rao DS, Wallace EA, Antonelli RF, Talpos GB, Ansari MR, Jacobsen G, Divine GW, Parfitt AM 2003 Forearm bone density in primary hyperparathyroidism: long-term follow-up with and without parathyroidectomy. Clin Endocrinol (Oxf) 58:348–354 [DOI] [PubMed] [Google Scholar]
- Leppla DC, Snyder W, Pak CY 1982 Sequential changes in bone density before and after parathyroidectomy in primary hyperparathyroidism. Invest Radiol 17:604–606 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Rao DS, Kleerekoper M 1991 Asymptomatic primary hyperparathyroidism discovered by multichannel biochemical screening: clinical course and considerations bearing on the need for surgical intervention. J Bone Miner Res 6(Suppl 2):S97–S101 [DOI] [PubMed] [Google Scholar]
- Elvius M, Lagrelius A, Nygren A, Alveryd A, Christensson TA, Nordenstrom J 1995 Seventeen year follow-up study of bone mass in patients with mild asymptomatic hyperparathyroidism some of whom were operated on. Eur J Surg161:863–869 [PubMed] [Google Scholar]
- Rao DS, Wilson RJ, Kleerekoper M, Parfitt AM 1988 Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: evidence for biphasic disease course. J Clin Endocrinol Metab 67:1294–1298 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Bilezikian JP 2003 “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab 88:5348–5352 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Silverberg SJ 2004 Clinical practice. Asymptomatic primary hyperparathyroidism. N Engl J Med 350:1746–1751 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Potts Jr JT, Fuleihan Gel H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA 2002 Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361 [DOI] [PubMed] [Google Scholar]
- Christiansen P, Steiniche T, Mosekilde L, Hessov I, Melsen F 1990 Primary hyperparathyroidism: changes in trabecular bone remodeling following surgical treatment–evaluated by histomorphometric methods. Bone 11:75–79 [DOI] [PubMed] [Google Scholar]
- Christiansen P, Steiniche T, Brixen K, Hessov I, Melsen F, Heickendorff L, Mosekilde L 1999 Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone 25:237–244 [DOI] [PubMed] [Google Scholar]
- Dalen N, Hjern B 1974 Bone mineral content in patients with primary hyperparathyroidism without radiological evidence of skeletal changes. Acta Endocrinol (Copenh) 75:297–304 [DOI] [PubMed] [Google Scholar]
- Almqvist EG, Becker C, Bondeson AG, Bondeson L, Svensson J 2004 Early parathyroidectomy increases bone mineral density in patients with mild primary hyperparathyroidism: a prospective and randomized study. Surgery 136:1281–1288 [DOI] [PubMed] [Google Scholar]
- Rao DS, Phillips ER, Divine GW, Talpos GB 2004 Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 89:5415–5422 [DOI] [PubMed] [Google Scholar]
- Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, Oppo A, Miccoli P, Berti P, Bilezikian JP, Pinchera A, Marcocci C 2007 Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab 92:3114–3121 [DOI] [PubMed] [Google Scholar]
- Bollerslev J, Jansson S, Mollerup CL, Nordenstrom J, Lundgren E, Torring O, Varhaug JE, Baranowski M, Aanderud S, Franco C, Freyschuss B, Isaksen GA, Ueland T, Rosen T 2007 Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J Clin Endocrinol Metab 92:1687–1692 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP 2003 Bone strength in primary hyperparathyroidism. Osteoporos Int 14(Suppl 5):113–117 [DOI] [PubMed] [Google Scholar]
- Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK 2003 Bone loss and bone size after menopause. N Engl J Med 349:327–334 [DOI] [PubMed] [Google Scholar]
- Charopoulos I, Tournis S, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis GP 2006 Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab 91:1748–1753 [DOI] [PubMed] [Google Scholar]
- Roschger P, Dempster DW, Zhou H, Paschalis EP, Silverberg SJ, Shane E, Bilezikian JP, Klaushofer K 2007 New observations on bone quality in mild primary hyperparathyroidism as determined by quantitative backscattered electron imaging. J Bone Miner Res 22:717–723 [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Mollerup CL, Frokjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L 2000 Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ 321:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Wermers RA, Crowson CS, O'Fallon W, Riggs B 1999 Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res 14:1700–1707 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A 2002 Clinical presentation of primary hyperparathyroidism in Europe-nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res 17(Suppl 2):N68–N74 [PubMed] [Google Scholar]
- Wermers RA, Khosla S, Atkinson EJ, Grant CS, Hodgson SF, O'Fallon WM, Melton 3rd LJ 1998 Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med 104:115–122 [DOI] [PubMed] [Google Scholar]