Abstract
Context: Mild primary hyperparathyroidism (PHPT) is characterized by asymptomatic hypercalcemia, most commonly in the absence of classical signs and symptoms. Hence, there is need to characterize this disorder with particular attention to the skeleton.
Design: We determined the ratio of pyridinium and dehydrodihydroxylysinonorleucine collagen cross-links in 46 iliac crest bone biopsies from patients with PHPT (14 men, aged 28–68 yr; 32 women, aged 26–74 yr) by Fourier transform infrared imaging. The results were compared with previously reported collagen cross-links ratio determined in iliac crest biopsies from normal subjects.
Results: PHPT patients exhibited significantly lower pyridinium to dehydrodihydroxylysinonorleucine collagen cross-links ratio, compared with normal controls. Parathyroidectomy restored values to those comparable with normal controls. Moreover, the differences among PHPT subjects were gender dependent, with female PHPT patients having a statistically significant lower ratio, compared with either male PHPT patients or normal controls. Comparison of the obtained outcomes with histomorphometry showed that the collagen cross-link ratio was strongly correlated with rate of bone formation, and mineralizing surface, in individual patients. This ratio was also correlated with bone mineralization density distribution parameters obtained in the same patients. The strongest correlations were with bone mineralization density distribution variables reflecting heterogeneity of mineralization and primary mineralization parameters.
Conclusions: The results are consistent with the high turnover state manifested in PHPT patients. Reduced collagen cross-link ratio in patients with PHPT would be expected to reduce the stiffness of bone tissue. These observations provide a more complete assessment of bone material properties in this disorder.
In patients with mild primary hyperparathyroidism, the collagen cross-link ratio (pyr and deH-DHLNL), as measured by Fourier transformed infrared imaging, was lower, consistent with the high bone turnover state in these patients.
Mild primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by excessive secretion of PTH and hypercalcemia (1,2,3). Most commonly, PHPT presents as asymptomatic hypercalcemia in which the serum calcium concentration is typically within 1 mg/dl above the normal limits. In contrast to the older, classical presentation of PHPT, overt renal complications are uncommon and radiological skeletal signs are rare. Nevertheless, bone densitometry and analysis of histomorphometric indices by the bone biopsy reveal abnormalities in both cortical and trabecular bone. Typically, the cortex is thin, but cancellous bone volume is preserved and some microarchitectural variables are maintained or improved. Dynamic indices of bone formation and resorption confirm a high turnover state (4,5,6,7,8,9,10). The dual actions of PTH to resorb endocortical bone and deposit new bone at the periosteal surface lead to bone that, despite have a thinner cortical shell, is greater in cross-sectional diameter (11,12).
Bone strength depends on bone quantity and bone quality. Elements of bone quality are multifactorial, including not only geometry and microarchitecture but also material properties of bone such as crystal structure, collagen, and microdamage (13). Type I collagen, the principal component of the organic matrix, is a large fibrous protein with a highly repetitive amino acid sequence [Gly (glycine) − X − Y]n, where X and Y depict proline and hydroxyproline, respectively (14). This repetitive sequence allows three polypeptide chains (two α1- and one α2-chains) to fold into a unique triple-helical structure. The most distinct feature of type I collagen in mineralized tissue can be seen in its cross-linking chemistry and molecular packing structure. Intermolecular cross-linking endows the fibrillar matrices with various mechanical properties such as tensile strength and viscoelasticity (14).
Fourier transform infrared imaging analysis (FTIRI) is a validated spectroscopic technique that measures the ratio of pyridinium (pyr; nonreducible) to dehydrodihydroxylysinonorleucine (deH-DHLNL; reducible) collagen cross links in mineralized sections of bone at about 6.3 μm spatial resolution (15). The technique, which gives information about the relative maturity of bone, has been used to discern differences between normal and osteoporotic bone (15,16,17). In the current study, FTIRI analysis was performed on thin tissue sections obtained from subjects with PHPT to determine the pyr to deH-DHLNL collagen cross-link ratio, an index reflecting tissue age and collagen maturation (14,15). The results provide new information on material properties of collagen in this disorder.
Subjects and Methods
Study subjects
The study was approved by the Institutional Review Boards of Columbia University and the Helen Hayes Hospital, and all subjects provided written informed consent. Percutaneous transiliac bone biopsies were obtained from 46 unselected subjects with mild PHPT. Their clinical characteristics and histomorphometric profiles have been published elsewhere in detail (5,7,8,9,10). Although biopsies were not obtained from all 116 subjects in this study cohort, the individuals from whom biopsies were obtained were indistinguishable from the entire group in terms of demographic features (age, gender distribution, racial distribution), serum calcium, PTH levels, or bone mineral density (BMD). Thirty-nine of the biopsies were from untreated patients. Two patients were biopsied both before and approximately 3 yr after parathyroidectomy (PTX), and seven patients, including the two with the 3-yr paired biopsies, were biopsied after an average of 6 yr after PTX. As expected from current guidelines for surgery in asymptomatic PHPT, patients who underwent PTX were younger and had higher serum calcium levels and lower T-scores than did those who were followed up medically. Forty biopsies were obtained from patients followed up without surgery. All biopsies were fixed and dehydrated in ethanol, embedded in polymethylmethacrylate, and processed as described earlier for histomorphometric analysis (5,7,8,9,10). For comparison purposes, a set of normal (based on either histological/histomorphometric evaluations, or a normal clinical evaluation including history, physical, laboratory, and x-ray examinations) human iliac crest biopsies was also analyzed (age range 51–70 yr, six males, 16 females) to provide a normative reference. We already reported on a cohort from this group (16). Because the published analysis involved line scans extracted from spectral images, the acquired FTIRI were reprocessed as outlined below.
Histomorphometry
Bone histomorphometry was performed as previously described (5,7,8,9,10). All variables were calculated and expressed according to the guidelines of the American Society for Bone and Mineral Research Nomenclature Committee (18).
FTIRI Analyses
Two thin tissue sections (∼4 μm) were taken from each biopsy block. For FTIRI analysis, trabeculae were selected so that they were devoid of resorption pits and one of the surfaces exhibited primary mineralization. Based on these selection criteria, the data are directly comparable with previously published studies (16,17). In all instances, three trabeculae per section were analyzed.
FTIRI data acquisition and spectral image analysis
Spectral images were obtained on a Bruker Equinox 55 (Bruker Optics, Ettlingen, Germany) spectrometer interfaced to a mercury cadmium telluride focal plane array detector (64 × 64 array) imaged onto the focal plane of an infared microscope (Hyperion 3000; Bruker Optics). Each area imaged was 400 × 400 μm, corresponding to an optimal spatial resolution of about 6.3 × 6.3 μm. Spectral resolution was 8 cm−1. Background spectral images were collected under identical conditions from the same BaF2 windows at the beginning and end of each experiment to ensure instrument stability. The spectrometer was continuously powered to minimize warm-up instabilities and purged with dry air (Bruker Optics) to minimize the water vapor and CO2 interference.
For FTIRI analysis, spectral images were acquired of three areas (each 400 × 400 μm) on separate trabeculae devoid of resorption pits and with primary mineralization evident on at least one surface. After acquisition, spectral images were transferred to an off-line computer (Precision 650; Dell, Austin, TX), and were zero corrected for the baseline in the spectral areas of Amide I and II (∼1490–1700 cm−1). Water vapor and polymethylmetacrylate spectral interference was corrected as published elsewhere (15). The relative ratio of pyr and deH-DHLNL collagen cross-links was expressed as the absorbance ratio at two specific wavelengths (1660 and 1690 cm−1) (15). For each section analyzed, absorbance ratios were expressed as color-coded images (using the same scale for all data; Sigmaplot 10.0; Systat Software, Inc., San Jose, CA) to depict spatial distribution of outcomes. In the spectral images, pixels devoid of bone (no mineral and/or matrix spectral signature) were set equal to zero and excluded from calculations.
Statistical analyses
For imaging analyses, three trabeculae per section were analyzed. For each section, the average of these values was treated as a single value for calculation of the group mean (± sd) data. The tests used for the various comparisons included paired and unpaired t test, ANOVA with Bonferroni’s multiple comparisons test, and nonparametric correlation (Spearman) test (Prism 4.0; GraphPad Software Inc., San Diego, CA). Differences were considered statistically significant at P < 0.05 on a two-tailed test.
Results
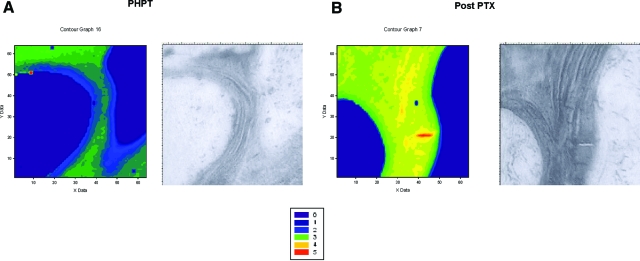
Figure 1 shows typical spectral images from a patient with PHPT before (Fig. 1A) and after PTX (Fig. 1B). An increase in the pyr to deH-DHLNL collagen cross-link ratio was observed after PTX (Fig. 1B).
Figure 1.
Spatial distribution of the pyr to deH-DHLNL collagen cross-link ratio in a subject with PHPT before (A) and 3 yr after PTX (B). In each case, the left panel is the FTIRI image, whereas the right panel is a light micrograph obtained through the camera attached to the FTIR microscope. The color-coded scale for the FTIRI spectral images is provided. The collagen cross-link ratio increased after PTX. The images shown are representative of the data set for each group (see Fig. 2).
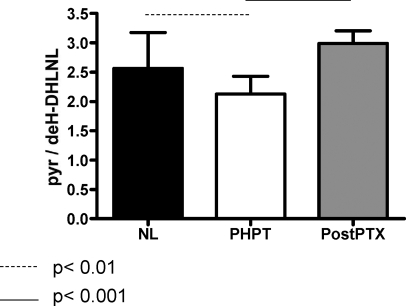
Data for the group means are plotted in Fig. 2 and evaluated for statistical significance using ANOVA with Bonferroni’s multiple comparison test. PHPT subjects had significantly lower cross-link ratio (P < 0.01) than the normal group (NL). In the post-PTX group (PTX), the cross-link ratio was higher than in PHPT (P < 0.001) and was indistinguishable from the NL group.
Figure 2.
Collagen cross-link ratio by FTIRI in NL untreated PHPT and after surgery (PostPTX). For each group, the mean and sd are plotted. Bonferroni’s multiple comparison test revealed that the PHPT group had a lower collagen cross-link ratio than NL, whereas this was reversed in the PostPTX group.
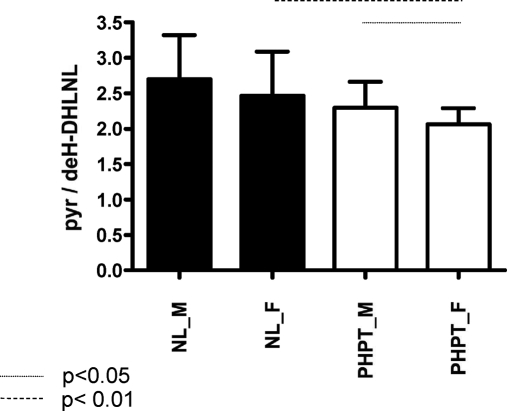
Gender differences were seen in the PHPT subjects (Fig. 3). Whereas there was no difference between the NL male and female groups, there was a significant difference (P < 0.01) between the NL female and PHPT female subjects, with the latter exhibiting lower collagen cross-link ratio values. There was also a significantly lower cross-link ratio (P < 0.05) in PHPT female subjects when they were compared with PHPT male subjects. These differences were not dependent on the age of the male and female subjects (data not shown).
Figure 3.
Dependence of the collagen cross-link ratio in NL and PHPT groups as a function of gender [male (M) and female (F) subjects]. The group means and sds are plotted. Unpaired t test comparisons revealed that whereas there was no difference between the NL-M and NL-F groups, there were statistical differences (P < 0.01) between the NL-F and PHPT-F groups as well as between the M and F PHPT groups.
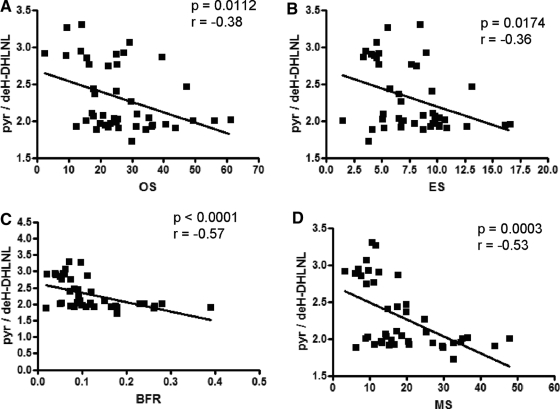
Comparison of classical histomorphometric features with FTIRI analyses on the same biopsy specimens (Fig. 4) demonstrated that collagen cross-link ratio correlated significantly with osteoid surface (Spearman r = −0.38, P = 0.0112), eroded surface (Spearman r = −0.357, P = 0.0174), bone formation rate (Spearman r = −0.57, P < 0.0001), and mineralizing surface (Spearman r = −0.53, P = 0.0003).
Figure 4.
Correlation in the same patient biopsies between FTIRI and histomorphometric variables in PHPT. Histomorphometric variables include osteoid surface (OS), eroded surface (ES), bone formation rate (BFR), and mineralizing surface (MS). Nonparametric correlation analysis showed the collagen cross-link ratio in these patients is strongly correlated with MS and BFR.
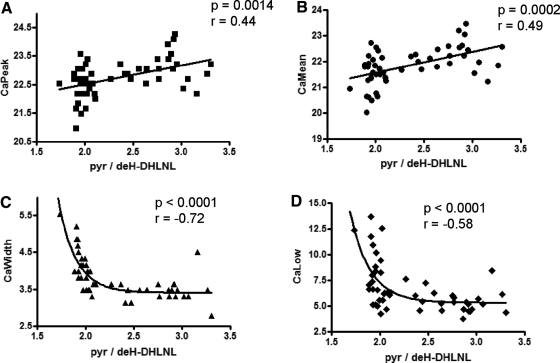
Measurements of bone mineralization density distribution (BMDD) in PHPT, as recently reported (19), are also available on the same biopsies on which the FTIRI analyses were made. As shown in Fig. 5, the FTIRI-derived collagen cross-links ratio was correlated with four major outcomes of BMDD analysis. The collagen cross-link ratio correlated significantly with CaPeak (Spearman r = 0.44, P = 0.0014) and CaMean (Spearman r = 0.49, P = 0.0002) and was strongly correlated with CaWidth (Spearman r = −0.73, P < 0.0001), and CaLow (Spearman r = −0.58, P < 0.0001). Interestingly, whereas the best fit for the correlation between collagen cross-link ratio and CaMean and CaPeak was linear, it was one phase decay when CaWidth (Y0 = 28835, plateau = 3.406, K = 5.452, half-life = 0.1271, τ = 0.1834, span = 28832, r2 = 0.61) and CaLow (Y0 = 32386, plateau = 5.311, K = 4.859, half-life = 0.1426, τ = 0.2058, span = 32381, r2 = 0.40) were considered.
Figure 5.
Correlation in the same patient biopsies between FTIRI and qBEI analyses. The quantitative backscattered electron imaging indices include CaMean, CaPeak, CaWidth, and CaLow. Nonparametric correlation analysis showed that the strongest correlations were between the collagen cross-link ratio and CaWidth and CaLow. K, Decay rate constant; Ca, calcium.
Discussion
Guidelines for the management of asymptomatic PHPT are based on readily measurable indices that are thought to be relevant to potential clinical complications of PHPT (20,21). BMD by dual-energy x-ray absorptiometry, for example, is used as an index of fracture risk. The guidelines recommend surgery in asymptomatic patients if their T-score by dual-energy x-ray absorptiometry is less than −2.5 at any site. However, it is not known whether BMD predicts fracture risk in PHPT as well as it does in postmenopausal women. The uncertainty is due to other skeletal properties in PHPT in which bone size and microarchitecture could favor a reduction in fracture risk. Without prospective fracture incidence studies, one has to rely on these surrogate markers of bone strength, despite the fact that mechanical variables that directly relate to fracture risk are either independent of (22) or not totally accounted for by bone mass (23,24,25,26,27). Epidemiological evidence also shows considerable overlap in BMD values between those who do and do not sustain fragility fractures, suggesting that other properties of bone are important measures that help to define bone quality (28,29). It is becoming evident then, that in addition to BMD, other aspects of bone quality should be considered in the overall assessment of bone strength. Examples of the properties of bone that contribute to bone strength include bone size, bone turnover, microarchitecture, and other factors (4,5,7,13,30). Abnormal biomechanical properties of bone due to altered collagen cross-links and structure have been described in osteogenesis imperfecta, lathyrism, and certain models of pyridoxine deficiency (31,32,33,34,35,36,37,38).
The most distinct feature of type I collagen in mineralized tissues can be seen in its cross-linking chemistry and molecular packing structure (14). All the known cross-links of type I collagen are condensation products between the prosthetic groups of juxtaposed specific peptidyl residues of lysine, hydroxylysine, and histidine. The process of cross-linking is initiated by the enzymatic oxidative deamination by lysyl oxidase of ε-amino groups on specific peptidyl lysine and hydroxylysine to aldehyde. The aldehydes formed then undergo a series of condensation reactions to form complex intra- and intermolecular cross-links in the fibril. The minimum divalent intermolecular cross-links seem to be the first to form and then mature into more complex multivalent cross-links. Because cross-link condensation reactions, except for the initial oxidation step, are spontaneous, turnover rate is an important factor in regulating cross-link maturation. At present, seven major naturally occurring intermolecular collagen cross-links have been established (14). FTIRI analysis of human iliac crest biopsies has shown abnormal collagen cross-linking in patients with fragility fractures (17).
In the present study, we used FTIRI analysis of iliac crest biopsies obtained from untreated PHPT patients and those who underwent successful parathyroidectomy. We calculated the ratio of the two major bone collagen cross-links, pyr to deH-DHLNL. In PHPT, this ratio was lower than in normal human subjects, an observation consistent with the high turnover characteristics of these patients as determined by histomorphometry and descriptive of a less mature collagen phenotype. After successful PTX, the ratio increased to values statistically similar to normal subjects. Women with PHPT had lower ratios than men with PHPT. Because younger women were similar to older women, the menopause cannot account for these observations. At present we do not have a plausible explanation for this gender discrepancy. The collagen cross-link ratio in the PHPT group was strongly correlated with mineralizing surface and bone formation rate as determined by histomorphometry.
Interestingly, the opposite trend was observed in the case of high turnover osteoporosis (17), strongly suggesting that posttranslational modifications of collagen are not solely dependent on bone turnover. In osteoporosis, several clinical studies have shown that fractures are associated with homocysteine levels (a well known lathyrogen, which interferes with posttranslational modifications of collagen) (39,40). Thus, although in PHPT collagen quality is dependent on mean bone tissue age, this may not be the case in high-turnover osteoporosis. All four parameters of mineralization density (BMDD) were also associated with the cross-link ratio by FTIRI, supporting again the idea that the high turnover state affects bone quality in PHPT.
The results are consistent with previous observations of increased bone turnover in PHPT and, consequently, reduced mean age of bone tissue. Reduced mineralization density and collagen cross-links ratio (pyr to deH-DHLNL) in patients with PHPT would be expected to reduce the stiffness of bone tissue. Because there are no prospective studies of fracture risk in PHPT, it is not clear how these new observations will relate to the incidence of fracture in this disorder. In the absence of prospective data, one must take into account data on collagen quality, as reported here; BMD as reported previously (19); bone size; and microarchitectural features of bone in PHPT as well as BMDD (41,42,43,44). Together, these properties of bone in PHPT are nevertheless providing a more complete assessment of bone material properties in this disorder.
It is not clear how these observations in the hypercalcemic presentation of PHPT will relate to a new phenotype of PHPT that has recently been observed in which PTH levels are elevated, without a secondary cause, in the absence of frank elevations in the serum calcium concentration (45).
Acknowledgments
We thank Gerda Dinst, Sabrina Thon, Daniela Gabriel, and Phaedra Messmer for careful sample preparations for FTIRI measurements at the bone material laboratory of the Ludwig Boltzmann Institute of Osteology (Vienna, Austria).
Footnotes
This work was supported by the Austrian Social Insurance for Occupational Risk, the Social Health Insurance Vienna, the Austrian Science Fund (FWF) Project P16880-B13 (to P.R., K.K.), and National Institutes of Health Grant DK 32333 (to J.P.B., D.W.D., S.J.S., and E.S.).
Disclosure Statement: None of the authors has anything to declare.
First Published Online July 1, 2008
Abbreviations: BMD, Bone mineral density; BMDD, bone mineralization density distribution; deH-DHLNL, dehydrodihydroxylysinonorleucine; FTIRI, Fourier transform infrared imaging analysis; NL, normal group; PHPT, primary hyperparathyroidism; PTX, parathyroidectomy; pyr, pyridinium.
References
- Arnold A 2006 Familial hyperparathyroid syndromes. In: Favus M, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research; 185–188 [Google Scholar]
- Bilezikian J, Silverberg S 2006 Primary hyperparathyroidism. In: Favus M, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research; 181–185 [Google Scholar]
- Prince R 2006 Secondary and tertiary hyperparathyroidism. In: Favus M, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research; 190–195 [Google Scholar]
- Charopoulos I, Tournis S, Trovas G, Raptou P, Kaldrymides P, Skarandavos G, Katsalira K, Lyritis G 2006 Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab 91:1748–1753 [DOI] [PubMed] [Google Scholar]
- Dempster D, Parisien M, Silverberg S, Liang X-G, Schnitzer M, Shen V, Shane E, Kimmel D, Recker R, Lindsay R, Bilezikian J 1999 On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 84:1562–1566 [DOI] [PubMed] [Google Scholar]
- Eriksen E 2002 Primary hyperparathyroidism: lessons from bone histomorphometry. J Bone Miner Res 17:N95–N97 [PubMed] [Google Scholar]
- Parisien M, Cosman F, Mellish R, Schnitzer M, Nieves J, Silverberg S, Shane E, Kimmel D, Recker R, Bilezikian J, Dempster D 1995 Bone structure in postmenopausal hyperparathyroid, osteoporotic and normal women. J Bone Miner Res 10:1393–1399 [DOI] [PubMed] [Google Scholar]
- Parisien M, Mellish R, Silverberg S, Shane E, Lindsay R, Bilezikian J, Dempster D 1992 Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res 7:913–919 [DOI] [PubMed] [Google Scholar]
- Parisien M, Silverberg S, Shane E, De La Cruz L, Lindsay R, Bilezikian J, Dempster D 1990 The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab 70:930–938 [DOI] [PubMed] [Google Scholar]
- Silverberg S, Shane E, De La Cruz L, Dempster D, Feldman F, Seldin D, Jacobs T, Siris E, Cafferty M, Parisien M, Lindsay R, Clemens T, Bilezikian J 1989 Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4:283–281 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton JI 2002 Fracture risk in primary hyperparathyroidism. J Bone Miner Res 17:N103–N107 [PubMed] [Google Scholar]
- Vestergaard P, Mosekilde L 2003 Fractures in patients with primary hyperparathyroidism: nationwide follow-up study of 1201 patients. World J Surg 27:343–349 [DOI] [PubMed] [Google Scholar]
- Fratzl P, Gupta H, Paschalis E, Roschger P 2004 Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 14:2115–2123 [Google Scholar]
- Yamauchi M 1996 Collagen: The major matrix molecule in mineralized tissues. In: Anderson JJB, Garner SC, eds. Calcium and phosphorus in health and disease. New York: CRC Press; 127–141 [Google Scholar]
- Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M 2001 Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res 16:1821–1828 [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Recker R, DiCarlo E, Doty SB, Atti E, Boskey AL 2003 Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res 18:1942–1946 [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL 2004 Bone fragility and collagen cross-links. J Bone Miner Res 19:2000–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, Ott S, Recker R 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Roschger P, Dempster D, Zhou H, Paschalis E, Silverberg S, Shane E, Bilezikian J, Klaushofer K 2007 New observations on bone quality in mild primary hyperparathyroidism as determined by quantitative backscattered electron imaging. J Bone Miner Res 22:717–723 [DOI] [PubMed] [Google Scholar]
- 2005 Hyperparathyroidism AATFoP 2005. The American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract 11:49–54 [DOI] [PubMed] [Google Scholar]
- Bilezikian J, Potts J, Fuleihan G-H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg S, Udelsman R, Wells S 2002 Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361 [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Schaffler MB Bone mass does not adequately predict variations in bone fragility: a genetic approach. Proc 47th Annual Meeting of the Trans Orthop Res Soc, San Francisco, CA, 2001, (Abstract 114) [Google Scholar]
- Kanis JA, Melton LJI, Christiansen C, Johnston CJ, Haltaev N 1994 Perspective: the diagnosis of osteoporosis. J Bone Miner Res 9:1137–1142 [DOI] [PubMed] [Google Scholar]
- Kann P, Graeben S, Beyer J 1994 Age-dependence of bone material quality shown by the measurement of frequency of resonance in the ulna. Calcif Tissue Int 54:96–100 [DOI] [PubMed] [Google Scholar]
- McCabe F, Zhou LJ, Steele CR, Marcus R 1991 Noninvasive assessment of ulnar bending stiffness in women. J Bone Miner Res 6:53–59 [DOI] [PubMed] [Google Scholar]
- Mosekilde L, Mosekilde L, Danielsen CC 1987 Biomechanical competence of vertebral trabecular bone in relation to ash density and age in normal individuals. Bone 79–85 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 1987 Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol 30:789–811 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P, et al. 1990 Appendicular bone density and age predict hip fracture in women: the study of osteoporotic fractures research group. JAMA 263:665–668 [PubMed] [Google Scholar]
- Ott SM 1993 When bone mass fails to predict bone failure. Calcif Tiss Int 53(Suppl):S7–S13 [DOI] [PubMed] [Google Scholar]
- McCreade RB, Goldstein AS 2000 Biomechanics of fracture: is bone mineral density sufficient to assess risk? J Bone Miner Res 15:2305–2308 [DOI] [PubMed] [Google Scholar]
- Phillips CL, Bradley DA, Schlotzhauer CL, Bergfeld M, Libreros-Minotta C, Gawenis LR, Morris JS, Clarke LL, Hillman LS 2000 Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone 27:219–226 [DOI] [PubMed] [Google Scholar]
- Rauch F, Travers R, Parfitt AM, Glorieux FH 2000 Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 26:581–589 [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Schaffler MB, Kuhn JL, Goulet RW, Bonadio J, Goldstein SA 1997 Type I collagen mutation alters the strength and fatigue behavior of Mov13 cortical tissue. J Biomech 30:1141–1147 [DOI] [PubMed] [Google Scholar]
- Cassella JP, Pereira R, Prockop DJ, Ali SY 1996 Mineral changes in a transgenic mouse model for osteogenesis imperfecta. Br J Biomed Sci 53:108–115 [PubMed] [Google Scholar]
- Pereira RF, Hume EL, Halford KW, Prockop DJ 1995 Bone fragility in transgenic mice expressing a mutated gene for type I procollagen (COL1A1) parallels the age-dependent phenotype of human osteogenesis imperfecta. J Bone Miner Res 10:1837–1843 [DOI] [PubMed] [Google Scholar]
- Marion MJ, Gannon FH, Fallon MD, Mennuti MT, Lodato RF, Kaplan FS 1993 Skeletal dysplasia in perinatal lethal osteogenesis imperfecta. A complex disorder of endochondral and intramembranous ossification. Clin Orthop 327–337 [PubMed] [Google Scholar]
- Alman B, Frasca P 1987 Fracture failure mechanisms in patients with osteogenesis imperfecta. J Orthop Res 5:139–143 [DOI] [PubMed] [Google Scholar]
- Oxlund H 1986 Relationships between the biomechanical properties, composition and molecular structure of connective tissues. Connect Tissue Res 15:65–72 [DOI] [PubMed] [Google Scholar]
- McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP 2004 Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049 [DOI] [PubMed] [Google Scholar]
- Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS 2007 Plasma homocysteine, folate and vitamin B12 and the risk of hip fracture. The Hordaland Homocysteine Study. J Bone Miner Res 22:747–756 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Müller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP 2007 Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 41:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SJ, Bilezikian JP 2006 Primary hyperparathyroidism. In: Seibel MJ, Robins SP, Bilezikian JP, eds. Dynamics of bone and cartilage metabolism. San Diego: Elsevier Press 767–778 [Google Scholar]
- Bilezikian JP 2007 Primary hyperparathyroidism. In: DeGroot L, ed; Arnold A, section ed. www.endotext.org (version of January 1, 2007) (mdtext.com, Inc.) [Google Scholar]
- Bilezikian JP, Fitzpatrick LA, Silverberg SJ 2007 The skeletal actions of parathyroid hormone in primary hyperparathyroidism and in osteoporosis. In: Marcus R, Feldman D, Nelson D, Rosen C, eds. Osteoporosis. 3d ed. San Diego: Elsevier; 1227–1246 [Google Scholar]
- Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ 2007 Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab 92:3001–3005 [DOI] [PubMed] [Google Scholar]