Abstract
Background
This study investigated basal and stress-induced HPA axis alterations in dissociative disorders (DD).
Methods
Forty-six subjects with DD without lifetime PTSD, 35 subjects with PTSD, and 58 HC subjects, free of current major depression, were studied as inpatients. After a 24-hour urine collection and hourly blood sampling for ambient cortisol determination, a low-dose dexamethasone suppression test was administered, followed by the Trier Social Stress Test.
Results
The DD group had significantly elevated urinary cortisol compared to the HC group, more pronounced in the absence of lifetime major depression, whereas the PTSD and HC groups did not differ. The DD group demonstrated significantly greater resistance to, and faster escape from, dexamethasone suppression compared to the HC group, whereas the PTSD and HC groups did not differ. The three groups did not differ in cortisol stress reactivity, but both psychiatric groups demonstrated a significant inverse correlation between dissociation severity and cortisol reactivity, after controlling for all other symptomatology. The PTSD subgroup with comorbid DD tended to have blunted reactivity compared to the HC group.
Conclusions
The study demonstrates a distinct pattern of HPA axis dysregulation in DD, emphasizing the importance of further study of stress response systems in dissociative psychopathology.
Keywords: cortisol, HPA axis, neuroendocrinology, dissociative disorders, PTSD, stress
Introduction
Dissociative disorders (DD), along with PTSD, comprise a major group of psychiatric disorders whose pathogenesis is intimately linked to traumatic stress. About one-third of seriously victimized children followed prospectively into adulthood manifest lifetime PTSD (Widom 1999); dissociative conditions are at least as common a sequel to such victimization (Putnam 1985; Chu and Dill 1990), or a complex admixture of both types of symptoms can occur (Zlotnick et al 1996). Although frequently overlapping or alternating, “dissociative” versus “posttraumatic stress” states at their purest present with “opposite” phenomenological patterns (amnesia, depersonalization, and decreased arousal in DD versus intrusions, hypermnesia, and hyperarousal in PTSD), implying distinct and possibly contrasting neurobiological underpinnings.
The hypothalamic-pituitary-adrenal (HPA) axis plays a central role in the regulation of the stress response. The studies of pituitary-adrenocortical hormones that have been conducted in stress-spectrum disorders involve mostly PTSD, major depression, and non-psychiatric individuals with trauma histories (Yehuda 1997; Yehuda et al 1990; Young and Breslau 2004a; Bremner et al 1997; Smith et al 1989; Bremner et al 2003; Yehuda et al 1993; Sachar et al 1970; Carroll 1982; Heim et al 2001). There has been very limited investigation of individuals manifesting primarily with dissociative psychopathology, and the few HPA axis studies in DD are largely confounded by comorbidity. A study of 19 adult women with childhood sexual abuse reported hypersuppression in response to low-dose dexamethasone, (Stein et al 1997); however 13 of the women had PTSD and 15 had DD, not permitting a dissection of the two diagnoses. On the other hand, a study of adult Dissociative Identity Disorder patients with extensive PTSD comorbidity reported resistance to dexamethasone suppression (Vermetten et al 2003). The latter finding is similar to a report of elevated ambient cortisol and resistance to low-dose dexamethasone in a dissociative sample of 9 depersonalization disorder subjects free of PTSD and current major depression (Simeon et al 2001).
Animal studies demonstrate that HPA axis responsivity to acute stressors is altered in animals that have previously been stressed. Adult rats subjected to neonatal maternal separation secrete increased ACTH in response to mild foot shock stress and have increased hypothalamic CRF concentrations (Ladd et al 1996). However, hyperreactivity is not the ubiquitous stress response in previously stressed animals; chronically severely stressed subordinate male rats have elevated ambient cortisol levels compared to dominant rats, yet in response to novel stressors some subordinate rats have a heightened while others have a blunted cortisol response (McKittrick et al 1995).
Human studies of HPA axis responsivity to psychosocial stress in trauma-spectrum disorders have generally revealed heightened reactivity. In a study of adult women, those with a history of childhood abuse, with or without current major depression, demonstrated heightened ACTH responsivity to psychosocial stress, while cortisol reactivity was heightened only in the abused group with major depression; many participants had PTSD comorbidity (Heim et al 2000). Similarly, adult women with abuse-related PTSD showed greater cortisol reactivity to trauma scripts during anticipation, exposure and recovery compared to control subjects (Elzinga et al 2003).
To our knowledge, there are no studies examining acute HPA axis reactivity to psychosocial stress as a function of dissociative symptoms. However, there is evidence of autonomic blunting to stressful stimuli in acute and chronic dissociative states which suggests that dissociative individuals are less acutely reactive to traumatic stress. In the acute aftermath of rape, women high in dissociation were reported to have decreased heart rates and galvanic skin responses (Griffin et al 1997).21 In the acute aftermath of motor vehicle accidents, 15-hour urine norepinephrine and epinephrine were inversely correlated with severity of peritraumatic dissociation (Delahanty et al. 2003). With respect to chronic dissociative symptoms, a sample of depersonalization disorder participants, compared to anxiety disorder and healthy controls, was characterized by reduced magnitude and increased latency of skin conductance responses to unpleasant stimuli (Sierra et al. 2002). Similarly in depersonalization disorder, 24-hour urine norepinephrine was strongly inversely correlated (r=−0.88) with depersonalization severity (Simeon et al. 2003).
The goal of the current study was to conduct the first extensive systematic investigation of HPA axis function in dissociative disorders. We predicted that the DD group would have elevated ambient cortisol compared to healthy volunteers whereas the PTSD group would have decreased ambient cortisol; that the DD group would demonstrate resistance to low-dose dexamethasone challenge whereas the PTSD group would show hypersuppression; and that the DD group would show blunted cortisol reactivity to psychosocial stress compared to controls, whereas the PTSD group would show heightened reactivity. We also predicted that dissociation severity would be associated with greater childhood trauma, elevated basal cortisol, resistance to dexamethasone suppression, and blunted cortisol stress reactivity.
Methods and Materials
Participants
Subjects were recruited into three groups, a dissociative disorder (DD) group free of lifetime PTSD, a PTSD group, and a healthy comparison (HC) group free of lifetime Axis I and II disorders. Participants were 18–60 years old. They were recruited via newspaper advertisements and postings or were self-referred via internet websites and other resources. Exclusion psychiatric criteria for DD and PTSD subjects were current major depression, eating disorder, or substance use disorder, and lifetime psychotic or bipolar I disorder. Subjects were medically and neurologically healthy; had no history of head trauma; had normal baseline physical examination and routine laboratory testing; were not taking any medications including psychotropic medications for at least 2 months; had negative urine toxicology testing; and had negative pregnancy testing. Cigarette smokers of more than 5 daily cigarettes were excluded. Women were tested irrespective of menopausal status and menstrual cycle timing. No over-the-counter medications or supplements were allowed for at least 3 days prior to admission. The study was approved by the institution’s review board, and all subjects signed written informed consent prior to evaluation.
Measures
Subjects were diagnostically evaluated with the Structured Clinical Interview for DSM-IV Dissociative Disorders (Steinberg 1994), the Structured Clinical Interview for DSM-IV Axis I disorders (First et al 2002), and the Structured Interview for DSM-IV Personality Disorders (Pfohl et al 1995). PTSD subjects were also evaluated with the Clinician-Administered PTSD Scale CAPS (Blake et al 1995), both to establish PTSD diagnosis and to measure symptom severity.
Subjects completed the Dissociative Experiences Scale DES (Bernstein-Carlson and Putnam 2003), a 28-item self-report measure assessing a wide range of dissociative symptoms. Items are responded to in 10% increments ranging from 0% to 100%, and total DES score is the mean of all items. The DES has good test-retest reliability (0.79–0.96), high internal consistency (Cronbach’s α 0.95), and strong convergent, discriminant and criterion validity. Participants also completed the Hamilton Rating Scale for Depression HAM-D (Hamilton 1960), the Hamilton Rating Scale for Anxiety HAM-A (Hamilton 1959), and the Liebowitz Social Anxiety Scale LSAS (Heimberg et al. 1999).
Two trauma measures were given, the clinician-administered Childhood Trauma Interview CTI (Fink et al 1995) and the self-report Childhood Trauma Questionnaire CTQ–short version (Bernstein et al 2003). The CTI is a detailed interview of childhood interpersonal trauma which quantifies frequency, duration, age range, severity, and perpetrator types for separations/losses, physical neglect, emotional abuse, physical abuse, witnessing violence and sexual abuse; it has high interrater reliability and good construct validity. The CTQ-short consists of 25 items rated on a 5-point scale; it has high internal consistency (Cronbach’s alpha 0.95), good test-retest reliability (ICC=0.88), and good convergence with the CTI. Five questions pertain to each of 5 types of trauma, emotional abuse, emotional neglect, physical neglect, physical abuse and sexual abuse, and confirmatory factor analysis has supported its latent structure (Bernstein et al 2003).
GCRC Procedures
Subjects were admitted to the Mount Sinai GCRC from Day 1, 6pm to Day 3, 4pm. Subjects ate standardized meals at fixed hours (8am, 12pm, 6pm), and were allowed low-level ambulatory activity or bed rest during daytime hours. Sleeping time was set at 11pm to 7:30am. Cigarette smoking was not allowed. An intravenous catheter lock was placed in subjects’ forearms by 8pm of Day 1, and a 24-hour urine collection commenced at 10pm. Starting at 8am on Day 2, 16 hourly serial blood samples were drawn for determination of ambient plasma cortisol levels. At 11pm subjects received a single oral 0.5 mg dexamethasone dose. The low rather than the standard 1mg dose was selected in order to better distinguish between increased and decreased suppression, facilitating distinction from the hypersuppression pattern that has been reported in PTSD (Yehuda et al 1993). On Day 3, plasma cortisol was measured at 8am and 2pm, with concomitant measurement of plasma dexamethasone levels.
At 2pm of Day 3, participants underwent a standardized psychosocial stress test, the Trier Social Stress Test (TSST), which has been shown to induce mild to moderate psychosocial stress in healthy individuals (Kirschbaum et al 1993). The TSST is a public performance test, and consisted of a 5-minute anticipation/preparation phase and a 10-minute presentation phase (speech and math task). Plasma cortisol levels were measured following the anticipation phase (2:10pm), following the presentation phase (2:20pm), and during recovery (2:50pm, 3:20pm). After TSST completion participants rated the stressor on a 7-point Likert scale (1–hardly any, 4-moderate, 7–worse ever).
Sample analyses
Blood samples were thoroughly mixed with anticoagulant and centrifuged at 3000 rpm for 15 min at 4°C. Plasma was transferred to plastic tubes and immediately frozen at −80°C. Urine samples collected during any 24-hour period were added to a 2-liter polyethylene container refrigerated for the duration of the collection. Subsequently the entire urine sample was mixed thoroughly, volume was recorded, and samples were transferred into aliquots and stored at −80°C. Samples were assayed for cortisol and dexamethasone using standard radioimmunoassay technique. For plasma cortisol, intra-assay coefficient of variation was 5% and inter-assay coefficient of variation was 7.5%, with a lower detection limit of 0.16 μg/dL. For urinary cortisol, intra-assay coefficient of variation was 8.4% and inter-assay coefficient of variation was 11.6%. For plasma dexamethasone, intra-assay coefficient of variation was 4.9% and inter-assay coefficient of variation was 5.9%.
Statistical analyses
Analyses of covariance were used to compare symptom and trauma scores between groups. Three sets of group comparisons were conducted for cortisol measures (DD-HC, PTSD-HC, DD-PTSD), as we were interested in differences between group pairs rather than in three-way patterns of comparison. Univariate or repeated-measures analyses of covariance were employed, as appropriate, all covarying for age, gender, and body mass index (BMI). The DST repeated-measures analyses employed the 8am and 2pm time points, covarying for 8am and 2pm dexamethasone levels and for pre-DST 8am and 2pm cortisol levels. The TSST repeated-measures analyses employed 4 time points (2:10pm, 2:20pm, 2:50pm, 3:20pm), covarying for 2pm cortisol levels. “Peak cortisol stress reactivity” was defined as the difference in plasma cortisol levels between 2:20pm and 2:00pm. Pearson’s correlations were used to examine relationships between dissociation severity and cortisol measures within each group; partial correlations were used to control for the impact of posttraumatic stress symptoms, depression, anxiety, and social anxiety.
The following secondary analyses were performed. To investigate the impact of lifetime major depression (LMD) on cortisol measures, we examined the PTSD and DD subgroups with and without LMD, using the analyses described above. To investigate the impact of DD comorbidity on PTSD findings, we examined the PTSD subgroups with and without DD, using the analyses described above.
Two PTSD subjects had missing time points for serial plasma cortisol determinations and were not included in this analysis. One DPD and 2 PTSD subjects were removed from the DST analyses due to undetectable 8am dexamethasone levels. One HC subject withdrew before the DST, and one HC subject withdrew before the TSST. One DD and two PTSD subjects did not complete the TSST. BMI was not available for 2 DD, 2 PTSD, and 3HC subjects. These missing data account for the minor discrepancies in sample sizes below.
Results
Demographic and Clinical Characteristics
A total of 139 subjects were recruited and participated in at least a portion of the biological study, 46 DD, 35 PTSD, and 58 HC subjects. Ethnic distribution was 57.6% White, 22.3% African-American, 5.8% Hispanic, 13.6% Asian, and 0.7% American Indian. Groups differed significantly in years of age [DD: 31.2 ± 10.3; PTSD: 41.5 ± 11.5; HC: 32.8 ± 10.8; F(2) = 10.28, p <0.001] but not gender [χ2(2) = 1.60, p = 0.45; DD: 26 women, 20 men; PTSD: 19 women, 16 men; HC: 26 women, 32 men].
Axis II personality disorders in the DD and PTSD groups were, respectively: paranoid 11% vs 29%; schizoid 15% vs 3%; schizotypal 2% vs 0%; antisocial 2% vs 0%: borderline 11% vs 29%; histrionic 7% vs 3%; narcissistic 2% vs 6%; avoidant 20% vs 26%; dependent 4% vs 3%; and obsessive-compulsive 35% vs 35%. The following index traumas characterized the PTSD group: accidents – 3, assaults – 3, witnessing violence – 4, fires – 2, military trauma – 3, adult rape – 3, September 11 – 2, traumatic separation – 1, traumatic death of close others – 5, childhood sexual abuse – 7, childhood physical abuse – 2.
Table 1 displays group comparisons for symptoms and childhood trauma. In the DD group, 36 participants had a diagnosis of depersonalization disorder (DPD) and 10 had Dissociative Disorder Not Otherwise Specified (DDNOS). DDNOS participants had significantly earlier age of disorder onset than DPD participants [DDNOS: 10.1 ± 8.7 years; DPD: 17.3 ± 9.0 years; t(44) = 2.26, p = 0.029]; higher dissociation scores [DDNOS: 43.2 ± 17.4; DPD: 25.0 ± 14.1; t(44) = 3.43, p = 0.001]; and greater childhood trauma scores [DDNOS: 56.3 ± 18.1; DPD: 40.5 ± 13.3; t(44) = 2.96, p = 0.005].
Table 1.
Clinical characteristics, psychopathology, and childhood trauma in the Dissociative Disorder, Posttraumatic Stress Disorder, and Healthy Comparison groups
| Variable | DD (n = 46) | PTSD (n = 35) | HC (n = 58) | p |
|---|---|---|---|---|
| Onset (age) | 15.7 ± 9.3 | 27.1 ± 14.2 | N/A | < 0.001 |
| Duration (years) | 15.5 ± 13.4 | 14.4 ± 13.1 | N/A | 0.73 |
| DES | 29.0 ± 16.5 | 23.4 ± 15.4 | 3.0 ± 2.8 | < 0.001 |
| CAPS | N/A | 66.7 ± 13.1 | N/A | N/A |
| HRSA | 12.0 ± 5.0 | 13.9 ± 5.5 | 2.0 ± 2.6 | < 0.001 |
| HRSD | 9.8 ± 5.0 | 11.5 ± 4.9 | 2.1 ± 1.6 | < 0.001 |
| LSAS | 43.2 ± 35.4 | 48.2 ± 44.8 | 6.9 ± 10.3 | < 0.001 |
| CTQ total | 43.7 ± 15.6 | 61.5 ± 26.1 | 33.8 ± 9.2 | < 0.001 |
| CTQ - physical abuse | 7.1 ± 3.2 | 11.0 ± 5.7 | 6.2 ± 2.5 | < 0.001 |
| CTQ – emotional abuse | 10.8 ± 5.1 | 14.0 ± 7.0 | 6.9 ± 2.5 | < 0.001 |
| CTQ – physical neglect | 7.5 ± 3.1 | 9.5 ± 4.3 | 6.3 ± 2.1 | < 0.001 |
| CTQ – sexual abuse | 6.2 ± 3.2 | 11.8 ± 8.2 | 5.6 ± 2.3 | < 0.001 |
| CTQ – Emotional neglect | 12.0 ± 5.2 | 15.1 ± 6.3 | 9.0 ± 3.8 | < 0.001 |
| CTI total | 487.2 ± 553.7 | 804.0 ± 655.0 | 236.4 ± 403.5 | < 0.001 |
| CTI – separations | 22.1 ± 21.6 | 20.5 ± 23.4 | 23.5 ± 24.6 | 0.84 |
| CTI – physical neglect | 64.5 ± 95.6 | 78.7 ± 137.8 | 16.2 ± 30.2 | 0.003 |
| CTI – emotional abuse | 190.5 ± 195.2 | 306.5 ± 280.9 | 85.9 ± 160.6 | < 0.001 |
| CTI – physical abuse | 87.5 ± 123.6 | 207.2 ± 229.8 | 52.5 ± 123.8 | < 0.001 |
| CTI – sexual abuse | 7.7 ± 25.9 | 28.2 ± 58.2 | 1.5 ± 5.8 | 0.001 |
| CTI – witnessing violence | 114.8 ± 274.0 | 163.0 ± 196.8 | 56.8 ± 133.8 | 0.057 |
In the PTSD group, 9 participants had comorbid DD (PTSD-DD) while 26 participants did not (PTSD-noDD). The two PTSD subgroups did not significantly differ in age [t(33) = 1.28, p = 0.21] or gender [χ2(1)=0.47, p = 0.49]. Comorbid DD diagnoses in the PTSD-DD subgroup were DPD (n = 4), DDNOS (n = 3), and Dissociative Identity Disorder (n = 2). The PTSD-DD subgroup had a significantly earlier age of onset of PTSD (18.1 ± 14.3years) compared to the PTSD-noDD subgroup (30.2 ± 13.0years) [t(33) = 2.35, p = 0.025]. Five of the 9 PTSD-DD participants had childhood-onset PTSD (before age 18) in contrast to only 4 of the 26 PTSD-noDD participants. The PTSD-DD subgroup also had significantly greater posttraumatic stress, dissociation, and childhood trauma compared to the PTSD-noDD subgroup.
Basal Urinary Cortisol (Figure 1)
Figure 1.
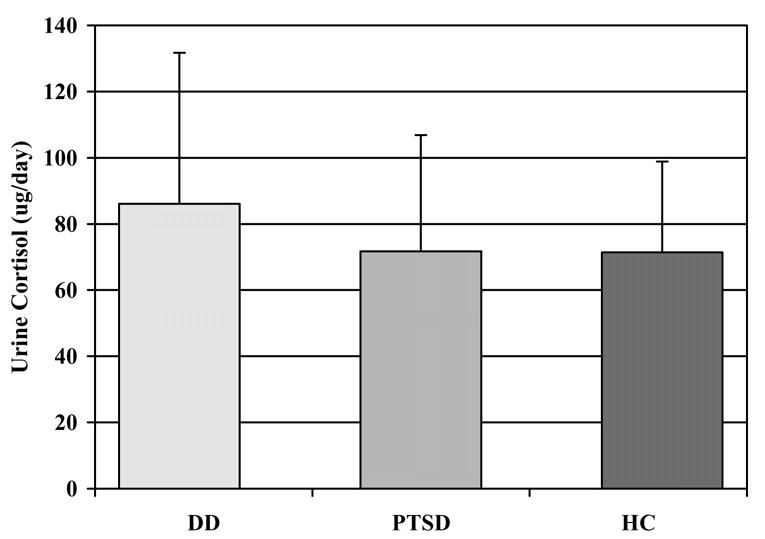
24-hour basal urinary cortisol in the Dissociative Disorder (n = 44), Posttraumatic Stress Disorder (n = 32), and Healthy Comparison (n = 56) groups
The DD group had higher basal urine cortisol than the HC group [F(1,95) = 3.84, p = 0.05], whereas the PTSD group did not differ from the HC [F(1,83) =0.20, p = 0.65) or the DD [F(1,71) = 1.80, p = 0.18] groups. There were no significant covariate effects.
Basal Plasma Cortisol (Figure 2)
Figure 2.
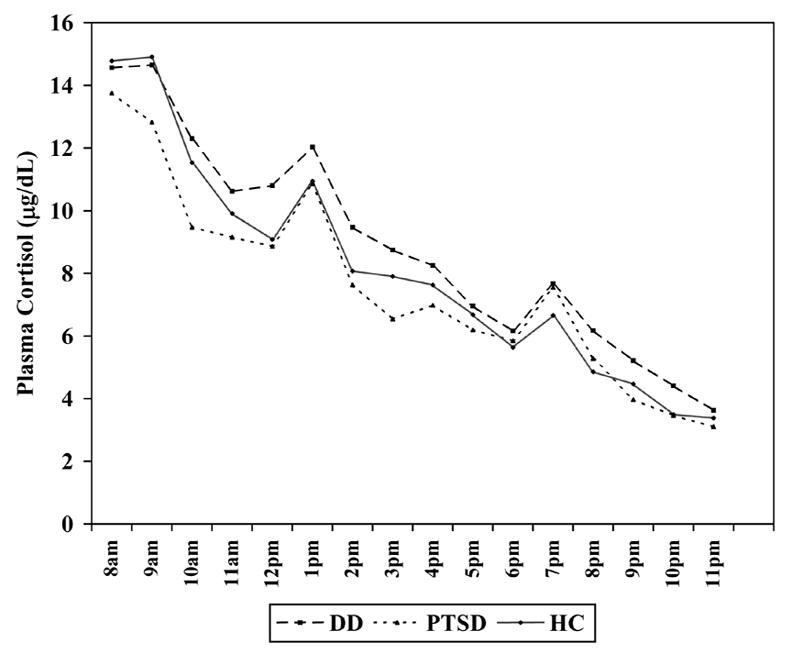
Day 2 basal hourly plasma cortisol levels in the Dissociative Disorder (n = 44), Posttraumatic Stress Disorder (n = 31), and Healthy Comparison (n = 56) groups
There were no significant average group effects or group × time interaction effects, for any of the group pairs [DD-HC: group F(1,95) =1.93, p = 0.17, group × time F(15, 1425) =1.23, p = 0.24; PTSD-HC: group F(1,82) = 0.00, p = 0.99; group × time F(15, 1230) = 1.36, p = 0.16; DD-PTSD: group F(1,70) = 1.33, p = 0.25, group × time F(15, 1050) = 1.12, p = 0.34].
Dexamethasone Suppression Test (Figure 3)
Figure 3.
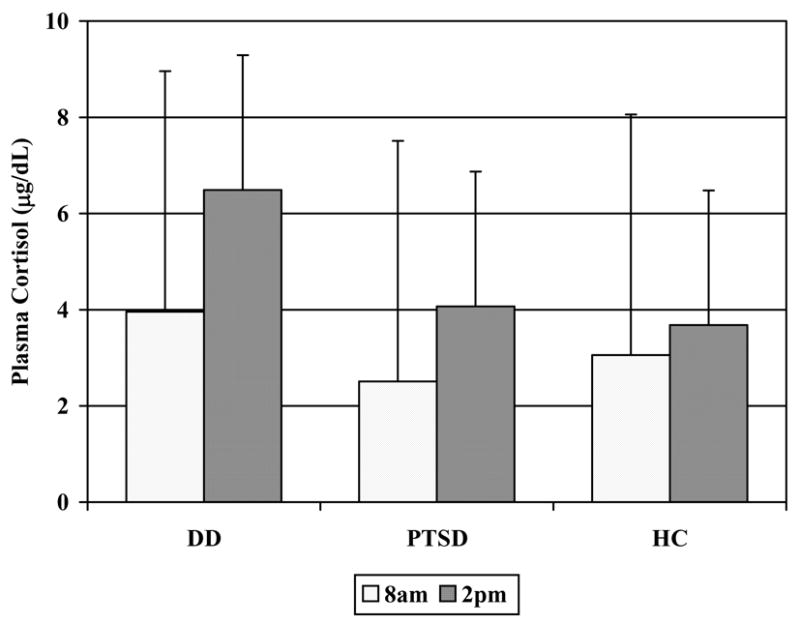
Plasma cortisol levels at 8am and 2pm in response to an 11pm 0.5mg oral dexamethasone challenge in the Dissociative Disorder (n = 43), Posttraumatic Stress Disorder (n = 28), and Healthy Comparison (n = 56) groups
Compared to the HC group, the DD group showed significant resistance to dexamethasone suppression [group: F(1,90) = 5.71, p = 0.019], and a significant group × time interaction effect [F(1,90) = 4.61, p = 0.034] indicating faster escape from suppression in DD subjects between 8am and 2pm. The PTSD group did not differ from the HC group [group: F(1,75) = 0.02, p = 0.903; group × time: F(1,75) = 1.50, p=0.224] or from the DD group [group: F(1,62) = 2.69, p = 0.106, group × time: F(1,62) = 0.70, p = 0.405]. There were significant gender effects in all comparisons, with females demonstrating greater suppression than males across diagnostic groups.
Trier Social Stress Test
The TSST induced significant increases in plasma cortisol in all groups [p < 0.001]. There were no differences in cortisol stress reactivity between any group pairs (Figure 4)[DD-HC: group × time F(3,276) = 0.33, p = 0.80; PTSD-HC: group × time F(3,234) = 0.47, p = 0.70; DD-PTSD: group × time F(3,198) = 1.09, p = 0.36]. Similarly, peak cortisol stress reactivity did not differ significantly between any groups [DD: 4.5 ± 4.1 μg/dL; PTSD: 2.5 ± 3.9 μg/dL; HC: 3.6 ± 3.7 μg/dL; DD-HC: F(1,94) = 1.05, p = 0.31; PTSD-HC: F(1,81) = 0.33, p = 0.57; DD-PTSD: F(1,68) = 1.32, p = 0.25]. There were no significant age or gender effects.
Figure 4.
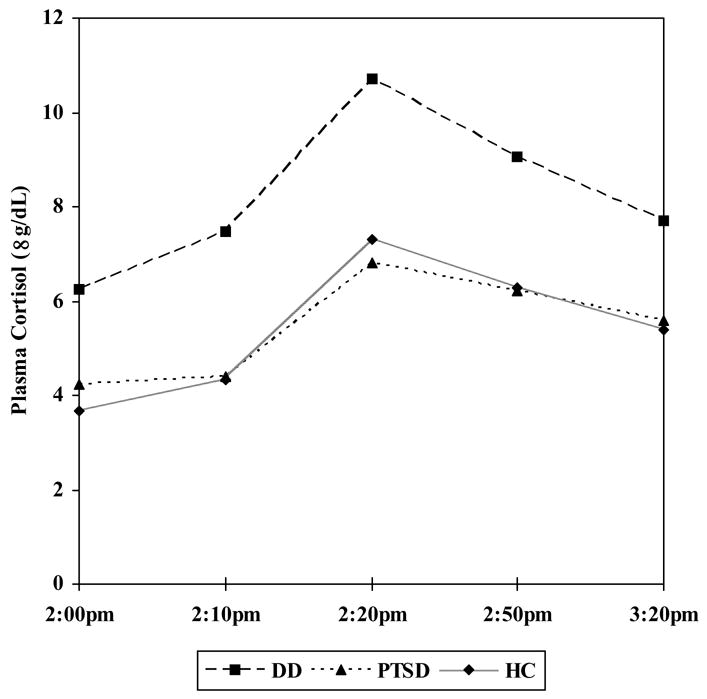
Plasma cortisol levels in response to the Trier Social Stress Test in the Dissociative Disorder (n = 43), Posttraumatic Stress Disorder (n = 29), and Healthy Comparison (n = 55) groups
Subjective stress experienced during the TSST differed among groups [DPD: 4.7 ± 1.1; PTSD: 5.1 ± 1.1, HC: 3.9 ± 1.0; F(2) = 10.58, p = 0.001]. Gender had a significant effect on stress scores with females experiencing greater stress than males [F(1,131) = 6.18, p = 0.01], whereas age did not. Stress ratings were not significantly correlated with peak cortisol stress reactivity in any group.
Relationships between cortisol measures in the three groups
Table 2 presents the relationships between the various cortisol measures within each of the three study groups.
Table 2.
Relationships between cortisol measures (urinary cortisol – UC, mean plasma cortisol – MPC, % 8am dexamethasone suppression (DST), TSST cortisol stress reactivity – TSST) in the Dissociative Disorders DD (n = 46), Posttraumatic Stress Disorder PTSD (n = 35) and Healthy Control (n = 58) groups; Pearson’s correlation is followed by two-tailed p value is parentheses (t = trend significance, * < .05; ** < .001)
| UC | MPC | DST | |
|---|---|---|---|
| MPC | DD: 0.04 (0.79)
PTSD: 0.49 (.004) ** HC: 0.31 (0.02) * |
||
| DST | DD: 0.14 (0.37)
PTSD: −0.10 (0.58) HC: −0.24 (0.07) t |
DD: −0.10 (0.49)
PTSD: −0.05 (0.80) HC: 0.17 (0.22) |
|
| TSST | DD: −0.07 (0.66)
PTSD: −0.05 (0.78) HC: 0.27 (0.04) * |
DD: −0.06 (0.69)
PTSD: 0.17 (0.34) HC: 0.09 (0.49) |
DD: −0.02 (0.89)
PTSD: 0.01 (0.96) HC: −0.35 (0.008) ** |
Impact of lifetime major depression on cortisol measures
Twenty-five DD participants had comorbid LMD, while 21 did not. The DD-noLMD subgroup tended to have higher urinary cortisol than the DD-LMD subgroup [DD-noLMD: 101.7 ± 52.8μg/day; DD-LMD: 71.9 ± 33.1μg/day; F(1,39) = 3.68, p = 0.06], and significantly higher than the HC group [F(1,72) = 9.54, p = 0.003]. The two DD subgroups did not significantly differ in hourly plasma cortisol diurnal pattern [F(15,585) = 0.73. p = 0.76], and neither DD subgroup differed significantly from the HC group [DD-noLMD: F(15,1080) = 1.39, p = 0.14; DD-LMD: F (15, 1110) =1.03, p = 0.42]. The two DD subgroups did not differ in DST 8am suppression [DD-noLMD: 72.6% ± 29.0%; DD-LMD: 70.2% ± 38.0%; F(1,37) = 0.20, p = 0.66] or in TSST peak cortisol stress reactivity [DD-noLMD: 4.6 ± 4.9μg/dL; DD-LMD: 4.3 ± 3.2μg/dL; F(1,38) = 0.001, p = 0.97].
Twenty-five PTSD participants had comorbid LMD while 10 did not. The two PTSD subgroups did not differ in urinary cortisol from each other [PTSD-noLMD: 69.7 ± 21.6μg/day; PTSD-LMD: 72.5 ± 39.6μg/day; F(1,27) = 0.30, p = 0.59], or from the HC group [PTSD-noLMD: F(1,60) = 0.06, p = .80; PTSD-LMD: F(1,74)=0.35, p = .56]. The two PTSD subgroups also did not differ from each other in diurnal plasma cortisol pattern [F(15,390) = 1.28, p = 0.21], and the PTSD-noLMD subgroup did not differ from the HC group [F(15,900)=0.70, p = 0.79] The PTSD-LMD subgroup showed a diurnal pattern significantly different from the HC group [F(15,1095) = 1.74, p = 0.039], characterized by greater reactivity surrounding the physiologic stressor of the three mealtimes.
The PTSD-LMD subgroup tended to have significantly greater suppression compared to the PTSD-noLMD subgroup [PTSD-LMD: 86.2% ± 12.7%; PTSD-noLMD: 69.5% ± 31.3%; F(1,24) = 3.39, p = 0.07]; comparison to the HC group (77.7% ± 27.7%) did not reveal differences for either subgroup [PTSD-LMD: F(1,72) = 1.83, p = 0.18; PTSD-noLMD: F(1,58) = 0.02, p = 0.88]. Similarly the two PTSD groups did not differ in TSST peak cortisol stress reactivity [PTSD-noLMD: 1.5 ± 3.5 μg/dL; PTSD-LMD: 3.0 ± 4.1 μg/dL; F(1,25) = 0.46, p = 0.51].
PTSD subgroups without and with DD comorbidity
The two PTSD subgroups did not differ from each other in any cortisol measures. Compared to the HC group, the PTSD-DD subgroup showed a tendency toward lower basal urinary cortisol [56.3 ± 20.7 μg/day; t(65) = 2.01, p = 0.07], and a tendency toward blunted peak cortisol stress reactivity [1.2 ± 3.3 μg/dL; t(63) = 1.79, p = 0.08].
Relationships between childhood trauma, dissociation, and cortisol
The CTQ and CTI were highly intercorrelated [r(131) = 0.70, p < 0.001] and yielded similar associations to other variables; therefore only CTQ analyses are presented for brevity. Dissociation was significantly correlated with childhood trauma in the DD [r(43) = 0.56, p < 0.001] and the PTSD [r(34) = 0.46, p = 0.005] groups, but not the HC group [r(53) = 0.06, p = 0.69]. Dissociation severity and childhood trauma were not significantly associated with basal cortisol or with response to dexamethasone in any group.
With respect to the TSST, in the DD group both dissociation severity and childhood trauma were significantly inversely correlated with maximal cortisol stress reactivity [DES: r(43) = −0.32, p = 0.03; CTQ: r(41) = −0.43, p = 0.004]. In the PTSD group, dissociation severity was significantly inversely correlated with maximal cortisol stress reactivity [r(31) = −0.38, p=0.03], with a similar tendency after controlling for PTSD symptom severity [partial r(30) = −0.33, p = 0.06], whereas childhood trauma was not [r(31) = −0.27, p = 0.14]. Controlling for depression, anxiety, and social anxiety scores preserved the inverse relationship between dissociation severity and cortisol stress reactivity in both psychiatric groups [DD: partial r(39) = −0.38, p = 0.015; PTSD: partial r(39) = −0.38, p = 0.038].
Discussion
This study comprises the first demonstration of HPA axis dysregulation in a large dissociative disorder sample free of major comorbidity. The main findings were as follows. 1) The DD group had higher basal urinary cortisol than the healthy comparison group, especially marked in the absence of lifetime major depression. 2) The DD group showed greater resistance to and faster escape from dexamethasone suppression than the comparison group. 3) Although the three groups did not differ in cortisol reactivity to psychosocial stress, both the DD and the PTSD groups demonstrated a significant inverse relationship between dissociation severity and cortisol stress reactivity, even when controlling for all other symptomatology.
Ambient and DST cortisol findings in the DD group replicate an earlier pilot study (Simeon et al 2001), revealing basal HPA axis hyperactivity with elevated cortisol and diminished pituitary negative feedback inhibition. The ambient and DST cortisol findings are similar to those encountered in a sizable portion of individuals with major depression (Sachar et al 1970; Heim et al 2001), even when depression is in remission (Young et al 2000; Young and Breslau 2004a, b), and are compelling given the exclusion of current depression and the examination of the impact of lifetime depression in our dissociative sample.
The psychosocial stressor induced significant increases in plasma cortisol in all groups, along with considerable subjectively perceived stress. However, the failure to demonstrate blunted stress reactivity in the DD group compared to controls, as we originally hypothesized, is difficult to interpret given performance of the TSST while the HPA axis was still impacted by the DST, resulting in the assessment of an admixture of reactivity and escape from suppression. The HC and PTSD groups were significantly more suppressed at the start of the TSST, thus not precluding the possibility that they might otherwise have demonstrated a more robust cortisol response than the DD group. Another possible explanation of the failure to show group differences may be the measurement of only cortisol and not ACTH (Heim et al 2000). Yet another explanation may relate to the exclusion of lifetime PTSD from the DD group, resulting in a more “pure” but markedly less “severe” dissociative group with only moderately elevated dissociation and childhood trauma scores. As a result, the PTSD subgroup with comorbid DD had markedly higher dissociation and trauma scores than the DD group, and did show a tendency toward blunted cortisol stress reactivity. Given that PTSD is reported to be associated with heightened reactivity (Heim et al 2000; Elzinga et al 2003), the PTSD-DD subgroup finding of the current study could be accounted for by the more severe dissociative symptoms. In support of this hypothesis, we also found a significant relationship between dissociation severity and blunted cortisol stress reactivity in both the DD and the PTSD groups, even after controlling for PTSD, depression, anxiety, and social anxiety severity. The finding that dissociation and childhood trauma were negatively associated with cortisol reactivity contrasts a report in which childhood trauma and major depression were associated with heightened HPA axis reactivity to psychosocial stress (Heim et al 2002). One plausible explanation of this difference may relate to sampling, i.e. a primarily “dissociative” sample versus a primarily “depressive” sample, suggesting that HPA axis reactivity to stress may fundamentally differ in dissociation (hyporeactive) versus depression (hyperreactive).
The relationship between the various cortisol measures of this study is of interest, and differed between groups. In the healthy control group the ambient urinary, dexamethasone suppression and TSST cortisol levels were significantly interrcorrelated, reflecting a well-regulated HPA axis; individuals with higher basal cortisol had enhanced negative feedback inhibition and heightened stress reactivity, reflecting a more active and reactive HPA axis. These intercorrelations were not as robust in the two psychiatric groups, presumably reflecting some degree of HPA axis dysregulation. Of particular interest, ambient urinary and plasma cortisol levels were significantly intercorrelated in the PTSD and HC groups, but not in the DD group. The most likely explanation for this finding is that urine sampling occurred over a 24-hour period whereas blood sampling took place only from 8am and 11pm. Therefore, the unsampled 8-hour period from 11pm to 7am may have been characterized by elevated cortisol secretion in the DD group such as has been previously described in major depression, with elevated cortisol nadir values during the late night and early morning hours (Yehuda et al. 1996). If so, higher cortisol production during this phase of the diurnal cycle in the DD group would have a two-fold consequence; a weaker correlation between urinary and plasma cortisol levels as sampled in this study, and less pronounced (and not statistically significant) plasma cortisol elevations in the DD group compared to the HC group, in contrast to the significant urinary cortisol elevation.
Although the primary focus of this study was to elucidate HPA function in DD, we briefly comment on the PTSD cortisol findings. The absence of basal cortisol or DST abnormalities in the group as a whole mirrors the considerable inconsistencies in the PTSD HPA axis literature; many variables are reported to potentially influence cortisol findings in PTSD (Yehuda 2002; Rasmusson et al 2003). Notably, a large community survey did not reveal abnormal ambient cortisol levels in PTSD (Young and Breslau 2004a, b). The current finding of a tendency toward hypersuppression in the PTSD participants with comorbid lifetime depression is consistent with several reports of hypersuppression in PTSD (Yehuda 2002), possibly reflecting more severe illness in this subgroup.
All study findings taken together suggest a preliminary model of HPA axis dysregulation in dissociative disorders characterized by basal hyperactivity yet blunted acute reactivity to acute psychosocial stressors, at least as a function of increasing dissociation severity. The study demonstrates clear differences in HPA axis dysregulation between dissociative disorders and PTSD, highlighting the need for further extensive study of stress response systems in dissociative psychopathology, whether it occurs independently of and in the context of PTSD.
Important strengths of the study include large sample sizes, strict selection and diagnostic criteria, control of several major confounds, and several cortisol measures that examine various components of HPA axis regulation. The two most important limitations of the study were the timing of the TSST, as well as the measurement of cortisol but not ACTH as the sole pituitary-adrenocortical hormone. A minor limitation was not controlling for stage of menstrual cycle or for menopause. With respect to menopausal status, reanalysis of the data after excluding the 2 DD, 5 PTSD, and 3 HC menopausal women did not affect the findings. Although we administered a low dose of dexamethasone, in accord with the PTSD comparison group and related literature, administration of the standard DST challenge dose (1mg) would be of interest given the DD group’s resistance to suppression.
Another “limitation” inherent to the nature of the disorders under study, rather than the study design itself, is the propensity of individuals who are highly and chronically traumatized early in life to have both PTSD and DD, sometimes referred to as “complex PTSD,” “complex trauma,” or “Disorders of Extreme Stress” (van der Kolk et al 2005), and to manifest the greatest severity of both sets of symptoms. In this study we chose to examine a “pure” dissociative disorders sample that was restricted in its composition, consisting largely of depersonalization disorder participants with a minority of Dissociative Disorder NOS. These individuals manifested more modest degrees of “traditional” childhood maltreatment, as has been previously described (Simeon et al. 2001). Also, certain types of traumatic stressors commonly encountered in such DD groups are not tapped by standard childhood maltreatment scales, such as sudden death of family and close friends, growing up with severely mentally ill parents, or experiencing a severe traumatic episode of mental illness that triggers chronic depersonalization (Simeon et al. 2003). In addition to maltreatment, prospective studies have found that serious disruptions of the early mother-infant dyad contribute to increased dissociation scores in late adolescence and adulthood (Hesse and Main 2006, Lyons-Ruth et al. 2006, Ogawa et al. 1997, Carlson 1998). Genetic contributions to dissociation have also been reported by some researchers (Becker-Blease et al. 2004, Jang et al. 1998).
Thus, the DD group exclusion criterion of lifetime PTSD resulted in a dissociative sample that was not representative of more extreme dissociative manifestations such as Dissociative Identity Disorder, typically characterized by very severe childhood trauma and very high PTSD comorbidity. Our preliminary finding of a tendency towards blunted cortisol stress reactivity in the PTSD subgroup with DD, as well as the finding of blunted cortisol stress reactivity as a function of increasing dissociation severity, suggest that this “comorbid” and prevalent DD + PTSD population merits further investigation. A future study could examine this “complex trauma” group on its own standing, comparing it to “pure” DD and PTSD samples. The effort to procure purer DD versus PTSD samples in research has to be carefully weighed against the clinical reality of their frequently shared traumatic antecedents and phenomenological overlaps.
Acknowledgments
This research was in part supported by NIMH RO1 MH62414 to Dr. Simeon and NIH MO1 RR0071 to the Mount Sinai School of Medicine General Clinical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker-Blease KA, Deater-Deckard K, Eley T, Freyd JJ, Stevenson J, Plomin R. A genetic analysis of individual differences in dissociative behaviors in childhood and adolescence. J Child Psychol Psychiatry. 2004;45:522–532. doi: 10.1111/j.1469-7610.2004.00242.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bernstein-Carlson E, Putnam FW. An update on the Disssociative Experiences Scale. Dissociation. 1993;6:16–27. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingaam M, Anderson G, Vermetten E, McGlashan T, Heninger G, et al. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol Psychiatry. 2003;54:710–718. doi: 10.1016/s0006-3223(02)01912-1. [DOI] [PubMed] [Google Scholar]
- Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Dev. 1998;69:1107–28. [PubMed] [Google Scholar]
- Carroll BJ. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- Chu JA, Dill DL. Dissociative symptomms in relation to childhood physical and sexual abuse. Am J Psychiatry. 1990;147:887–892. doi: 10.1176/ajp.147.7.887. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Royer DK, Raimonde AJ, Spoonster E. Peritraumatic dissociation is inversely related to catecholamine levels in initial urine samples of motor vehicle accident victims. J Trauma Dissociation. 2003;4:65–80. [Google Scholar]
- Elzinga BM, Schmahl CGT, Vermetten E, van Dyck R, Bremner DJ. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacol. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the Childhood Trauma Interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (SCID-P), version 2. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Griffin MG, Resick PA, Mechanic MB. Objective assessment of peritraumatic dissociation: Psychophysiological indicators. Am J Psychiatry. 1997;154:1081–1088. doi: 10.1176/ajp.154.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurology, Neurosurgery and Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport JD, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DF, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Hesse E, Main M. Frightened, threatening, and dissociative parental behavior in low-risk samples:Description, discussion, and interpretations. Dev Psychopathol. 2006;18:309–343. doi: 10.1017/S0954579406060172. [DOI] [PubMed] [Google Scholar]
- Jang KL, Paris J, Zweig-Frank H, Livesley WJ. Twin study of dissociative experience. J Nerv Ment Dis. 1998;186:345–351. doi: 10.1097/00005053-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychoendocrinol. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Ownes MJ, Nemeroff CB. Persistent changes in corticotrophin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Dutra L, Schuder MR, Bianchi I. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29:63–86. doi: 10.1016/j.psc.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard C, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Ogawa JR, Sroufe LA, Weinfield NS, Carlson EA, Egeland B. Development and the fragmented self: longitudinal study of dissociative symptomatology in a nonclinical sample. Dev Psychopathol. 1997;9:855–79. doi: 10.1017/s0954579497001478. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality Disorders SIDP-IV. Iowa: Department of Psychiatry, University of Iowa; 1995. [Google Scholar]
- Putnam FW. Dissociation as a response to extreme trauma. In: Kluft RP, editor. The Childhood Antecedents of Multiple Personality. Washington, DC: American Psychiatric Press Inc; 1985. pp. 65–97. [Google Scholar]
- Rasmusson AM, Vythilingam M, Morgan CA. The neuroendocrinology of posttraumatic stress disorder: new directions. CNS Spectr. 2003;8:651–667. doi: 10.1017/s1092852900008841. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Hellman K, Fukushima DK, Gallagher TF. Cortisol production in depressive illness. Arch Gen Psychiatry. 1970;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- Sierra M, Senior C, Dalton J, McDonough M, Bond A, Phillips ML, O’Dwyer AM, David AS. Autonomic response in depersonalization disorder. Arch Gen Psychiatry. 2002;59:833–838. doi: 10.1001/archpsyc.59.9.833. [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Knutelska M, Hollander E, Schmeidler J. Hypothalamic-pituitary-adrenal axis dysregulation in depersonalization disorder. Neuropsychopharmacol. 2001;25:793–795. doi: 10.1016/S0893-133X(01)00288-3. [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Schmeidler J, Sirof B, Knutelska M. The role of childhood interpersonal trauma in depersonalization disorder. Am J Psychiatry. 2001;158:1027–1033. doi: 10.1176/appi.ajp.158.7.1027. [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Knutelska M, Yehuda R, Schmidler J. Basal norepinephrine in depersonalization disorder. Psych Res. 2003;121:93–97. doi: 10.1016/s0165-1781(03)00205-1. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Nelson D, Guralnik O. Feeling unreal: a depersonalization disorder update of 117 cases. J Clin Psychiatry. 2003;64:990–997. doi: 10.4088/jcp.v64n0903. [DOI] [PubMed] [Google Scholar]
- Smith MA, Davidson J, Ritchie JC, Kudler H, Lipper S, Chappell P, et al. The corticotrophin-releasing hormone test in patients with posttraumatic stress disorder. Biol Psychiatry. 1989;26:349–355. doi: 10.1016/0006-3223(89)90050-4. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Steinberg M. Structured Clinical Interview for DSM-IV Dissociative Disorders-Revised (SCID-D) Washington, DC: American Psychiatric Press Inc; 1994. [Google Scholar]
- van der Kolk B, Roth S, Pelcovitz D, Sunday S, Spinazzola J. Disorders of Extreme Stress: the empirical foundation of a complex adaptation to trauma. J Traumatic Stress. 2005;18:389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Wilson K, Zdunek C, Lowenstein R, Payne C, et al. Neurobiological correlates of DID in comparison with PTSD and BPD; Presented at the 19th Annual Meeting of the International Society for Traumatic Stress Studies; Chicago, IL. October 29–November 1.2003. [Google Scholar]
- Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Annals NY Acad Sci. 1997;821:57–75. doi: 10.1111/j.1749-6632.1997.tb48269.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Waahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JM, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in combat veterans with posttraumatic stress disorder and major depressive disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Young EA, Aggen SH, Prescott CA, Kendler KS. Similarity in saliva cortisol measures in monozygotic twins and the influence of past major depression. Biol Psychiatry. 2000;48:70–74. doi: 10.1016/s0006-3223(00)00842-8. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder. Arch Gen Psychiatry. 2004a;61:34–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol Psychiatry. 2004b;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Zakriski AL, Shea MT, Costello E, Begin A, Pearlstein T, et al. The long-term sequelae of sexual abuse: support for a complex posttraumatic stress disorder. J Traumatic Stress. 1996;9:195–205. doi: 10.1007/BF02110655. [DOI] [PubMed] [Google Scholar]


