Abstract
T cell-to-T cell Ag presentation is increasingly attracting attention. In this study, we demonstrated that active CD4+ T (aT) cells with uptake of OVA-pulsed dendritic cell-derived exosome (EXOOVA) express exosomal peptide/MHC class I and costimulatory molecules. These EXOOVA-uptaken (targeted) CD4+ aT cells can stimulate CD8+ T cell proliferation and differentiation into central memory CD8+ CTLs and induce more efficient in vivo antitumor immunity and long-term CD8+ T cell memory responses than OVA-pulsed dendritic cells. They can also counteract CD4+25+ regulatory T cell-mediated suppression of in vitro CD8+ T cell proliferation and in vivo CD8+ CTL responses and antitumor immunity. We further elucidate that the EXOOVA-uptaken (targeted)CD4+ aT cell’s stimulatory effect is mediated via its IL-2 secretion and acquired exosomal CD80 costimulation and is specifically delivered to CD8+ T cells in vivo via acquired exosomal peptide/MHC class I complexes. Therefore, EXO-targeted active CD4+ T cell vaccine may represent a novel and highly effective vaccine strategy for inducing immune responses against not only tumors, but also other infectious diseases.
Dendritic cells (DC)3 process exogenous Ags in endosomal compartments such as multivesicular endosomes (1) which can fuse with plasma membrane, thereby releasing Ag-presenting vesicles called “exosomes” (EXO) (2, 3). EXO are 50- to 90-nm diameter vesicles containing Ag-presenting (MHC class I, class II, CD1, heat shock protein 70–90), tetraspan (CD9, CD63, CD81), adhesion (CD11b, CD54), and costimulatory (CD86) molecules (4, 5), i.e., the necessary machinery required for generating potent immune responses.
Zitvogel et al. (3) first demonstrated vaccination of DC-derived EXO in eradication of tumors in animal models. Subsequently, EXO-based vaccines have been confirmed to stimulate strong CTL responses and induce antitumor immunity in different animal models (6, 7). However, its therapeutic efficiency is still limited to only production of prophylactic immunity against tumors possibly due to lacking capacity of breaking immune tolerance (8). Exosomal peptide/MHC class I and II (pMHC I and II) complexes are functional, but require transfer to DC to promote T cell activation leading to tumor eradication (9–11). Therefore, the potential pathway of in vivo EXO-mediated antitumor immunity may be through uptake of EXO by host immature DC that, in turn, stimulate Ag-specific T lymphocytes via the pMHC complexes and costimulatory molecules on EXO-uptaken DC. In addition, Kennedy et al. (12) have also demonstrated that CD4+ T cells can acquire APC membrane molecules in vivo and induce memory CTL responses. We have recently demonstrated that mature DC with uptake of OVA-pulsed DC (DCOVA)-derived EXO (EXOOVA) express a higher level of pMHC I and costimulatory CD40, CD54, and CD80 molecules and can strongly stimulate OVA-specific CD8+ CTL responses and antitumor immunity (13). Recently, we have also demonstrated that CD4+ Th cells can acquire membrane molecules from DC via DC activation, and act as Th-APCs (14). These Th-APC with acquired pMHC I and costimulatory CD54 and CD80 molecules can stimulate tumor-specific CD8+ CTL responses and induce antitumor immunity. One of the potential mechanisms of CD4+ T cell acquisition of DC molecules is uptake of DC-released EXO by CD4+ T cells. Therefore, these results clearly indicate that DC-derived EXO can transfer the Ag-presenting activity of DCs to either DC or CD4+ T cells through EXO uptake by these cells. However, the EXO-targeted CD4+ T cell vaccine and its potential immune mechanism in induction of antitumor CTL responses have not been studied and elucidated.
In this study, we demonstrated that EXOOVA-uptaken (targeted) active CD4+ T (aTEXO) cells can 1) stimulate central memory CD8+ CTL responses, more efficient antitumor immunity, and T cell memory than DCOVA and 2) counteract CD4+25+ regulatory T (Tr) cell-mediated immune suppression. We also elucidated that the aTEXO stimulatory effect is mediated via its IL-2 secretion and acquired exosomal CD80 costimulation and is specifically delivered to CD8+ T cells in vivo via acquired exosomal pMHC I complexes.
Materials and Methods
Reagents, cell lines, and animals
OVA was obtained from Sigma-Aldrich. OVA I (SIINFEKL) and OVA II (ISQAVHAAHAEINEAGR) which are OVA peptides specific for H-2Kb and Iab, respectively (15, 16). Mut I (FEQNTAQP) peptide is specific for H-2Kb of an irrelevant 3LL lung carcinoma (11). All peptides were synthesized by Multiple Peptide Systems. Biotin-labeled or FITC-labeled Abs specific for H-2Kb (AF6-88.5), Iab (AF6-120.1), CD4 (GK1.5), CD8 (53-6.7), CD11c (HL3), CD25 (7D4), CD40 (IC10), CD44 (IM7), CD54 (3E2), CD62L (MEL-14), CD69 (H1.2F3), CD80 (16-10A1), IL-7R (4G3), and Vα2Vβ5+ TCR (MR9-4), as well as FITC-conjugated avidin, were all obtained from BD Pharmingen. Anti-LFA-1 Ab, CTLA-4/Ig fusion protein, recombinant mouse IL-4, and GM-CSF were purchased from R&D Systems. The mouse thymoma cell line EL4 and OVA-transfected EL4 (EG7) cell line were obtained from American Type Culture Collection. The highly lung metastasis OVA-transfected BL6 –10OVA melanoma cell line was generated in our own laboratory (14). Female C57BL/6 (B6, CD45.2+), C57BL/6.1 (B6.1, CD45.1+), OVA-specific TCR-transgenic OT I and OT II mice, H-2Kb, Iab, IL-2, IFN-γ, TNF-α, CD40, CD54, and CD80 gene knockout (KO) mice were obtained from The Jackson Laboratory. Homozygous OT II/H-2Kb−/−, OT II/CD40−/−, OT II/CD54−/−, OT II/CD80−/−, OT II/IL-2−/−, OT II/IFN-γ, and OT II/TNF-α −/− mice were generated by backcrossing the designated gene KO mice onto the OT II background. All mice were treated according to animal care committee guidelines of the University of Saskatchewan.
DC generation
DCs were generated as described previously (17). Briefly, spleen cells were prepared in PBS with 5 mM EDTA, washed, and incubated in culture medium with 7% FCS at 37°C for 2 h. Nonadherent cells were removed by gentle pipetting with warm serum-free medium. Adherent cells were cultured overnight in medium with 1% normal mouse serum, GM-CSF (1 ng/ml), and OVA (0.3 mg/ml). These DCs were termed as DCOVA. DC generated from H-2Kb, CD54, CD40, and CD80 gene KO mice were referred to as (Kb−/−)DCOVA, (CD40−/−)DCOVA, (CD54−/−)DCOVA and (CD80−/−)DCOVA, respectively.
EXO preparation
Preparation and purification of EXO derived from the culture supernatants of DCOVA were previously described (13), an overage of 5 μg of EXO was recovered from an overnight culture of 1 × 106 DCOVA in 1.0 ml of AIM-V serum-free medium containing GM-CSF (10 ng/ml). EXO derived from DCOVA were termed EXOOVA. Similar to DCOVA, EXOOVA also expressed MHC class I (H-2Kb) and class II (Iab), CD11c, CD40, CD54, CD80, and the pMHC I complex, but in a lesser content, compared with DCOVA (13). EXO derived from (Kb−/−) DCOVA, (CD40−/−)DCOVA, (CD54−/−) DCOVA, and (CD80−/−)DCOVA were termed (K b−/−)EXOOVA, (CD40−/−)EXOOVA, (CD54−/−)EXOOVA, and (CD80−/−)EXOOVA, respectively. To generate CFSE-labeled EXO, DCOVA were stained with 0.5 μM CFSE at 37°C for 20 min (16) and washed three times with PBS, and then pulsed with OVA protein in AIM-V serum-free medium overnight. The CFSE-labeled EXO (EXOCFSE) was harvested and purified from the culture supernatants as previously described above.
CD4+ T cell preparation and characterization
Naive OVA-specific T (nT) cells were isolated from OVA-specific TCR-transgenic OT I and OT II mouse spleens, enriched by passage through nylon wool columns (C&A Scientific), and then purified by negative selection using anti-mouse CD8(Ly2) or CD4 (L3T4) paramagnetic beads (Dynal Biotech) to yield populations that were >98% CD4+/Vα2Vβ5+ or CD8+/Vα2Vβ5+, respectively (14). To generate active OT II CD4+ T cells, the spleen cells from OT II mice were cultured in RPMI 1640 medium containing IL-2 (20 U/ml) and Con A (1 μg/ml) for 3 days. The Con A-activated CD4+ T (aT) cells were purified using nylon wool columns and then CD4 microbeads (Miltenyi Biotec). The CD4+ aT cells derived from OT II/H-2Kb−/−, OT II/CD40−/−, OT II/CD54−/−, and OT II/ CD80−/−mice were termed aT(H-2Kb−/−), aT(CD40−/−), aT(CD54−/−), and aT(CD80−/−), respectively. The above naive and Con A-activated CD4+ T cells were analyzed using a panel of Abs by flow cytometry. To assess the potential counteraction of CD4+25+ Tr cell-induced suppression by EXO-targeted CD4+ T cells, CD4+CD25+ Tr cells were purified from C57BL/6 mouse spleen T cells using CD25 microbeads (clone 7D4; Milte-nyi Biotec) (18). The purified CD4+CD25+ Tr cells were stained with PE-anti-CD25 (clone PC61.5), FITC-CD4 (clone GK1.5), and energy-coupled dye (ECD)-Foxp3 (clone FJK-16S) using Regulatory T Cell Staining kit No. 3 (eBioscience), and then analyzed by flow cytometry.
Exosomal molecule uptake by CD4+ T cells
First, the OT II CD4+ nT and aT cells were incubated with EXOCFSE (10 μg/1 × 106 T cells in 200 μl of AIM-V serum-free medium containing IL-2 (20 U/ml)) at 37°C for 5 h and then analyzed for CFSE staining by flow cytometry. In another set of experiments, the OT II CD4+ aT cells were incubated with EXOCFSE under the above same condition for 1, 3, and 5 h, and then assessed by confocal fluorescence microscopy. To further determine the transfer of exosomal molecules to T cells, the CD4+ nT and aT cells from OT II mice or OT II mice with different gene KO were incubated with EXOOVA, and then analyzed for expression of H-2Kb, CD40, CD54, CD80, and pMHC I by flow cy-tometry or analyzed for expression of CD40 and CD80 by confocal fluorescence microscopy. The OT II CD4+ nT and aT cells cocultured with EXOOVA were termed nTEXO and aTEXO cells, respectively. The CD4+ aT(H-2Kb−/−), aT(CD40−/−), aT(CD54−/−), and aT(CD80−/−) cells were cocultured with (Kb−/−)EXOOVA, (CD40−/−)EXOOVA, (CD54−/−)EXOOVA, and (CD80−/−)EXOOVA and termed CD4+ aTEXO (Kb−/−), aTEXO(CD40−/−), aTEXO(CD54−/−), and aTEXO(CD80−/−) cells, respectively. The CD4+ aT cells from OT II/IL-2−/−, OT II/ IFN-γ, and OT II/TNF-α −/− mice were cocultured with EXOOVA and termed CD4+ aTEXO(IL-2−/−), aTEXO(IFN-γ −/−), and aTEXO(TNF-α −/−) cells, respectively. The cytokine profiles of aTEXO(K b−/−), aTEXO (CD40−/−), aTEXO(CD54−/−), and aTEXO(CD80−/−) cells are similar to that of aTEXO cells, whereas the cytokine profiles of aTEXO(IL-2−/−), aTEXO(IFN-γ −/−), and aTEXO(TNF-α −/−) cells are also similar to that of aTEXO cells except for the specific cytokine (IL-2 or IFN-γ or TNF-α) deficiency (data not shown). For blocking assays, OT II CD4+ aT cells were incubated with anti-H-2Kb, anti-Iab, and anti-LFA-1 Abs (12 μg/ml) or CTLA-4/Ig (12 μg/ml), respectively, on ice for 30 min, then were cocultured with EXOCFSE for 5 h at 37°C. The cells were harvested and analyzed for CFSE expression by flow cytometry.
T cell proliferation assays
To assess the functional effect of CD4+ nTEXO and aTEXO cells, we performed an in vitro CD8+ T cell proliferation assay. CD4+ nTEXO and aTEXO (0.3 × 105 cells/well) cells derived from OT II mice and their 2-fold dilutions were cultured with a constant number of naive OT I CD8+ T cells (1 × 105 cells/well) in presence or absence of CD4+CD25+ Tr cells (0.3 × 105 cells/well) derived from C57BL/6 mice. To examine the molecular mechanism, a panel of reagents including anti-H-2Kb, I-Ab, and LFA-1 Abs and CTLA-4/Ig fusion protein (each 10 μg/ml), a mixture of the above reagents (as mixed reagents), and a mixture of isotype-matched irrelevant Abs (as control reagents) were added to the cell cultures, respectively. After culturing for 3 days, thymidine incorporation was determined by liquid scintillation counting (19). In the in vivo OVA-specific CD8+ T cell proliferation assay, C57BL/6 mice (six mice per group) were i.v. immunized with irradiated (4000 rad) stimulators including DCOVA, CD4+ aTEXO cells (1 × 106 cells/mouse), or CD4+ aTEXO cells (1 × 106 cells/ mouse) with different gene KO. In another set of experiments, C57BL/6 mice (six mice per group) were i.v. immunized with irradiated (4000 rad) DCOVA, CD4+ aT, and aTEXO cells (1 × 106 cells/mouse) alone or together with CD4+25+ Tr cells (3 × 106 cells/mouse). For evaluation of in vivo OVA-specific CD8+ T cell proliferation, the tail blood samples derived from mice 6 days after immunization were incubated with PE-H-2Kb/ OVAI tetramer and FITC-anti-CD8 Ab (Beckman Coulter) and analyzed by flow cytometry (13).
Cytotoxicity assay
In vivo cytotoxicity assay was performed as previously described (14). Briefly, wild-type C57BL/6 or Iab−/− mice (six mice per group) were i.v. immunized with the above stimulator cells (1 × 106 cells/mouse), respectively. In another set of experiments, C57BL/6 mice (six mice per group) were i.v. injected with irradiated (4000 rad) DCOVA and CD4+ aTEXO cells (1 × 106 cells/mouse) alone or together with CD4+25+ Tr cells (3 × 106 cells/mouse). Splenocytes were harvested from naive mouse spleens and incubated with either high (3.0 μM, CFSEhigh) or low (0.6 μM, CFSElow) concentrations of CFSE, to generate differentially labeled target cells. The CFSEhigh cells were pulsed with OVA I peptide, whereas the CFSElow cells were pulsed with Mut 1 peptide and served as internal controls. These peptide-pulsed target cells were washed extensively to remove free peptides, and then i.v. coinjected at a 1:1 ratio into the immunized mice 6 days after immunization. Sixteen hours after the target cell delivery, the spleens of immunized mice were removed and residual CFSEhigh and CFSElow target cells remaining in the recipients’ spleens were analyzed by flow cytometry.
Animal studies
To examine the antitumor protective immunity conferred by EXO-targeted CD4+ T cells, wild-type C57BL/6 mice (n = 8) were injected i.v. with irradiated (4000 rad) DCOVA, aT, and aTEXO cells (1 × 106 cells/mouse) with or without coinjection of CD4+25+ Tr cells (3 × 106 cells/mouse) aTEXO cells with various gene KO (1 × 106 cells/mouse), respectively. The mice were injected with PBS as a control. To assess the antitumor immunity, the immunized mice were challenged i.v. with 0.5 × 106 BL6–10OVA tumor cells 6 days subsequent to the immunization. To assess the long-term antitumor immunity, wild-type C57BL/6 mice were first immunized with irradiated (4000 rad) DCOVA and aTEXO cells (1 × 106 cells/mouse). The immunized mice were then challenged i.v. with 2 × 106 BL6–10OVA cells 3 mo subsequent to the immunization to assess development of tumor-specific CD8+ memory T (Tm) cells. The mice were sacrificed 4 wk after tumor cell injection and two individuals counted the lung metastasis tumor colonies in a blind fashion. BL6–10OVA-derived metastases on freshly isolated lungs appeared as discrete black-pigmented foci that could be distinguishable from normal lung tissues and all were confirmed by histological examination. Metastasis foci too numerous to count were assigned an arbitrary value of >100 (14).
Results
CD4+ T cells uptake exosomal pMHC I and costimulatory molecules in both Ag-specific and nonspecific manners
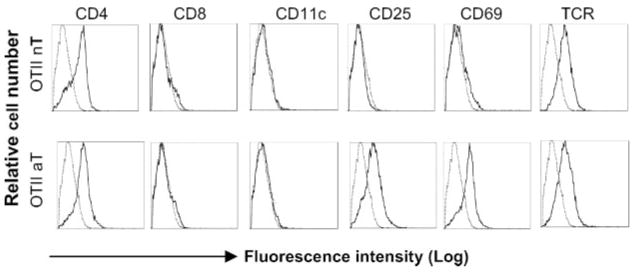
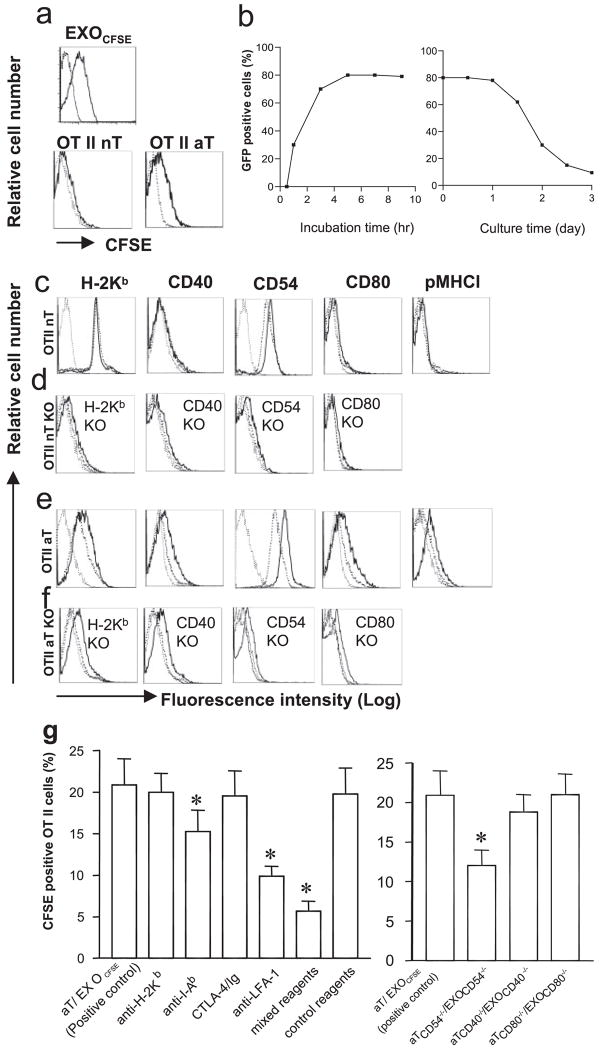
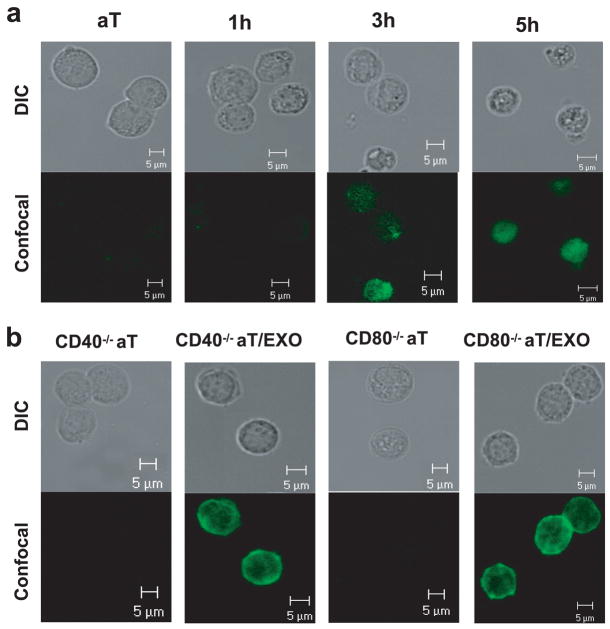
The nT and Con A-stimulated aT cells derived from transgenic OT II mice expressed both CD4 and TCR. The latter ones also expressed active T cell markers (CD25 and CD69) (Fig. 1) and secreted IL-2 (~2.4 ng/ml/106 cells/24 h), IFN-γ (~2.0 ng/ml/106 cells/24 h), and TNF-α (~1.7 ng/ml/106 cells/24 h), but no IL-4 and IL-10, indicating that they are active type Th1 cells. In addition, there was no CD11-positive splenic DC contamination in the purified CD4+ aT population (Fig. 1). To assess EXO uptake, CD4+ nT and aT cells derived from OT II and wild-type C57BL/6 (B6) mice were incubated with EXOCFSE expressing CFSE (Fig. 2a) for various times and then analyzed by flow cytometry and confocal fluorescence microscopy. As shown in Fig. 2a, the CFSE dye was minimally and apparently detectable on OT II CD4+ nT and aT cells, respectively. The uptake of EXOCFSE by OT II CD4+ aT cells increased with incubation time and reached a maximal level (80% CFSE-positive cells) after a 5-h incubation (Fig. 2b), which was also confirmed by confocal fluorescence microscopic analysis (Fig. 3a). The CFSE-positive cells declined with time when culturing them in medium, but were still detectable >3 days in culture, indicating that the uptaken exosomal molecules on CD4+ T cells are quite stable, which is consistent with a previous report by Undale et al. (20). Similar to CFSE dye, other exosomal molecules such as H-2Kb, CD40, CD54, and CD80 molecules were also minimally and apparently transferred from EXOOVA onto OT II CD4+ nT and aT cells, respectively (Fig. 2, c and e). In addition, the pMHC I complexes were also transferred onto CD4+ aT cells. To further confirm it, we incubated EXOOVA with OT II CD4+ T cells with different gene KO and then analyzed by flow cytometry or confocal fluorescence microscopy. The original OT II CD4+ nT and aT cells with different gene KO did not express endogenous H-2Kb, CD40, CD54, and CD80, respectively. However, after incubation with EXOOVA, T cells (especially the CD4+ aT cells) did display the above exosomal molecules (Fig. 2, d and f), indicating that increased expression of the above molecules on CD4+ T cells is due to an uptake of EXO molecules rather than an endogenous up-regulation. This was also confirmed by confocal fluorescence microscopic analysis. As shown in Fig. 3b, CD4+ aT cells with deficiency of endogenous CD40 and CD80 expression displayed CD40 and CD80 expression after uptake of EXOOVA. To elucidate the molecular mechanism involved in EXO uptake, we used a panel of reagents in a blocking assay. The anti-Iab and LFA-1 Abs, but not the CTLA-4/Ig fusion protein and the anti-H-2Kb Ab, were able to significantly block EXO uptake ( p < 0.05) (Fig. 2g). This was further confirmed by a significant decrease in EXO uptake ( p < 0.05) when incubation of aT(CD54−/−), but not aT(CD40−/−) and aT(CD80−/−), cells with EXOCFSE derived from CFSE-labeled (CD54−/−)DCOVA, (CD40−/−)DCOVA, and (CD80−/−) DCOVA, respectively, indicating that the EXOOVA uptake by CD4+ T cells is mediated by both OVA-specific Iab/TCR and nonspecific CD54/LFA-1 interactions, but not by H-2Kb/TCR and CD80/CD28 interactions, which is consistent with previous reports by Hwang et al. (21, 22).
FIGURE 1.
Phenotypic analysis of CD4+ T cells by flow cytometry. Flow cytometric analysis of OT II CD4+ T cells. The nT OT II and aT OT II cells (thick solid lines) were stained with a panel of Abs and then analyzed by flow cytometry. These cells were also stained with isotype-matched irrelevant Abs, respectively, and used as control populations (thin dotted lines). One representative experiment of three is shown.
FIGURE 2.
EXO uptake by CD4+ T cells. a, Both naive and active OT II and C57BL/6 CD4+ T cells with (thick solid lines) and without (thin dotted lines) uptake of EXOCFSE were analyzed for CFSE expression by flow cytometry. b, Kinetic study of EXO uptake by CD4+ T cells. Active OT II CD4+ T cells (2 × 106 cells) were incubated with EXOCFSE (20 μg) in DMEM (0.2 ml) containing 10% FCS and IL-2 (10 U/ml) at 37°C for different times. CFSE-positive T cells were detected by fluorescence microscopy and the percentages of CFSE-positive T cells were calculated. In another set of experiments, active OT II CD4+ T cells with incubation of EXOCFSE for 5 h were cultured in DMEM containing 10% FCS and IL-2 (10 U/ml) at 37°C for different times. CFSE-positive T cells were detected by fluorescence microscopy and the percentages of CFSE-positive T cells were calculated. c and e, Both naive and active OT II CD4+ T cells with (thick solid lines) and without (thick dotted lines) uptake of EXOOVA were analyzed for expression of a panel of surface molecules including H-2Kb, CD40, CD54, CD80, and pMHC I by flow cytometry. Irrelevant iso-type-matched Abs were used as controls (thin dotted lines). d and f, Both naive and active OT II CD4+ cells from H-2Kb, CD40, CD54, and CD80 gene KO mice were also cocultured with (thick solid lines) and without (thick dotted lines) EXOOVA and then analyzed for expression of H-2Kb, CD40, CD54, and CD80 by flow cytometry, respectively. Irrelevant isotype-matched Abs were used as controls (thin dotted lines). g, In the blocking assay, OT II CD4+ aT cells were treated with anti-H-2Kb, anti-Iab, and anti-LFA-1 Abs, and CTLA-4/Ig fusion protein, a mixture of these reagents or a mixture of matched isotype Abs (as control reagents) or PBS (as positive control) on ice for 30 min, respectively, and then incubated with EXOCFSE. In addition, OT II CD4+ aT cells with CD54 deficiency were incubated with EXOCFSE derived from CFSE-labeled (CD54−/−) DCOVA. The fractions of CFSE-positive T cells were analyzed after coculture for 5 h at 37°C. *, p < 0.05 vs cohorts of the positive control (aT/EXOCFSE) (Student’s t test). One representative experiment of three is displayed.
FIGURE 3.
Membrane molecule acquisition analysis by confocal fluorescence microscopy. a, CD4+ aT cells derived from OT II mice were incubated with EXOCFSE for 1, 3, and 5 h and then examined under differential interference contrast (DIC) and by confocal fluorescence microscopy. b, CD4+ aT cells derived from OT II mice with CD40 or CD80 gene deficiency were incubated with EXOOVA for 5 h, stained with FITC-conjugated anti-CD40 or anti-CD80 Ab, and then examined under DIC and by confocal fluorescence microscopy. One representative experiment of two is displayed.
CD4+ aTEXO cells counteract in vitro CD4+25+ Tr cell-mediated suppression of naive CD8+ T cell proliferation
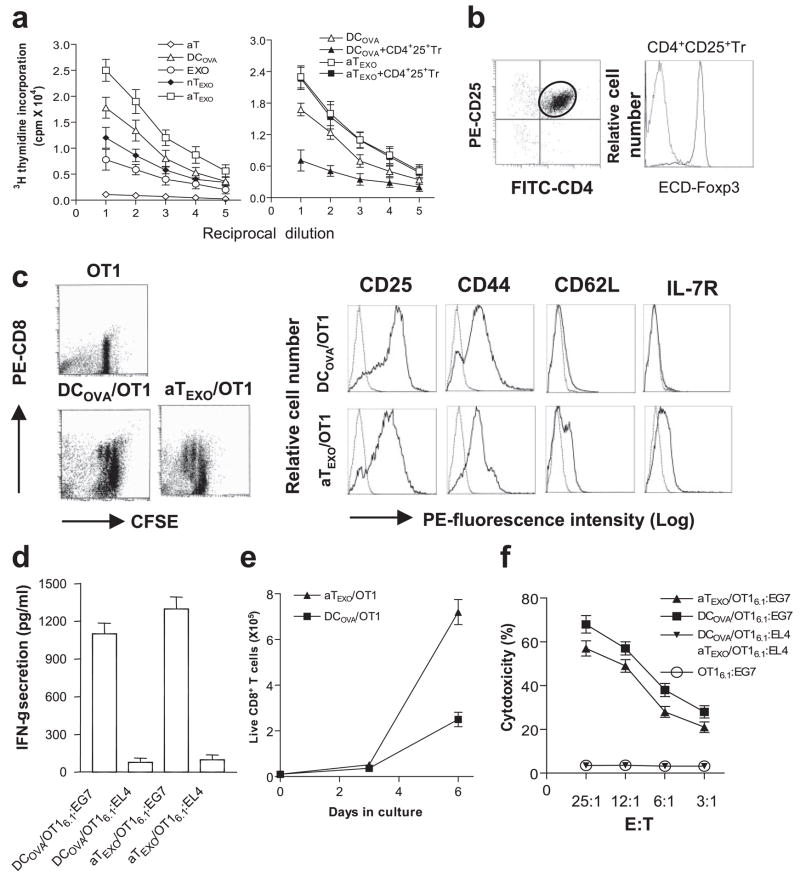
We then examined the stimulatory effect of EXOOVA-targeted active CD4+ T (aTEXO) cells. As shown in Fig. 4a, EXOOVA could prime CD8+ T cell proliferation in vitro, which is consistent with previous reports by Hwang et al. (21, 22), but in to a lesser extent compared with DCOVA. Of interest, CD4+ aTEXO is a stronger stimulator in CD8+ T cell proliferation than DCOVA, whereas nTEXO is a relatively weak stimulator. CD4+25+ Tr cells expressing CD4, CD25, and Foxp3 (Fig. 4b) inhibited DCOVA-stimulated CD8+ T cell proliferation (Fig. 4a). However, aTEXO cells maintained their stimulatory effect in presence of CD4+25+ Tr cells, indicating that aTEXO cells can counteract in vitro CD4+25+ Tr cell-mediated suppression of naive CD8+ T cell proliferation.
FIGURE 4.
Stimulation of CD8+ memory T cell responses in vitro. a, In vitro CD8+ cell proliferation assay. EXOOVA (10 μg/ml), DCOVA, nTEXO, aTEXO, and Con A-activated OTII T (aT) cells and their 2-fold dilutions were cocultured with a constant number of OT I CD8+T cells in presence or absence of CD4+25+ Tr cells. After 3 days, the proliferation response of CD8+ T cells was determined by [3H]thymidine uptake assay. b, Phenotypic analysis of CD4+25+ Tr cells. The CD4+25+ Tr cells were purified from C57BL/6 mouse splenocytes using CD25 microbeads, stained with PE-CD25, FITC-CD4, and ECD-Foxp3 Abs, and then analyzed by flow cytometry. The FITC-CD4- and PE-CD25-positive T cells were grouped for analysis of ECD-Foxp3 expression (solid line). Irrelevant isotype-matched Ab was used as a control (dotted line). c, Phenotypic analysis of in vitro aTEXO-primed CD8+ T cells. CFSE-labeled naive T cells derived from OT I mice were primed with irradiated DCOVA and aTEXO for 2 days in vitro and stained for CD8, CD25, CD44, CD62L, and IL-7R, respectively. Dot plots of CFSE-positive CD8+ T cells stained with PE-anti-CD8 Ab are shown, indicating that the CFSE-labeled CD8+ T cells underwent some cycles of cell division, and were sorted by flow cytometry for assessment of CD25, CD44, CD62L, and IL-7R expression using PE-labeled Abs (solid lines) or PE-isotype-matched irrelevant Abs (dotted lines) by flow cytometry. d, The in vitro DCOVA- and aTEXO-activated OT I CD8+CD45.1+ T cells were purified using biotin anti-CD45.1 Ab and anti-biotin microbeads (Miltenyi Biotec) and are referred to as DCOVA/OT I6.1 and aTEXO/OT I6.1, respectively. They were then incubated with irradiated (4,000 rad) EG7 and EL4 for 24 h. The supernatants in wells containing DCOVA/OT I6.1 plus EG7 or EL4 cells (DCOVA/OT I6.1:EG7 or DCOVA/OT I6.1:EL4) and aTEXO/OT I6.1 plus EG7 or EL4 cells (aTEXO/OT I6.1:EG7 or aTEXO/OT I6.1:EL4) were examined for IFN-γ expression by ELISA. e, T cell proliferation assay. In vitro DCOVA- and aTEXO-activated CD8+CD45.1+ T cells (0.4 × 105 cells/well) derived from OT I/B6.1 mouse OTI CD8+ T cells, primed on day 0 with irradiated DCOVA (■) or aTEXO (▴), were maintained in cultures for 1 wk with the indicated cytokines (IL-2 (50 U/ml) and IL-7 (10 ng/ml)) added on days 3 and 5. Live CD8+ T cells with trypan blue exclusion for each culture done in triplicate were counted at the indicated time points. f, In vitro cytotoxicity assay. The above DCOVA/OT I6.1 (■) and aTEXO/OT I6.1 (▴) cells were used as effector cells, whereas 51Cr-labeled EG7 or EL4 cells were used as target cells in a chromium release assay. One representative experiment of three is displayed.
CD4+ aTEXO cells stimulate in vitro CD8+ T cell proliferation and differentiation into central memory T cells
We next conducted phenotypic characterization of the above in vitro aTEXO-primed CD8+ T cells. Our data showed that DCOVA or aTEXO priming resulted in several cycles of CD8+ CFSE-T cell division, whereas nontreated CD8+ CFSE-T cells were not divided (Fig. 4c). The primed T cells displayed expression of CD25, CD44 (Tm marker) (23) and CD62L (Fig. 4c). However, aTEXO-primed CD8+ T cells expressed IL-7R and higher CD62L than DCOVA-primed ones with no IL-7R expression, indicating that they may be prone to becoming long-lived Tm cells. We then examined whether aTEXO-primed CTL exhibited any other functional traits attributed to typical Tm cells. These traits include 1) secretion of IFN-γ upon Ag stimulation, 2) the enhanced survival and proliferation in response to IL-7 (24), and 3) the capacity to generate Ag-specific CTL. Our data showed that both DCOVA- and aTEXO-primed CD8+ T cells secrete IFN-γ upon Ag stimulation by EG7 tumor cells expressing OVA (Fig. 4d). However, aTEXO-primed CTL expanded better in the presence of IL-2 and IL-7 than DCOVA-primed ones (Fig. 4e). In a chromium release assay, aTEXO-primed CTL (aTEXO/OT I6.1) showed cytotoxicity to EG7 tumor cells, but at a relatively lower level than DCOVA-primed ones (DCOVA/OT I6.1) (Fig. 4f). In addition, the nontreated OT I6.1 cells used as a control did not show any killing activity to EG7 tumor cells. Taken together, our results indicate that DCOVA-primed CD44+CD62LlowIL-7R−and aTEXO-primed CD44+CD62LhighIL-7R+ CTL, which have high and low cytotoxicity to tumor cells, are consistent with typical effector and central memory CTL (emCTL and cmCTL), respectively (12, 25).
CD4+ aTEXO cells stimulate in vivo CD4+ T cell-independent CD8+ T cell proliferation
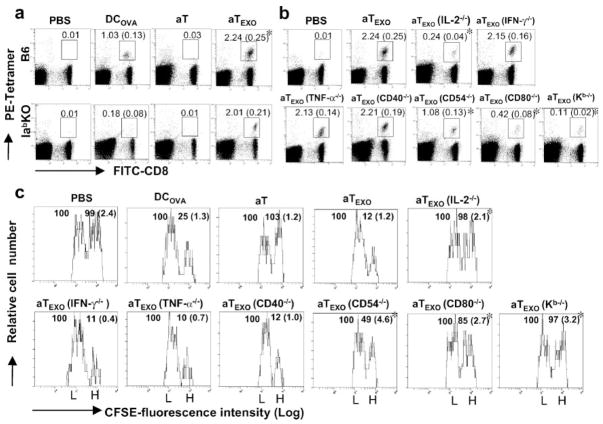
We then performed a tetramer staining assay to detect OVA-specific CD8+ T cells in wild-type C57BL/6 (B6) or MHC class II (Iab) gene KO mice. As shown in Fig. 5a, DCOVA and aTEXO cells stimulated proliferation of H-2Kb /OVA257–264 tetramer-positive CD8+ T cells accounting for 1.03 and 2.24% of the total spleen CD8+ T cell population in B6 mice, respectively, indicating that aTEXO is a significantly stronger stimulator than DCOVA ( p < 0.05). In Iab gene KO mice lacking CD4+ T cells, however, only aTEXO cells (2.01%), but not DCOVA, could stimulate OVA-specific CD8+ T cell responses, indicating that the aTEXO-induced CD8+ T cell response is CD4+ T cell independent, whereas those of DCOVA are CD4+ T cell dependent.
FIGURE 5.
Stimulation of CD8+ T cell proliferation and differentiation into CTL in vivo. Wild-type C57BL/6 or Iab−/− gene KO mice were i.v. immunized with irradiated (a) DCOVA, aT and aTEXO, and (b) aTEXO with various gene KO, respectively. Six days after immunization, the tail blood samples of immunized mice were incubated with PE-H-2Kb/OVAI tetramer and FITC-anti-CD8 Ab (Beckman Coulter) according to the company’s protocol, then analyzed by flow cytometry. The value in each panel represents the percentage of tetramer-positive CD8+ T cells vs the total CD8+ T cell population. The value in parentheses represent the SD. *, p < 0.05 vs cohorts of the positive control (aT/EXO) (Student’s t test). c, In an in vivo cytotoxicity assay, the above immunized mice were i.v. coinjected at a 1:1 ratio of splenocytes labeled with high (3.0 μM, CFSEhigh) and low (0.6 μM, CFSElow) concentrations of CFSE and pulsed with OVAI and Mut 1 peptide, respectively, 6 days after immunization with aT, aTEXO, and aTEXO with various gene KO, respectively. Sixteen hours after target cell delivery, the residual CFSEhigh and CFSElow target cells remaining in the recipients’ spleens were sorted and analyzed by flow cytometry. The value in each panel represents the percentage of CFSEhigh cells vs CFSElow cells remaining in the spleens. *, p < 0.05 vs cohorts of the positive control (aT/EXOCFSE) (Student’s t test). One representative experiment of three in the above different experiments is shown.
CD4+ aTEXO cell-induced CTL responses and antitumor immunity are mediated by IL-2 and acquired exosomal CD80 specifically delivered via acquired exosomal pMHC I
To elucidate the immune mechanism involved in aTEXO-induced CTL responses, we performed animal studies by using aTEXO with different gene KO. The stimulation of OVA-specific CD8+ T cell responses by aTEXO(CD54−/−) was reduced to more than half (1.08%) of the original one by aTEXO (2.24%) (Fig. 5b), possibly due to lacking CD54/LFA-1 interaction leading to decreased EXO uptake by aTEXO(CD54−/−) cells. Interestingly, the stimulation of OVA-specific CD8+ T cell responses by aTEXO(IL-2−/−) (0.24%) and aTEXO(CD80−/−) (0.42%) cells, but not by aTEXO(IFN-γ −/−) (2.15%), aTEXO(TNF-α −/−) (2.13%) and aTEXO(CD40−/−) (2.21%), was greatly lost and significantly less than that by aTEXO ( p < 0.05), indicating that the aTEXO stimulatory effect is mediated by its IL-2 and acquired CD80. Furthermore, aTEXO(K b−/−) cells (0.11%) with similar cytokine profiles as aTEXO, but without acquired pMHC I complexes, also completely lost their stimulatory effect, indicating that the aTEXO stimulatory effect is specifically delivered to CD8+ T cells in vivo via acquired exosomal pMHC I complexes. To assess aTEXO-induced CD8+ T cell differentiation into CTL, we adoptively transferred OVAI peptide-pulsed splenocytes that had been strongly labeled with CFSE (CFSEhigh), as well as the control peptide Mut 1-pulsed splenocytes that had been weakly labeled with CFSE (CFSElow), into the recipient mice that had been vaccinated with DCOVA and CD4+ aTEXO cells, respectively. As expected, the mice immunized with aTEXO had larger loss of CFSE high cells than DCOVA (75%) and CD4+ aTEXO cells (88%) (Fig. 5c), indicating that CD4+ aTEXO cells can most efficiently stimulate CD8+ T cell differentiation into CTL effectors. Interestingly, the OVA-specific cytotoxicity was substantially lost in CD4+ aTEXO(IL-2−/−)-(2%) and aTEXO (CD80−/−)-immunized (15%) mice and significantly less than the CD4+ aTEXO-induced one (p < 0.05), but not in CD4+ aTEXO (IFN-γ −/−)-(89%), aTEXO(TNF-α −/−)-(90%), and aTEXO (CD40−/−)-immunized (88%) mice, thus further confirming that CD4+ the aTEXO stimulatory effect is mediated by its IL-2 secretion and acquired CD80 costimulation. In addition, the CD4+ aTEXO(K b−/−)-vaccinated mice did not display any killing activity (3%), again confirming that the acquired pMHC I complexes play a critical role in targeting the CD4+ aTEXO stimulatory effect to OVA-specific CD8+ T cells in vivo. Furthermore, the results derived from our in vivo antitumor immunity studies are also consistent with the above aTEXO-induced in vivo CD8+ CTL responses (Fig. 5a). Our data showed that 1) CD4+ aTEXO cells induced stronger antitumor immunity than DCOVA (Expt. I of Table I), and 2) IL-2 and acquired exosomal CD80 of CD4+ aTEXO are specifically delivered to CD8+ T cells in vivo via acquired exosomal pMHC I (Expt. II of Table I).
Table I.
EXO-targeted CD4+ T cell vaccine protects against lung tumor metastases
| Vaccinesa | Tumor Growth Incidence (%) | Median Number of Lung Tumor Colonies |
|---|---|---|
| Expt. 1 | ||
| DCOVA | 2/8 (25) | 17 ± 6 |
| aTEXO | 0/8 (0) | 0 |
| aT | 8/8 (100) | >100 |
| PBS | 8/8 (100) | >100 |
| Expt. 2 | ||
| aTEXO(IL-2−/−) | 7/8 (88) | 78 ± 20 |
| aTEXO(IFN-γ −/−) | 0/8 (0) | 0 |
| aTEXO (TNF-α −/−) | 0/8 (0) | 0 |
| aTEXO (CD40−/−) | 0/8 (0) | 0 |
| aTEXO (CD54−/−) | 3/8 (38) | 26 ± 8 |
| aTEXO (CD80−/−) | 5/8 (63) | 47 ± 23 |
| aTEXO (K b−/−) | 7/8 (88) | 84 ± 17 |
| PBS | 8/8 (100) | >100 |
| Expt. 3 | ||
| DCOVA | 4/8 (50) | 14 ± 9 |
| aTEXO | 0/8 (0) | 0 |
| PBS | 8/8 (100) | >100 |
| Expt. 4 | ||
| DCOVA | 2/8 (25) | 17 ± 6 |
| DCOVA plus Tr cells | 8/8 (100) | >100 |
| aTEXO | 0/8 (0) | 0 |
| aTEXO plus Tr cells | 0/8 (0) | 0 |
| PBS | 8/8 (100) | >100 |
In experiment 1, the wild-type C57BL/6 (B6) mice (n = 8) were i.v. immunized with DCOVA, aT and aTEXO cells, or PBS. In experiment 2, the wild-type C57BL/6 mice (n = 8) were i.v. immunized with either aTEXO cells or aTEXO cells with various gene KO. Six days after the immunization, each mouse was challenged i.v. with OVA-expressing (BL6–10OVA) tumor cells (0.5 × 106 cells/mouse). In experiment 3, the wild-type C57BL/6 mice (n = 8) were i.v. immunized with DCOVA, aTEXO, or PBS. Three months after the immunization, each mouse was challenged i.v. with BL6–10OVA tumor cells (2 × 106 cells/mouse). In experiment 4, the wild-type C57BL/6 mice (n = 8) were i.v. immunized with DCOVA, aTEXO cells alone or together with CD4+25+ Tr cells. Six days after the immunization, each mouse was challenged i.v. with OVA-expressing (BL6–10OVA) tumor cells (0.5 × 106 cells/ mouse). All the mice were sacrificed 4 wk after tumor cell challenge and the numbers of lung metastatic tumor colonies were counted. One representative experiment of three is shown.
CD4+ aTEXO cells induce efficient long-term OVA-specific CD8+ T cell memory
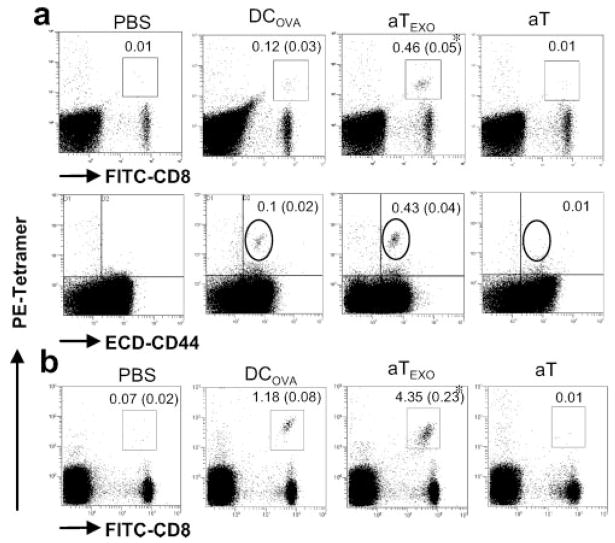
Active CD8+ T cells can become long-lived Tm cells after adoptive transfer in vivo (26). We then assessed whether these CD4+ aTEXO-primed CD8+T cells can also become long-lived Tm cells. As shown in Fig. 6a, we still detected 0.46% OVA-specific CD8+ T cells in peripheral blood of mice immunized with aTEXO, which is significantly stronger than that (0.12%) in mice immunized with DCOVA 3 mo after the immunization ( p < 0.05). These OVA-specific CD8+ T cells were also CD44+, indicating that they are OVA-specific CD8+ Tm cells. In addition, the survived CD4+ aTEXO-primed CD8+ Tm cells are nearly 4-fold compared with the survived DCOVA-stimulated ones, indicating that CD4+ aTEXO-primed CD44+CD62LhighIL-7R+ CTL with low cytotoxicity to tumor cells are long survival cmCTL. The recall responses were then assessed on day 4 after the boost of immunized mice with DCOVA. As expected, CD8+ Tm cells were expanded by 10-fold in these immunized mice after the boost (Fig. 6b), indicating that these CD8+ Tm cells are functional. In another set of experiments, the above immunized mice were challenged with a high dose (2 × 106 cells/mouse) of BL6–10OVA tumor cells. Only four of eight (50%) mice immunized with DCOVA were tumor free, whereas all eight of eight (100%) mice immunized with CD4+ aTEXO cells did not have any lung metastasis (Expt. III of Table I), indicating that CD4+ aTEXO cells can induce more efficient long-term CD8+ T cell memory than DCOVA.
FIGURE 6.
Development of Ag-specific CD8+ memory T cells. a, C57BL/6 mice were immunized with irradiated DCOVA and aTEXO, respectively. Three months later, the tail blood was taken from these immunized mice and stained with PE-H-2Kb/OVA tetramer, FITC-anti-CD8 and ECD-anti-CD44 Abs, and analyzed by flow cytometry. The value in each panel represents the percentage of tetramer-positive CD8+ T cells vs the total CD8+ T cell population. The value in parentheses represents the SD. The PE-tetramer- and FITC-CD8-positive cells in the squares were sorted and analyzed, showing they were also PE-tetramer- and ECD-CD44-positive cells in the circles. *, p < 0.05 vs cohorts of the positive control (DCOVA) (Student’s t test). b, The above immunized mice were boosted with DCOVA. Four days after the boost, the recall responses were examined using staining with PE-H-2Kb/OVA tetramer and FITC-anti-CD8 Ab and analyzed by flow cytometry. The value in each panel represents the percentage of tetramer-positive CD8+ T cells vs the total CD8+ T cell population. The value in parentheses represents the SD. The results presented are representative of five separate mice per group. One representative experiment of three is shown.
CD4+ aTEXO cells counteract in vivo CD4+25+ Tr cell-mediated suppression of OVA-specific CD8+ T cell proliferation and antitumor immunity
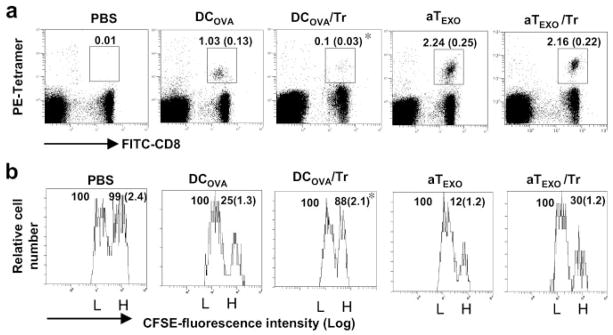
Because we demonstrated above the counteraction of in vitro CD4+25+ Tr cell-mediated suppression, we wanted to further assess the potential effect of CD4+ aTEXO cells in counteraction of in vivo CD4+25+ Tr cell-mediated suppression. We performed in vivo proliferation and cytotoxicity assays as described above. As shown in Fig. 7a, the stimulation of OVA-specific CD8+ T cell responses in mice by DCOVA (1.03%) was almost completely lost (0.10%) when mice were coinjected with CD4+25+ Tr cells ( p < 0.05), indicating that CD4+25+ Tr cells can significantly inhibit DCOVA-stimulated CD8+T cell responses. However, the OVA-specific CD8+ T cell responses in mice with CD4+ aTEXO stimulation in presence or absence of CD4+25+ Tr cells maintained at a similar level (2.24%). In the in vivo cytotoxicity assay, the effector CTL responses derived from DCOVA, but not CD4+ aTEXO cells, in presence of CD4+25+ Tr cells were lost (Fig. 7b). Furthermore, the results derived from our in vivo antitumor immunity studies (Expt. IV of Table I) are also consistent with the above aTEXO-induced in vivo CD8+ CTL responses (Fig. 5). Our data demonstrated that CD4+ aTEXO cells, but not DCOVA, induced efficient antitumor immunity in mice even with coinjection of CD4+25+ Tr cells, which was against the challenge of BL6–10OVA tumor cells from developing lung tumor metastasis (Expt. IV of Table I), indicating that CD4+ aTEXO cells can counteract in vivo CD4+25+ Tr cell-mediated immune suppression, possibly through bypassing the CD4+25+ Tr cell-mediated suppressive pathway.
FIGURE 7.
CD4+25+ Tr-mediated suppression of CD8+ T cell proliferation and differentiation in vivo. Wild-type C57BL/6 mice were i.v. immunized with irradiated (a) DCOVA, aT and aTEXO, alone or with CD4+25+ Tr cells, respectively. Six days after immunization, the tail blood samples of immunized mice were incubated with PE-H-2Kb/OVAI tetramer and FITC-anti-CD8 Ab according to the company’s protocol, then analyzed by flow cytometry. The value in each panel represents the percentage of tetramer-positive CD8+ T cells vs the total CD8+ T cell population. The value in parentheses represents the SD. *, p < 0.05 vs cohorts of the positive control (aTEXO in absence of Tr cells) (Student’s t test). b, In an in vivo cytotoxicity assay, the above immunized mice were i.v. coinjected at a 1:1 ratio of splenocytes labeled with high (3.0 μM, CFSEhigh) and low (0.6 μM, CFSElow) concentrations of CFSE and pulsed with OVAI and Mut 1 peptide, respectively, 6 days after immunization. Sixteen hours after target cell delivery, the residual CFSEhigh and CFSElow target cells remaining in the recipients’ spleens were sorted and analyzed by flow cytometry. The value in each panel represents the percentage of CFSEhigh cells vs CFSElow cells remaining in the spleens. *, p < 0.05 vs cohorts of the positive control (aTEXO in absence of Tr cells) (Student’s t test). One representative experiment of three in the above different experiments is shown.
Discussion
According to the progressive linear differentiation hypothesis (27), T cell differentiation involves a phase of proliferation preceding the acquisition of fitness and effector function. Primed CD8+ T cells reach a variety of differentiation stages that contain effector cells as well as cells that have been arrested at intermediate levels of differentiation. Thus, they retain a flexible gene imprinting. T cells that may survive after the retraction phase of an immune response can be resolved into distinct subsets of either cmCTLs representing cells at intermediate levels of differentiation or fully differentiated emCTLs with effector capacity (28, 29). It has been shown that a strong Ag presentation stimulates development of effector CTL, whereas a less efficient Ag presentation can lead to generation of central memory CTL (30). In this study, we demonstrated that CD4+ aTEXO cells were able to stimulate naive CD8+ T cell differentiation into CD8+44+62highIL-7R+ cmCTLs with less cytotoxicity and longer survival capacity leading to strong memory T cell responses, compared with DCOVA-primed CD8+44+62lowIL-7R+ emCTLs with high cytotoxicity and shorter survival capacity in vivo. Therefore, an EXO-targeted CD4+ T cell vaccine using peripheral blood CD4+ T cells of a cancer patient incubated with tumor cell-derived EXO from ascites of a cancer patient may be a useful strategy for EXO-based treatment of cancer.
Administration of attenuated T lymphocytes to animals has been shown to stimulate immune suppression and to prevent the development of experimental autoimmune diseases (31, 32). Vaccination using myelin basic protein autoreactive T cells has also been applied to clinical trial in multiple sclerosis (33). In this study, we clearly showed that CD4+ aTEXO cells can more strongly stimulate OVA-specific immunogenic CD8+ CTL responses, antitumor immunity, and CD8+ T cell memory in wild-type mice than EXO and DCOVA. Interestingly, the CD8+CTL responses stimulated by are found to be CD4+T cell independent. Thus, EXO-aTEXO targeted T cell vaccines may be very useful in induction of anti-HIV immunity in HIV patients with CD4+ T cell deficiency because HIV-1 infection is characterized by a gradual loss of CD4+ T cells and progressive immune deficiency (34). Furthermore, we also elucidated the molecular mechanism involved in aTEXO vaccines, which includes 1) the IL-2 secretion and the acquired exosomal CD80 costimulation that mediate the aTEXO stimulatory effect and 2) the acquired exosomal pMHC I complexes that play a critical role in targeting the aTEXO stimulatory effect to CD8+ T cells in vivo.
CD4+25+ Tr cells develop in the thymus and then enter peripheral tissues where they suppress activation of other self-reactive T cells (35, 36). It has been reported that an elevated number of Tr cells was detected in tumors (37, 38) which suppressed the anti-tumor immune responses by inhibition of CD4+T cell proliferation and a helper effect (39–41) as well as DC maturation (42). Therefore, how to combat immune tolerance becomes a critical challenge in cancer immunotherapy (43). A variety of stimuli that increase the potency of T cell stimulation have been shown to abrogate CD4+ Tr cell function including high Ag dose, TLR signals, CD28 engagement, and provision of IL-2 (44–48). In this study, we demonstrated that CD4+ aTEXO cells, but not DCOVA, can stimulate CD8+ T cell proliferation in the presence of CD4+25+ Tr cells in vitro and in vivo. These CD4+ aTEXO cells expressing exosomal pMHC I and CD80 and secreting IL-2 can break Tr cell-mediated immune tolerance, possibly due to its capacity to 1) directly stimulate CD8+ T cell responses in absence of CD4+ Th cells and DC, thus bypassing the above Tr cell-mediated suppressive pathways or 2) directly counteract CD4+ Tr suppression via CD28 engagement and IL-2 stimulation (44–48).
Taken together, our data show that DCOVA-derived EXOOVA can be uptaken by CD4+ T cells. EXOOVA-targeted CD4+ aTEXO cells expressing acquired exosomal pMHC I and CD80 can stimulate CD8+ cmCTL responses, more efficient in vivo antitumor immunity and long-term CD8+ T cell memory than DCOVA, and counteract CD4+25+ Tr cell-mediated suppression of OVA-specific CTL responses and antitumor immunity. The aTEXO stimulatory effect is mediated via its IL-2 secretion and acquired exosomal CD80 costimulation and is specifically targeted to CD8+T cells in vivo via acquired exosomal pMHC I complexes. Therefore, an EXO-targeted active CD4+ T cell vaccine may represent a novel and highly effective vaccine strategy for inducing immune responses not only against tumors, but also other infectious diseases.
Acknowledgments
We appreciate Mark Boyd for help in flow cytometry.
Footnotes
This work was supported by Research Grants MOP 67230 and 79415 from the Canadian Institutes of Health Research. S.H. was supported by a Postdoctoral Fellowship from Saskatchewan Health Research Foundation.
Abbreviations used in this paper: DC, dendritic cell; EXO, exosome; pMHC, peptide/MHC; DCOVA, OVA-pulsed DC; EXOOVA, DCOVA-derived EXO; aT, active CD4+ T; aTEXO, EXOOVA-uptaken (targeted) aT; KO, knockout; EXOCFSE, CFSE-labeled EXO; nT, naive OVA-specific T; Tr, regulatory T; nTEXO, nT cocultured with EXOOVA; Tm, memory T; emCTL, effector memory CTL; cmCTL, central memory CTL; ECD, energy-coupled dye.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 2.Kleijmeer MJ, Escola JM, UytdeHaag FG, Jakobson E, Griffith JM, Osterhaus AD, Stoorvogel W, Melief CJ, Rabouille C, Geuze HJ. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2:124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 4.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 5.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 6.Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 7.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 10.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 11.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy R, Undale AH, Kieper WC, Block MS, Pease LR, Celis E. Direct cross-priming by Th lymphocytes generates memory cytotoxic T cell responses. J Immunol. 2005;174:3967–3977. doi: 10.4049/jimmunol.174.7.3967. [DOI] [PubMed] [Google Scholar]
- 13.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120:90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–7505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 15.Slingluff CL., Jr Tumor antigens and tumor vaccines: peptides as immunogens. Semin Surg Oncol. 1996;12:446–453. doi: 10.1002/(SICI)1098-2388(199611/12)12:6<446::AID-SSU10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Huang H, Yuan J, Sun D, Hou WS, Gordon J, Xiang J. CD4−8− dendritic cells prime CD4+ T regulatory 1 cells to suppress antitumor immunity. J Immunol. 2005;175:2931–2937. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu J, Moriizumi E. CD4+CD25− T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–1682. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Chen Z, Li F, Kamencic H, Juurlink B, Gordon JR, Xiang J. Tumour necrosis factor-α (TNF-α) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-α. Immunology. 2003;108:177–188. doi: 10.1046/j.1365-2567.2003.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Undale AH, van den Elsen PJ, Celis E. Antigen-independent acquisition of MHC class II molecules by human T lymphocytes. Int Immunol. 2004;16:1523–1533. doi: 10.1093/intimm/dxh154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang I, Sprent J. Role of the actin cytoskeleton in T cell absorption and internalization of ligands from APC. J Immunol. 2001;166:5099–5107. doi: 10.4049/jimmunol.166.8.5099. [DOI] [PubMed] [Google Scholar]
- 23.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 24.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Xiang J. CD4+ T cell-independent maintenance and expansion of memory CD8+ T cells derived from in vitro dendritic cell activation. Int Immunol. 2006;18:887–895. doi: 10.1093/intimm/dxl025. [DOI] [PubMed] [Google Scholar]
- 27.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 28.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 29.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 30.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62Lhigh CD44high) subset. J Immunol. 2005;174:5341–5350. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 31.Shapira OM, Mor E, Reshef T, Pfeffermann RA, Cohen IR. Prolongation of survival of rat cardiac allografts by T cell vaccination. J Clin Invest. 1993;91:388–390. doi: 10.1172/JCI116211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishimoto C, Takada H, Hiraoka Y, Shinohara H, Kitazawa M. Protection against murine coxsackievirus B3 myocarditis by T cell vaccination. J Mol Cell Cardiol. 2000;32:2269–2277. doi: 10.1006/jmcc.2000.1253. [DOI] [PubMed] [Google Scholar]
- 33.Hong J, Zang YC, Nie H, Zhang JZ. CD4+ regulatory T cell responses induced by T cell vaccination in patients with multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5024–5029. doi: 10.1073/pnas.0508784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 35.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 36.Cozzo C, Larkin J, 3rd, Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–5682. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 37.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adeno-carcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 38.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 39.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Becourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J Immunol. 2004;172:3391–3398. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 42.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 43.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 44.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 46.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–511. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 47.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 48.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]