Abstract
Cytochrome P450 (CYP) genes catalyze formation of epoxyeicosatrienoic acids (EETs) from arachidonic acid (AA). The effects of 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET microinjected into the ventrolateral periaqueductal gray (vlPAG) on the thermally-produced tail-flick response were studied in male SD rats. 14,15-EET microinjected into vlPAG (3 to 156 pmol) dose-dependently inhibited the tail-flick response (ED50 = 32.5 pmol). In contrast, 5,6-EET, 8,9-EET and 11,12-EET at a dose of 156 pmol were not active when injected into the vlPAG. 14,15-EET failed to displace the radio-binding of [3H][D-Ala2,NHPe4,Gly-ol5]enkephalin (μ-opioid receptor ligand) or [3H]Naltrindole (δ-opioid receptor ligand) in crude membrane fractions of rat brain. Tail-flick inhibition produced by 14,15-EET from vlPAG was blocked by intra-vlPAG pretreatment with antiserum against β-endorphin or Met-enkephalin, or μ-opioid receptor antagonist CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) or δ-opioid receptor antagonist naltrindole, but not with dynorphin A [1-17] antiserum or κ-opioid receptor antagonist nor-binaltorphimine. Also, tail-flick inhibition produced by 14,15-EET treatment was blocked by intrathecal pretreatment with Met-enkephalin antiserum, naltrindole or CTOP, but not with β-endorphin antiserum. It is concluded that 1) 14,15-EET itself does not have any affinity for μ- or δ-opioid receptors, and 2) 14,15-EET activates β-endorphin and Met-enkephalin, which subsequently act on μ-and δ-opioid receptors to produce antinociception.
Introduction
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 (CYP) epoxygenase metabolites of the lipid arachidonic acid (AA) (Alkayed et al., 1996; Node et al., 1999; Bylund et al., 2002; Nelson et al., 2004). Four regioisomeric EETs: 5,6-, 8,9-, 11,12- and 14,15-epoxyeicosatrienoic acids (EETs) (Fig. 1) have been reported (Rifkind et al., 1995; Laethem et al., 1996; Makita et al., 1996; Ma et al., 1999). Until recently, the only CYP enzymes thought to play an important role in the formation of EETs were CYP2C11 and CYP2J, which are found in astrocytes of the brain (Alkayed et al., 1996; Node et al., 1999). Recently, an enzyme for a previously unidentified CYP gene that is specifically expressed in the rat brain was cloned and sequenced (Bylund et al., 2002). The enzyme was designated CYP4X1 by the committee on P450 nomenclature (Nelson et al., 2004). In situ hybridization demonstrated the expression of CYP4X1 in neurons with a wide pattern of distribution throughout the central and peripheral nervous systems (Bylund et al., 2002). However, it was not found in any other tissues including the liver in rats (Bylund et al., 2002). These data suggest that EETs can be synthesized in the brain. In supports of this, Junier et al (1990) reported that the total endogenous EETs concentration (a mixture of 8,9-, 11,12- and 14,15-EETs) in the hypothalamus was estimated to be 120 ng/g wet tissue.
Figure 1.
Chemical structures and metabolic pathways of 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET. EETs are formed by the cytochrome P450 2C11, 4X1 and 2J (CYP 2C11/4X1/2J) epoxygenase pathway of arachidonic acid.
While EETs are important modulators of cardiovascular and renal function, the pharmacological properties and the physiological functions of EETs in the brain are not known. We have found for the first time that one of these CYP metabolites of AA, 14,15-EET, when given into the ventrolateral periaqueductal gray area (vlPAG) of the mesencephalon produces potent antinociception. Mesencephalic vlPAG contains high concentration of endogenous opioid peptides β-endorphin and Met-enkephalin and their receptors, μ, δ and ε. Activation of any of these opioid receptors by β-endorphin, morphine or other opioids given into the vlPAG produces potent antinociception at the supraspinal sites and also activates the spinopetal descending pain control pathways, which are mediated by the rostral ventromedial medulla of the brainstem and project to the spinal and trigeminal dorsal horns for producing spinal analgesia (Basbaum and Fields, 1984; Pavlovic and Bodnar, 1998; Smith et al., 1988; Yaksh et al., 1988). In our study, Antisera against β-endorphin, Met-enkephalin and dynorphin A [1-17] and their respective selective receptor antagonists were used as pharmacological tools to delineate the neural mechanisms of antinociception produced by 14,15-EET microinjected into the vlPAG. We found that the antinociception produced by 14,15-EET from the vlPAG is mediated by the activation of β-endorphin and Met-enkephalin acting on μ- and δ-opioid receptors.
Methods
Animals
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing between 250 - 300 g at the time of surgery were housed in pairs before and after surgery. They were maintained in a room at 22 ± 0.5 °C with an alternating 12-h light/dark cycle. Food and water were available ad libitum. All experiments were approved by and conformed to the guidelines of the Animal Care Committee at the Medical College of Wisconsin.
Surgical Procedures
Rats were pretreated with methylatropine bromide (5 mg/kg, i.p.), anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A 23-gauge stainless steel guide cannula 12 mm in length was then implanted unilaterally 3 mm down from the surface of the skull and anchored to the skull with three stainless steel screws and dental cement. The stereotaxic coordinate for the placement of the cannula for vlPAG microinjection was 1.20 mm anterior to interaural point and 0.7 mm lateral to the midline (Paxinos and Watson, 1997). After a recovery period of at least 5 days animals were used for the experiments.
Assessment of Antinociception
Antinociceptive responses were measured with the tail-flick test (D'Amour and Smith, 1941). To measure the latency of tail-flick response, rats were gently held by hand and their tail positioned on the apparatus (model TF6; EMDIE Instrument Co., Maidens, VA). Tail-flick response was elicited by applying radiant heat to the dorsal surface of the tail. The intensity of the heat stimulus was set to provide a pre-drug tail-flick response time of 3 to 4 seconds. The cutoff time was set at 10 seconds to minimize tissue damage.
Drugs, Antisera and Drug Administration
Morphine sulfate, norbinaltorphimine (nor-BNI), and naltrindole (NTI) were obtained from the National Institution of Drug Abuse (Baltimore, MD).d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), polyclonal rabbit antisera against β-endorphin, Met-enkephalin, Leu-enkephalin and dynorphin A [1-17] were kindly donated by Dr. Leon F Tseng (Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, WI). The antiserum against endomorphin-1 was purchased from Phoenix Pharmaceuticals. 14,15-EET or other AA metabolites were kindly donated by Dr. John R. Falck (Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX). 14,15-EET or other AA metabolites were dissolved in a sterile saline solution containing 5% hydroxypropyl-β-cyclodextrin (CD). Morphine sulfate, CTOP, NTI and nor-BNI were dissolved in sterile saline solution (0.9% NaCl solution). Polyclonal rabbit antisera against β-endorphin, Met-enkephalin, Leu-enkephalin, endomorphin-1 and dynorphin A [1-17] were dissolved in sterile 0.9% NaCl solution and used as pharmacological tools to delineate the neural mechanism of 14,15-EET-produced tail-flick inhibition. Doses and times of administration of drugs given with each experiment are based on previous publications (Craft et al., 2001; Ohsawa et al., 2001; Terashvili et al., 2004; 2005). The binding potency and the cross-reactivity of the antiserum against β-endorphin, Met-enkephalin, Leu-enkephalin and dynorphin A [1-17] have been previously characterized and used to study the neural mechanisms of antinociception of opioid peptides and opiates (Tseng and Suh, 1989; Tseng et al., 2000; Wu et al., 2001). Previous studies with antiserum to inactivate the endogenous neuropeptides have indicated that 1 h of pretreatment time and the doses used are sufficient for their specific effects (Arts et al., 1992; Ohsawa et al., 2000; Wu et al., 2004; Terashvili et al., 2005).
Injections of drug solution into the vlPAG were carried out manually with a 30-gauge injection needle attached to a microsyringe via polyethylene tubing. The injection needle was inserted directly into the guide cannula. Injection volume for each microinjection was 0.5 μl and solution was administered over a 30 second period. The injection needle was left in place for an additional 60 seconds to ensure complete distribution. The stereotaxic coordinate of the vlPAG injection site was 1.20 mm anterior to interaural point, 0.7 mm lateral to the midline and 5.8 mm down from the surface of the skull (Paxinos and Watson, 1997).
The method for intrathecal injection of drug solution in rats was performed according to the procedure described by Hylden and Wilcox (1989) in mice with minor modification. Rats were anesthetized with isofluorane. A 25 μl Hamilton syringe with a 30-gauge needle was used for drug injection. The injection volume of drug solution was 20 μl.
Histological Identification of the Injection Site
At the end of the experiments, 0.5 μl of methylene blue solution (2%) was injected into the vlPAG. The rats were then sacrificed with CO2 (100%) 10 to 20 min after injection. The brains were removed, frozen, and sectioned sagittally for microscopic identification of the injection sites. The stereotaxic atlas of rats by Paxinos and Watson (1997) was used as a guide for the identification of anatomical injection sites. Only the data obtained from rats in which the injection sites were accurately identified to be in vlPAG were used for further statistical analysis.
Determination of the binding properties of 14,15-EET with μ- and δ-opioid receptors in the rat brain: Membrane Preparation
Anesthetized rats were killed by decapitation and the whole brain, excluding the cerebellum, was rapidly excised at 4°C. The tissue was homogenized using a Potter-Elvehjem tissue grinder with a Teflon pestle in 20 volumes (w/v) of ice-cold Tris buffer containing 50 mM Tris-HCl (pH 7.4) for the μ- and δ-opioid receptor binding assay. The homogenate was centrifuged at 4°C for 10 min at 48 000 × g. The pellet was resuspended in ice-cold Tris buffer and centrifuged at 4°C for 10 min at 48 000 × g. The resultant pellet was resuspended in ice-cold Tris buffer and stored at −80°C until further use.
Binding Assay
The membrane homogenate (900–1000 μg protein/assay) was incubated at 25°C for 2 h in 50 mM Tris–HCl buffer (pH 7.4) with 1 nM [3H]DAMGO (specific activity, 56.8 Ci/mmol) or 5nM [3H]Naltrindole (specific activity, 20.0 Ci/mmol, PerkinElmer Life and Analytical Sciences, Boston, MA) in a total volume of 1 ml. The reaction was terminated using a Brandel cell harvester (Model M-24, Brandel, MD) and the samples were filtered through Whatman GF/B glass filters pre-soaked in 50 mM Tris–HCl (pH 7.4) with 0.01% polyethylenimine at 4°C for 2 h. Filters were washed three times with 5 ml of Tris–HCl buffer (pH 7.4, 4°C) and then transferred to scintillation counting vials containing 0.5 ml of a tissue solubilizer (Soluene-350, Packard Instrument Company, Meriden, CT) and 4 ml of a scintillation cocktail (Hionic Fluor, Packard Instrument Company). Non-specific binding was measured in the presence of 10 μM unlabeled naloxone and NTI. Comparable results were obtained from at least three independent sets of experiments.
Statistical Analysis
The analgesic responses (tail-flick latency (sec)) were presented as the mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Dunnett's post-test, two-way ANOVA followed by Bonferroni's post-test or Student's t-test was used to determine the difference between groups. The “percent of maximum possible effect” (%MPE) was used to calculate the ED50 values. The % MPE was calculated as [(T1 − T0) / (T2 − T0)] × 100. T0 and T1 were tail-flick latencies. T0 represents tail-flick latency before drug injection and T1 represents tail-flick latency at 10 min after drug injection. T2 was the cutoff time, which was set at 10 sec. Nonlinear regression model was used to fit the dose–response curve and calculate the ED50 values and 95% confidence intervals for 14,15-EET and morphine produced antinociception. GraphPad Prism software was used to perform statistics (version 4.02; GraphPad Software, Inc., San Diego, CA).
Results
Effects of 5,6-EET, 8,9-EET, 11,12-EET or 14,15-EET microinjected into the vlPAG on thermally-induced tail-flick response
Groups of rats were microinjected into the vlPAG with 156 pmol of 5,6-EET, 8,9-EET, 11,12-EET or 14,15-EET or 0.5 μl of saline vehicle and tail-flick response was measured at 5, 10, 15, 20, 30, 40 and 60 min thereafter. Intra-vlPAG injection with 156 pmol of 14,15-EET time-dependently caused an increase of tail-flick inhibition; the tail-flick latency increased in 5 min, reached its maximal peak at 10 min, declined gradually and returned to the pre-injection level in 30-60 min. On the other hand, intra-vlPAG injection with the same dose (156 pmol) of 5,6-EET, 8,9-EET or 11,12-EET did not cause any significant increase of the latency of tail-flick response (Fig. 2).
Figure 2.
Effects of 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET microinjected into the vlPAG on tail-flick response. Groups of rats were microinjected with 156 pmol of 5,6-EET, 8,9-EET, 11,12-EET, 14-15-EET or vehicle. Tail-flick response was then measured at different time points. 156 pmol of 14,15-EET but not 5,6-EET, 8,9-EET, 11,12-EET microinjected into the vlPAG increased tail-flick inhibition. Data are represented as mean ± SEM, n= 5 to 6 animals per group. Two-way ANOVA followed by Bonferroni's post-test was used to determine the difference between groups. *P < 0.01.
The potency of 14,15-EET microinjected into the vlPAG for inhibition of the tail-flick response was then studied. The effect of morphine sulfate microinjected into the vlPAG to inhibit the tail-flick response was also performed in order to compare with that of 14,15-EET. Groups of rats were microinjected with various doses of 14,15-EET (3, 39, 78 or 156 pmol) or morphine sulfate (0.3, 1, 3, 9 nmol) into the vlPAG and tail-flick response was measured at 10 min after the injection. As shown in Figure 3, 14,15-EET at a dose from 39 to 156 pmol and morphine sulfate at a dose from 1 to 9 nmol dose-dependently inhibited the tail-flick response observed at 10 min after injection. The ED50 values for 14,15-EET and morphine sulfate were estimated to be 32.5 pmol and 1124 pmol, respectively. Thus, 14,15-EET is approximately 35-fold more potent than that of morphine in inhibiting the tail-flick response.
Figure 3.
Dose-response relationship of the inhibition of tail-flick response produced by 14,15-EET and morphine sulfate microinjected into the vlPAG. Groups of rats were microinjected with different doses of 14,15-EET (3, 39, 78 or 156 pmol), morphine (0.3, 1, 3 or 9 nmol) or vehicle. Tail-flick response was then measured 10 min after the injection. 14,15-EET and morphine microinjected into the vlPAG dose-dependently increased tail-flick latencies. Data are represented as mean ± SEM, n= 6 animals per group. One-way ANOVA followed by Dunnett's post-test was used to determine the difference between groups. *P < 0.05, **P < 0.01.
Effect of pretreatment with the μ-opioid receptor antagonist CTOP, δ-opioid receptor antagonist NTI or κ-opioid receptor antagonist nor-BNI microinjected into the vlPAG on tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG
Experiments were then performed to determine what types of opioid receptors, μ, δ or κ, in the vlPAG may be involved in tail-flick inhibition produced by 14,15-EET. Groups of rats were intra-vlPAG pretreated with various doses of μ-opioid receptor antagonist CTOP (9.4, 47.0 or 94.1 pmol) for 5 min (Ohsawa et al., 2001; Terashvili et al., 2004), δ-opioid receptor antagonist NTI (0.2, 1.1 or 2.2 nmol) for 10 min (Craft et al., 2001; Terashvili et al., 2005), or κ-opioid receptor antagonist nor-BNI (6.6 nmol) for 24 h (Craft et al., 2001; Terashvili et al., 2005) before the intra-vlPAG administration of 14,15-EET (156 pmol) and tail-flick response was measured 10 min thereafter. Intra-vlPAG pretreatment with CTOP at a dose from 9.4 to 94.1 pmol to block μ-opioid receptors in the vlPAG, dose-dependently attenuated the tail-flick inhibition produced by 14,15-EET and CTOP at a high dose (94.1 pmol) was found to completely block the 14,15-EET-produced tail-flick inhibition (Fig. 4A). Intra-vlPAG pretreatment with CTOP (94.1 pmol) alone did not affect the baseline tail-flick response in rats injected with saline into the vlPAG (Fig. 4A).
Figure 4.
Effects of pretreatment with the μ-opioid receptor antagonist, CTOP (A), δ-opioid receptor antagonist, NTI (B) or κ-opioid receptor antagonist, nor-BNI (C) microinjected into the vlPAG on tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Groups of rats were vlPAG pretreated with different doses of CTOP (9.4, 47.0 or 94.1 pmol) for 5 min, NTI (0.2, 1.1 or 2.2 nmol) for 10 min, nor-BNI (6.6 nmol) for 24 hr or vehicle before vlPAG microinjection of 14,15-EET (156 pmol). Tail-flick response was then measured 10 min after 14,15-EET (156 pmol) microinjection into the vlPAG. Separate groups of rats were vlPAG pretreated with CTOP (94.1 pmol, 5 min), NTI (2.2 nmol, 10 min) or nor-BNI (6.6 nmol, 24 h) before vlPAG microinjection of vehicle. Pretreatment with CTOP or NTI, but not nor-BNI attenuated tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Data are represented as mean ± SEM, n= 6 animals per group. One-way ANOVA followed by Dunnett's post-test and Student's t-test were used to determine the difference between groups. *P < 0.01.
Intra-vlPAG pretreatment with NTI at a dose from 0.2 to 2.2 nmol to block δ-opioid receptors in the vlPAG, also dose-dependently attenuated tail-flick inhibition produced by 14,15-EET. However, intra-vlPAG pretreatment with NTI even at a high dose (2.2 nmol) did not completely block the 14,15-EET-produced tail-flick inhibition (Fig. 4B). Intra-vlPAG pretreatment with NTI (2.2 nmol) alone did not affect the baseline tail-flick latency in rats injected with saline (Fig. 4B). Thus, CTOP appears to be more effective than NTI in attenuating tail-flick inhibition produced by 14,15-EET at the vlPAG site. On the other hand, intra-vlPAG pretreatment with nor-BNI (6.6 nmol) to block κ-opioid receptors in the vlPAG did not affect tail-flick inhibition produced by 14,15-EET (Fig. 4C). Intra-vlPAG pretreatment with nor-BNI (6.6 nmol) alone also did not affect the baseline tail-flick response in rats injected with saline into the vlPAG (Fig. 4C).
Effects of 14,15-EET in the displacement of [3H]DAMGO and [3H]Naltrindole binding in brain membrane preparation of the rat
The results of the experiments described above suggest that tail-flick inhibition produced by 14,15-EET from the vlPAG is mediated by activation of μ- and, to a lesser extent, δ-opioid receptors, but not by κ-opioid receptor. Radio-ligand receptor binding studies in the rat brain preparation were performed to explore the possibility that tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG is mediated by a direct stimulation of μ- or δ-opioid receptors by 14,15-EET. The effects of 14,15-EET on the displacement of the μ-opioid receptor radio-ligand, [3H]DAMGO, and δ-opioid receptor radio-ligand, [3H]naltrindole, in the rat brain membrane preparation was then determined. The effects of μ-opioid receptor ligands DAMGO and morphine on the displacement of the [3H]DAMGO receptor binding and the effect of δ-opioid receptor ligands Met-enkephalin and DPDPE on the displacement of [3H]Naltrindole receptor binding were also performed for comparison. 14,15-EET did not cause any displacement of the [3H]DAMGO binding, while DAMGO and morphine concentration-dependently displaced the [3H]DAMGO binding in the rat membrane preparation (Fig. 5A). 14,15-EET also did not cause any displacement of the [3H]naltrindole binding, while Met-enkephalin and DPDPE concentration-dependently displaced the [3H]naltrindole binding in the rat membrane preparation (Fig. 5B).
Figure 5.
Displacement of [3H]DAMGO μ-opioid radio-labeled binding by morphine or DAMGO, but not by 14,15-EET and [3H]Naltrindole δ-radio-labeled receptor binding by DPDPE or Met-enkephalin, but not by 14,15-EET. In Figure 5A the rat brain membranes were incubated with 1nM of [3H]DAMGO with different concentrations of morphine, DAMGO or 14,15-EET and in Figure 5B the rat brain membranes were incubated with 5 nM of [3H]naltrindole with different concentrations of DPDPE, Met-enkephalin or 14,15-EET in a final volume of 1.0 ml which contained 50 mM Tris–HCl buffer (pH 7.4) and 0.1 ml of the membrane homogenate (900–1000 μg) for 2 h at 25°C. Specific binding was defined as the difference in binding observed in the absence and presence of 10 μM unlabeled naloxone and NTI respectively. The results of a representative experiment that was replicated three times are shown.
Effects of intra-vlPAG pretreatment with antiserum against β-endorphin, Met-enkephalin, Leu-enkephalin, endomorphin-1 or dynorphin A [1-17] microinjected into the vlPAG on the tail-flick inhibition produced by 14,15-EET
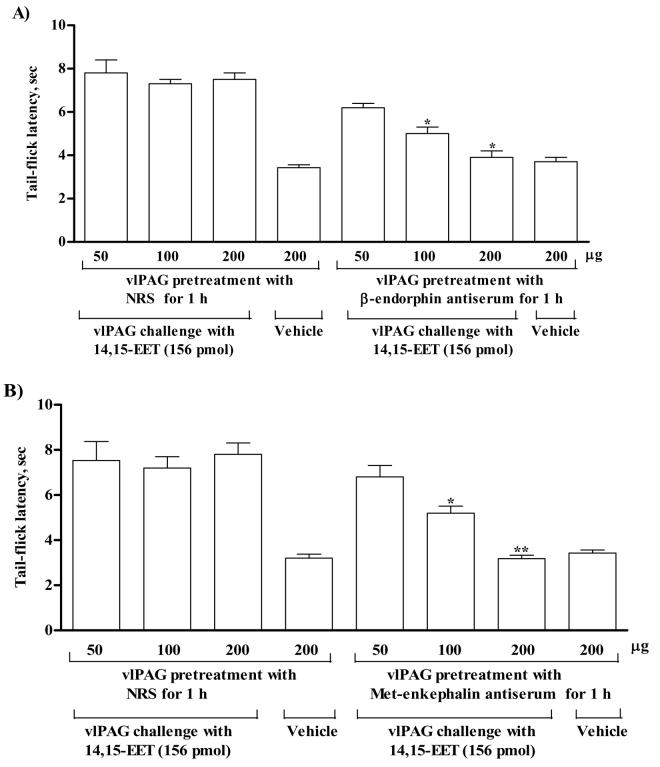
The results of the experiments described above indicate that 14,15-EET does not have any affinity for μ- and δ-opioid receptors. The finding excludes the possibility that tail-flick inhibition produced by 14,15-EET is mediated by a direct stimulation of μ- or δ-opioid receptors by 14,15-EET. The following experiments were then undertaken to determine whether the tail-flick inhibition produced by 14,15-EET is mediated indirectly by the activation of endogenous opioid peptides, β-endorphin, Met-enkephalin, Leu-enkephalin, endomorphin-1 or dynorphin A [1-17] at the vlPAG. Antisera against β-endorphin, Met-enkephalin, Leu-enkephalin, endomorphin-1 or dynorphin A [1-17] was used to determine if pretreatment with these antibodies to bind the extracellular opioid peptides would abolish tail-flick inhibition produced by 14,15-EET. Groups of rats were intra-vlPAG pretreated with various doses of β-endorphin antiserum (50, 100, 200 μg), Met-enkephalin antiserum (50, 100 or 200 μg), Leu-enkephalin antiserum (200 μg), endomorphin-1 antiserum (0.5 μl) or dynorphin A [1-17] antiserum (200 μg) 1h before intra-vlPAG administration of 14,15-EET (156 pmol) and tail-flick response was measured 10 min thereafter. Rats pretreated with normal rabbit serum served as control. Tail-flick inhibition produced by 14,15-EET was attenuated dose-dependently by intra-vlPAG pretreatment with β-endorphin antiserum (50 - 200 μg). Intra-vlPAG pretreatment with a high dose (200 μg) of β-endorphin antiserum completely abolished the 14,15-EET-produced tail-flick inhibition (Fig. 6A). Intra-vlPAG pretreatment with Met-enkephalin antiserum (50-200 μg) also dose-dependently attenuated tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG (Fig. 6B). Tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG was not affected by the pretreatment with normal rabbit serum (50-200 μg). Intra-vlPAG pretreatment with β-endorphin antiserum (200 μg) or Met-enkephalin antiserum (200 μg) for 1h did not affect the baseline tail-flick response in rats injected with saline vehicle microinjected into the vlPAG (Fig. 6AB). Tail-flick inhibition produced by 14,15-EET (156 pmol) microinjected into the vlPAG was not affected by intra-vlPAG pretreatment with Leu-enkephalin antiserum (200 μg; 7.0 ± 0.4, n=5), endomorphin-1 antiserum (0.5 μl; 6.6 ± 0.3, n=5), dynorphin A [1-17] antiserum (200 μg; 7.8 ± 1.0, n=5) or normal rabbit serum (200 μg; 7.8 ± 0.6, n=6).
Figure 6.
Effect of pretreatment with antiserum against β-endorphin (A) and antiserum against Met-enkepahin (B) microinjected into the vlPAG on tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Groups of rats were vlPAG pretreated with different doses of β-endorphin antiserum (50, 100 or 200 μg), Met-enkephalin antiserum (50, 100 or 200 μg) or NRS (50, 100 or 200 μg) 1h before vlPAG injection of 14,15-EET (156 pmol) and tail-flick response was measured 10 min after vlPAG microinjection of 14,15-EET (156 pmol). Separate groups of rats were vlPAG pretreated with β-endorphin antiserum (200 μg, 1 h) or Met-enkephalin antiserum (200 μg, 1 h) before vlPAG microinjection of vehicle. Pretreatment with β-endorphin antiserum or Met-enkephalin antiserum dose-dependently attenuated tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Data are represented as mean ± SEM, N=7 animals per group. Student's t-test was used to determine the difference between groups. * P < 0.01, ** P <0.001.
Effects of intrathecal pretreatment with antiserum against Met-enkephalin or β-endorphin or with NTI or CTOP on the tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG
The results of the experiments described above indicate that 14,15-EET microinjected into the vlPAG activates β-endorphin for producing tail-flick inhibition. It has been demonstrated previously that β-endorphin given supraspinally stimulates the ε-opioid receptors to activate the descending pain control system, which involves the release of Met-enkephalin acting on δ-opioid receptors in the spinal cord for producing antinociception (for reviews see Narita and Tseng, 1998; Tseng, 2001). Thus, inactivation of Met-enkephalin with Met-enkephalin antibody or the blockade of the δ-opioid receptors with δ-opioid receptor blocker NTI or μ-opioid receptor antagonist CTOP in the spinal cord would be expected to block the antinociception produced by 14,15-EET from vlPAG 14,15-EET. The effects of intrathecal pretreatment with antiserum against Met-enkephalin or β-endorphin, δ-opioid receptor antagonist NTI or μ-opioid receptor antagonist CTOP on the tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG were determined. Groups of rats were pretreated intrathecally with Met-enkephalin antiserum (50 or 200 μg) or β-endorphin antiserum (200 μg) for 1 hr or δ-opioid receptor antagonist NTI (0.2 or 2.2 nmol) for 10 min or μ-opioid receptor antagonist CTOP (9.4 or 94.1 pmol) for 5 min before intra-vlPAG administration of 14,15-EET (156 pmol) and tail-flick response was measured 10 min thereafter. Intrathecal pretreatment with Met-enkephalin antiserum at a dose of 200 μg, but not 50 μg, markedly attenuated the tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. On the other hand, intrathecal pretreatment with β-endorphin antiserum at a dose of 200 μg did not affect the tail-flick inhibition produced by 14,15-EET from the vlPAG (Fig. 7). Intrathecal pretreatment with Met-enkephalin antiserum (200 μg) did not affect the baseline tail-flick latency in rats injected with vehicle into the vlPAG. Intrathecal pretreatment with the normal rabbit serum did not affect tail-flick inhibition produced by 14,15-EET, nor did it affect the baseline tail-flick latency in rats injected with vehicle into the vlPAG (Fig. 7).
Figure 7.
Effects of intrathecal pretreatment with Met-enkephalin antiserum or β-endorphin antiserum on tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Groups of rats were pretreated intrathecally with different doses of Met-enkephalin antiserum (50 or 200 μg), β-endorphin antiserum (200 μg) or NRS (50 or 200 μg) 1h before vlPAG injection of 14,15-EET (156 pmol). Tail-flick response was then measured 10 min after microinjection of 14,15-EET into the vlPAG. Separate groups of rats were pretreated intrathecally with Met-enkephalin antiserum (200 μg, 1 h) or NRS (200 μg, 1 h) before vlPAG microinjection of vehicle. Intrathecal pretreatment with Met-enkephalin antiserum but not β-endorphin antiserum attenuated tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Student's t-test was used to determine the difference between groups. *P < 0.001.
Blockade of δ-opioid receptors in the spinal cord by intrathecal pretreatment with NTI (0.2 and 2.2 nmol) dose-dependently attenuated the tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG (Fig. 8A). However, blockade of μ-opioid receptors in the spinal cord by intrathecal pretreatment with 94 pmol, but not 9.4 pmol of CTOP, also attenuated the tail-flick inhibition produced by 14,15-EET from vlPAG (Fig. 8B). Intrathecal pretreatment of a high dose of NTI (2.2 nmol) or CTOP (94.1 pmo) did not effect the tail-flick latency in rats injected with vehicle into the vlPAG (Fig. 8AB).
Figure 8.
Effects of intrathecal pretreatment with the μ-opioid receptor antagonist CTOP (A) or δ-opioid receptor antagonist NTI (B) on tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Groups of rats were pretreated intrathecally with different doses of CTOP (9.4 or 94.1 pmol) or vehicle for 5 min, or different doses of NTI (0.2 or 2.2 nmol) or vehicle for 10 min before vlPAG microinjection of 14,15-EET (156 pmol). Tail-flick response was then measured 10 min after 14,15-EET (156 pmol) microinjection into the vlPAG. Separate groups of rats were pretreated intrathecally with CTOP (94.1 pmol, 5 min) or NTI (2.2 nmol, 10 min) before vlPAG microinjection of vehicle. Intrathecal pretreatment with CTOP or NTI attenuated tail-flick inhibition produced by 14,15-EET microinjected into the vlPAG. Data are represented as mean ± SEM, n= 6 animals per group. One-way ANOVA followed by Dunnett's post-test was used to determine the difference between groups. *P < 0.001.
Discussion
Biologically active lipid mediators derived from the precursor arachidonic acid have been described in several systems (Rifkind et al., 1995; Laethem et al., 1996; Makita et al., 1996; Ma et al., 1999). Recent attention has been focused on those metabolites derived via CYP-dependent metabolism of arachidonic acid by epoxygenases to EETs. EETs have been shown to produce vasodilation (Xiao et al., 1998; Chen et al., 1999), activate potassium channels (Gebremedhin et al., 1992), produce anti-inflammatory effects (Inceoglu et al., 2006) and stimulate angiogenesis (Pozzi et al., 2005). Additionally, this investigation supports the novel concept that EETs produce analgesia that is dependent upon the release of endogenous opioids. In support of these concepts the present study has identified that: 1) 14,15-EET, but not 11,12-, 8,9- or 5,6-EET dose-dependently inhibits thermally-induced pain using the tail-flick model, 2) The ED50 for 14,15-EET for tail-flick inhibition was estimated to be 32.5 pmol, which is 35-fold more potent than morphine for inhibiting tail-flick response, 3) antinociception produced by 14,15-EET from the vlPAG is mediated by the stimulation of μ- and δ-, but not κ-opioid receptors and 4) this effect may be mediated via the activation of β-endorphin and Met-enkephalin acting on μ- and δ-opioid receptors for producing antinociception at both supraspinal and spinal sites.
While this may be the first report for the analgesic function of EET in acute pain, Inceoglu et al. (2006) have also suggested a role for EETs in inflammatory pain. These authors reported that inhibition of soluble epoxide hydrolase (sEH), to indirectly increase endogenous EETs, reduce thermal and mechanical models of pain in an LPS-induced rat model of inflammatory pain (Inceoglu et al., 2006). The studies of Inceoglu further demonstrated the increased levels of endogenous EETs and decreased Prostaglandin D2 (PGD2) by soluble epoxide hydrolase inhibitors (sEHI). Similar observations were made in LPS treated mice with systemic administration of cyclooxygenase inhibitors in combination with sEHIs (Schmelzer et al., 2005). Prostaglandin E2 (PGE2) levels were decreased with concomitant increases in EETs. These studies indicate that shifting the balance of pro- and anti-inflammatory eicosanoids may ultimately be important for overall analgesic efficacy. However, a direct comparison can not be made due to the fact that the type of pain induced using the tail flick assay is different than that induced via inflammation.
This study found that 5,6-EET, 8,9-EET and 11,12-EET even at the highest dose tested (156 pmol) failed to inhibit the tail-flick response. This lack of efficacy among the EET regioisomers may be a function of their stereochemistry which could yield important new data regarding possible EET “receptors” (Gauthier et al., 2004). Some evidence suggests that EETs act in a receptor-dependent manner as their biological effects have been shown to be blocked by chemically synthesized EET antagonists like 14,15-EEZE (Gauthier et al., 2004).
In the present study we further attempted to explore the mechanism of action of 14,15-EET in analgesia. Antinociception produced by 14,15-EET from the vlPAG is mediated in part by the stimulation of μ- and also δ-opioid receptors at the vlPAG injected sites. This view is evident by the finding that the blockade of μ- or δ-opioid receptors in vlPAG by intra-vlPAG treatment with the μ-opioid receptor antagonist CTOP or δ-opioid receptor antagonist NTI dose-dependently blocked antinociception produced by 14,15-EET. CTOP was found to be more effective than NTI in attenuating 14,15-EET-produced antinociception, suggesting that μ-opioid receptors may play a more important role than δ-opioid receptors in the 14,15-EET-produced antinociception at the supraspinal vlPAG sites. However, pretreatment with the κ-opioid receptor antagonist nor-BNI was not effective in attenuating antinociception produced by 14,15-EET, indicating that κ-opioid receptor at the vlPAG is not involved in 14,15-EET-produced antinociception.
Despite the fact that no specific EET receptor has yet been characterized, several studies suggest the possibility of both intracellular and membrane bound EETs receptors (Spector et al., 2004) and recent reports (Inceoglu et al., 2007) have shown binding in the micro molar range of EETs to peripheral benzodiazepine receptor (PBR), cannabinoid CB2 receptor, neurokinin NK2 receptor and Dopamine D3 receptors. It is also possible that EETs may be acting on neuronal ion channels (e.g. potassium and/or TRP).
The lack of direct binding of 14,15-EET to opioid recptors in our studies led us to explore the possibility of release of endogenous opioid peptides, such as β-endorphin, Met-enkephalin, Leu-enkephalin, endomorphin-1 or dynorphin A [1-17]. We found that intra-vlPAG pretreatment with antiserum against β-endorphin or Met-enkephalin, which bind extracellular β-endorphin and Met-enkephalin respectively, blocked the tail-flick inhibition produced by 14,15-EET from the vlPAG. This finding suggests that antinociception produced by 14,15-EET is mediated by the activation of β-endorphin and Met-enkephalin at the vlPAG sites. On the other hand, vlPAG pretreatment with antiserum against Leu-enkephalin, endomorphin-1 or dynorphin A [1-17] did not affect antinociception produced by 14,15-EET, excluding the possibility that these endogenous opioid peptides are involved in antinociception produced by 14,15-EET. In this regard, it is important to note that only 14,15-EET was an effective analgesic, while the other regioisomers were without effect.
β-Endorphin is a non-selective opioid receptor agonist, which stimulates μ-, δ- and ε-opioid receptors (Tseng, 2001). β-Endorphin when activated by 14,15-EET may directly stimulate μ- or δ-opioid receptors at the injected vlPAG sites. On the other hand, β-endorphin may also act on ε-opioid receptors and cause release of Met-enkephalin, which stimulates δ-opioid receptors (Tseng, 2001) for producing antinociception.
Next, we wanted to determine through which descending pain control system the activated β-endorphin produces antinociception. We found in the present studies that intrathecal pretreatment with Met-enkephalin antiserum, δ-opioid receptor antagonist NTI or μ-opioid receptor antagonist CTOP blocked antinociception produced by 14,15-EET microinjected into the vlPAG. The finding suggests that antinociception produced by 14,15-EET microinjected into the supraspinal vlPAG is mediated by the activation of Met-enkephalin and the stimulation of μ- and δ-opioid receptors in the spinal cord. On the other hand, intrathecal pretreatment with β-endorphin antiserum did not block antinociception produced by 14,15-EET microinjected into the supraspinal vlPAG, suggesting that β-endorphin is not involved in the 14,15-EET-prduced antinociception. These findings suggest that 14,15-EET microinjected into the vlPAG activates β-endorphin acting on the μ- and δ-opioid receptors at the supraspinal site, which in turn activates Met-enkephalin acting on μ- and δ-opioid receptors in the spinal cord for producing antinociception (Fig. 9).
Figure 9.
Schematic diagram of a possible mechanism of action of 14,15-EET activated descending pain control system. In this pathway, β-endorphin stimulated by 14,15-EET may activate Met-enkephalin via μ-, δ- and/or ε-opioid receptors. In addition, activated Met-enkephalin at either site, vlPAG or intrathecaly, are both necessary to produce antinociception and therefore could be acting additively. The “+” represents an additive effect of Met-enkephalins.
Also it has been previously reported that β-endorphin injected supraspinally stimulates the ε-opioid receptors and subsequently activates the descending pain control pathway, which involves the release of Met-enkephalin acting on μ- and δ-opioid receptors in the spinal cord for producing antinociception (for review see Narita and Tseng, 1998; Tseng, 2001). Based on their findings we would like to put forth the speculation that 14,15-EET-activated β-endorphin at the supraspinal site act on the ε-opioid receptor and subsequently release Met-enkephalin acting on μ- and δ-opioid receptors in the spinal cord for producing antinociception (Fig. 9). Although our work in conjunction with the work of Tseng (2001) suggests that μ/δ- and/or ε-opioid receptors may be important for the stimulation of the descending pain control system(s), this hypothesis still needs further investigation.
It has been previously demonstrated that simultaneous activation of the opioid receptors at both supraspinal and spinal sites by opioid receptor agonists induce a multiplicative or additive interaction for producing antinociception (Roerig et al., 1991; Yeung and Rudy, 1980). Based on this view we speculate that activation of μ- and δ-opioid receptors by Met-enkephalin both at supraspinal and spinal cord sites act additively to produce the antinociceptive action of 14,15-EET from the vlPAG (Fig. 9).
There is a striking similarity between 14,15-EET-produced analgesia and the analgesia induced by continuous cold-water swimming in a 2°C ice-water bath. The β-endorphin-mediated system has been suggested to be involved in the analgesia induced by cold-water swimming. Such a system involves the activation of β-endorphin acting on ε-opioid receptor at the supraspinal sites and the release of Met-enkephalin acting on δ-opioid recepors at the spinal sites to produce analgesia (Narita and Tseng, 1998; Tseng, 2001). Thus, it is speculated that synthesis of 14,15-EET may be stimulated by the cold-water bath to activate endogenous β-endorphin at the supraspinal sites which may be acting via the ε-receptor and subsequently induce spinal release of Met-enkephalin acting on δ- and μ-opioid receptors for regulation of pain in the brain.
In summary, our studies in conjunction with the work of other investigators, such as Inceoglu et al (2006) show that EETs are analgesic. While the precise mechanism of action has yet to be defined, our working hypothesis suggests it may be that EETs stimulate the release of β- endorphin which then stimulates the further release of endogenous opioids. Additional studies are necessary to precisely understand the biological effects of EETs as analgesics as they could become novel therapeutic agents for the treatment of pain.
Acknowledgments
This work was supported by NIH/NHLBI PO1 HL6876, PO1 HL59996, RO1 HL33833, Veterans administration grant 3440-06P (PI: David R. Harder).
Abbreviations
- vlPAG
ventrolateral periaqueductal gray
- CYP
Cytochrome P450
- EET
Epoxyeicosatrienoic Acids
- CTOP
d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2
- nor-BNI
norbinaltorphimine
- NTI
naltrindole
- AA
arachidonic acid
- DAMGO
[d-Ala2,N-MePhe4,Gly-ol5]enkephalin
- DPDPE
[D-Pen2,5]-enkephalin
- NRS
normal rabbit serum
References
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Arts KS, Fujimoto JM, Tseng LF. Involvement of dynorphin A and not substance P in the spinal antianalgesic action of capsaicin against morphine-induced antinociception in mice. J Pharmacol Exp Ther. 1992;261:643–651. [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bylund J, Zhang C, Harder DR. Identification of a novel cytochrome P450, CYP4X1, with unique localization specific to the brain. Biochem Biophys Res Commun. 2002;296:677–684. doi: 10.1016/s0006-291x(02)00918-x. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou D, Okubo T, Kao YC, Yang C. Breast tumor aromatase: functional role and transcriptional regulation. Endocr Relat Cancer. 1999;6:149–156. doi: 10.1677/erc.0.0060149. [DOI] [PubMed] [Google Scholar]
- Craft RM, Tseng AH, McNiel DM, Furness MS, Rice KC. Receptor- selective antagonism of opioid antinociception in female versus male rats. Behavioral Physiology. 2001;12:591–602. doi: 10.1097/00008877-200112000-00003. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–524. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins and other Lipid Mediators. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junier MP, Dray F, Blair I, Capdevila J, Dishman E, Falk JR, Ojeda SR. Epoxygenase products of arachidonic acid are endogenous constituents of the hypothalamus involved in D2 receptor-mediated, dopamine –induced release of somatostatin. Endocrinol. 1990;126:1534–1540. doi: 10.1210/endo-126-3-1534. [DOI] [PubMed] [Google Scholar]
- Laethem RM, Balazy M, Koop DR. Epoxidation of C18 unsaturated fatty acids by cytochromes P4502C2 and P4502CAA. Drug Metab Dispos. 1996;24:664–668. [PubMed] [Google Scholar]
- Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem. 1999;274:17777–17788. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- Makita K, Falck JR, Capdevila JH. Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. FASEB J. 1996;10:1456–1463. doi: 10.1096/fasebj.10.13.8940291. [DOI] [PubMed] [Google Scholar]
- Narita M, Tseng LF. Evidence for the existence of the beta-endorphin-sensitive “epsilon-opioid receptor” in the brain: the mechanisms of epsilon-mediated antinociception. Jap J Pharmacol. 1998;76:233–253. doi: 10.1254/jjp.76.233. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa M, Mizoguchi H, Narita M, Chu M, Nagase H, Tseng LF. Differential mechanisms mediating descending pain controls for antinociception induced by supraspinally administered endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther. 2000;294:1106–1111. [PubMed] [Google Scholar]
- Ohsawa M, Shiraki M, Mizoguchi H, Narita M, Kawai K, Nagase H, Cheng EY, Narita M, Tseng LF. Release of [Met5]enkephalin from the spinal cord by intraventricular administered endomorphin-2, but not endomorphin-1 in the anesthetized rat. Neuroscience Letters. 2001;316:1–4. doi: 10.1016/s0304-3940(01)02334-5. [DOI] [PubMed] [Google Scholar]
- Pavlovic ZW, Bodnar RJ. Opioid supraspinal analgesic synergy between the amygdala and periaqueductal gray in rats. Brain Res. 1998;779:158–169. doi: 10.1016/s0006-8993(97)01115-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Roerig SC, Hoffman RG, Takemori AE, Wilcox GL, Fujimoto JM. Isobolographic analysis of analgesic interactions between intrathecally and intracerebroventricularly administered fentanyl, morphine and D-Ala2-D-Leu5-enkephalin in morphine-tolerant and nontolerant mice. J Pharmacol Exp Ther. 1991;257:1091–1099. [PubMed] [Google Scholar]
- Rifkind AB, Lee C, Chang TK, Waxman DJ. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch Biochem Biophys. 1995;320:380–389. doi: 10.1016/0003-9861(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hommock BD. Soluble epoxide hydrolase is the therapeutic target for acute inflammation. Proc Natl Acad Sci. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Perotti JM, Crisp T, Cabral ME, Long JT, Scalziti JM. The mu opiate receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. Eur J Pharmacol. 1988;156:47–54. doi: 10.1016/0014-2999(88)90145-8. [DOI] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Progress Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Hung KC, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Sun HS, Clithero AD, Tseng LF. Differential mechanisms of antianalgesia induced by endomorphin-1 and endomorphin-2 in the ventral periaqueductal gray of the rat. J Pharmacol Exp Ther. 2005;312:1257–1265. doi: 10.1124/jpet.104.076224. [DOI] [PubMed] [Google Scholar]
- Tseng LF. The β-endorphin-sensitive opioid ε-receptor-mediated antinociception. Trends Pharmacol Sci. 2001;22:623–630. doi: 10.1016/s0165-6147(00)01843-5. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Suh HH. Intrathecal [Met5]enkephalin antibody blocks analgesia induced by intracerebroventricular beta-endorphin but not morphine in mice. Eur J Pharmacol. 1989;173:171–176. doi: 10.1016/0014-2999(89)90515-3. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Narita M, Suganuma C, Mizoguchi H, Ohsawa M, Nagase H, Kampine JP. Differential antinociceptive effects of endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther. 2000;292:576–583. [PubMed] [Google Scholar]
- Wu H, Hung KC, Ohsawa M, Mizoguchi H, Tseng LF. Antisera against endogenous opioids increase the nocifensive response to formalin: demonstration of inhibitory beta-endorphinergic control. Eur J Pharmacol. 2001;421:39–43. doi: 10.1016/s0014-2999(01)00970-0. [DOI] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun H, Leitermann RJ, Fujimoto JM, Tseng LF. Nonopioidergic mechanism mediating morphine-induced antianalgesia in the mouse spinal cord. J Pharmacol Exp Ther. 2004;310:240–246. doi: 10.1124/jpet.104.065334. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+channels and contraction in mammalian heart cells. J Physiol. 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Al-Rodhan NR, Jensen TS. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371–394. doi: 10.1016/s0079-6123(08)62803-4. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Rudy TA. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of action as revealed by concurrent intrathecal and intracerebroventricular injection of morphine. J Pharmacol Exp Ther. 1980;215:633–642. [PubMed] [Google Scholar]